Oxidative Stress and the Nrf2/PPARγ Axis in the Endometrium: Insights into Female Fertility
Abstract
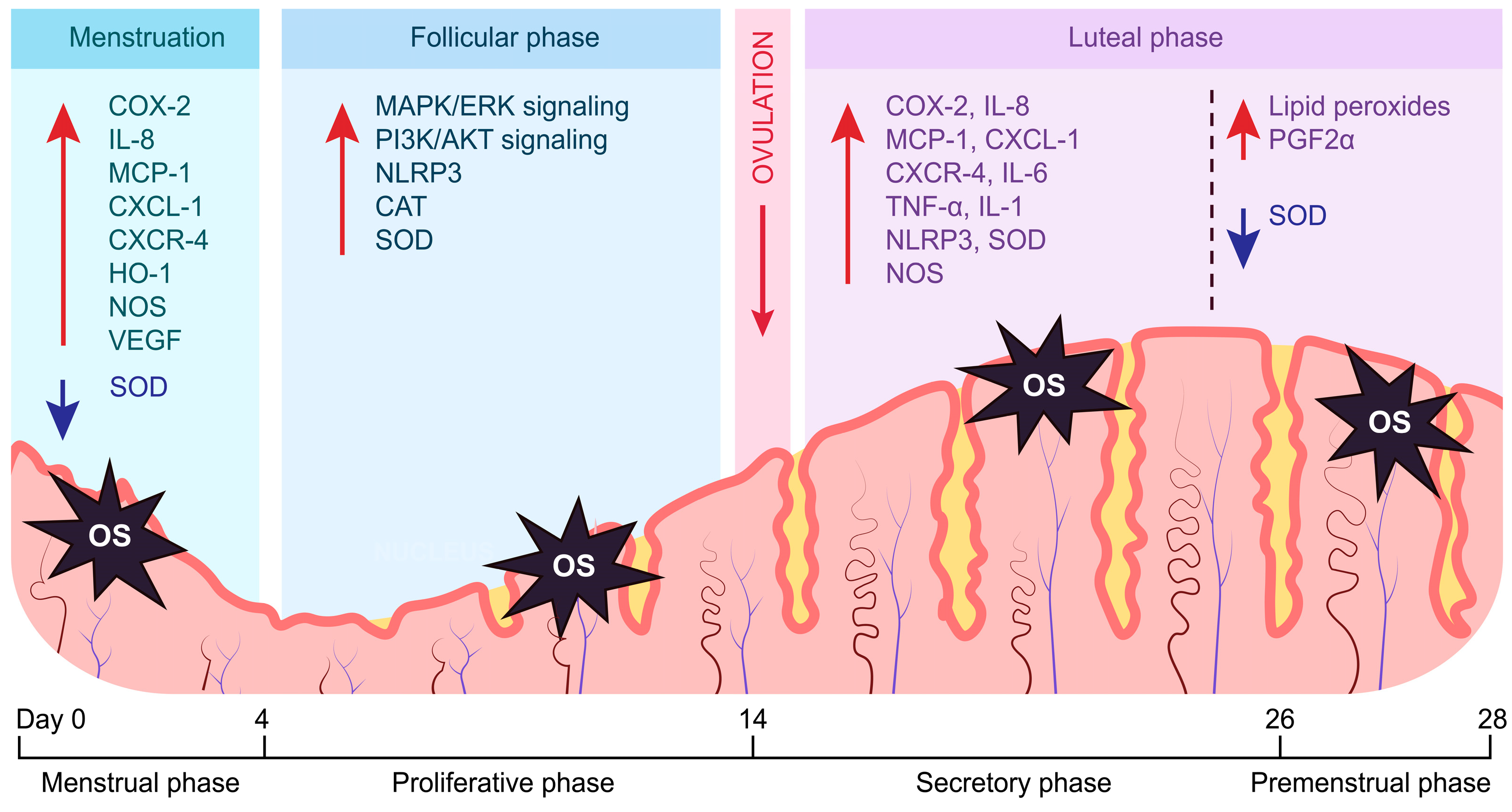
1. Introduction: Endometrial Cycle
2. The Role of Oxidative Stress in the Endometrium
2.1. Oxidative Stress Regulates Inflammation in the Endometrium
2.2. Oxidative Stress Regulates Angiogenesis in the Endometrium
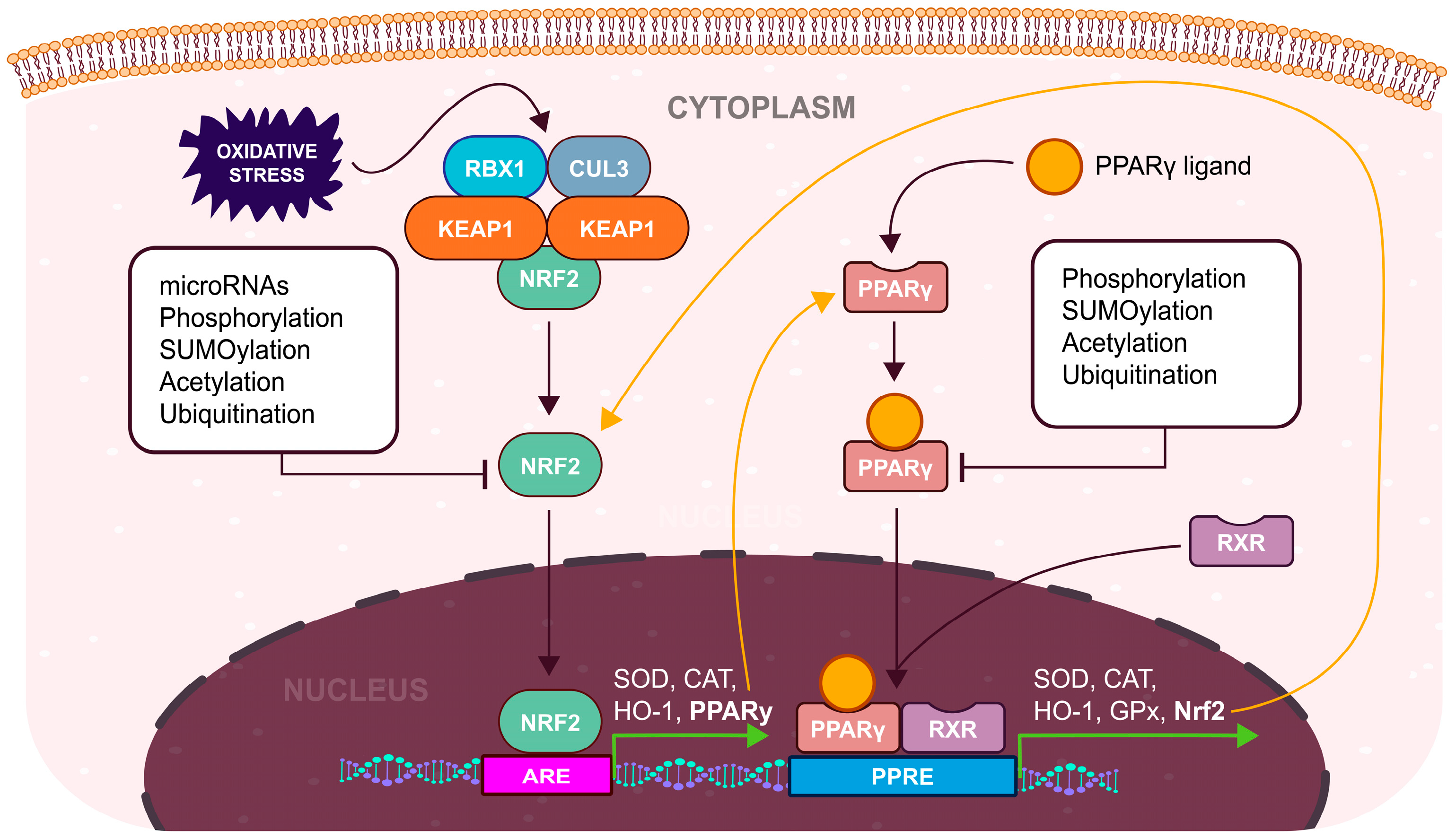
3. Antioxidant Defense and the Nrf2 Pathway
4. Biochemistry of PPARγ
5. Impact of Nrf2/PPARγ Pathway on the Endometrium
5.1. Negative Impact of the PPARγ Pathway on the Endometrium
5.2. Positive Impact of the PPARγ Pathway on the Endometrium
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sehring, J.; Beltsos, A.; Jeelani, R. Human Implantation: The Complex Interplay between Endometrial Receptivity, Inflammation, and the Microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Breindel, M.F.; Singh, M.; Kahn, J. Endometrial Receptivity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Makieva, S.; Giacomini, E.; Ottolina, J.; Sanchez, A.M.; Papaleo, E.; Viganò, P. Inside the Endometrial Cell Signaling Subway: Mind the Gap(s). Int. J. Mol. Sci. 2018, 19, 2477. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, S.; Kong, S. Psychological Stress and Functional Endometrial Disorders: Update of Mechanism Insights. Front. Endocrinol. 2021, 12, 690255. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen Receptor Signaling Mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-L.; Jeong, D.-U.; Kang, Y.; Kim, T.-H.; Lee, S.K.; Han, A.-R.; Kang, J.; Park, S.-R. Impairment of Decidualization of Endometrial Stromal Cells by Hsa-miR-375 through NOX4 Targeting. Reprod. Sci. 2022, 29, 3212–3221. [Google Scholar] [CrossRef]
- Gao, W.; Feng, F.; Ma, X.; Zhang, R.; Li, L.; Yue, F.; Lv, M.; Liu, L. Progress of Oxidative Stress in Endometrium Decidualization. J. Obstet. Gynaecol. 2022, 42, 3429–3434. [Google Scholar] [CrossRef] [PubMed]
- Cope, D.I.; Monsivais, D. Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success. Cells 2022, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Chodankar, R.R.; Maybin, J.A.; Critchley, H.O.D. Uterine Bleeding: How Understanding Endometrial Physiology Underpins Menstrual Health. Nat. Rev. Endocrinol. 2022, 18, 290–308. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, L.; Luo, X.; Chen, X. Adult Stem Cells in Endometrial Regeneration: Molecular Insights and Clinical Applications. Mol. Reprod. Dev. 2021, 88, 379–394. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of Oxidative Stress on Male and Female Germ Cells: Implications for Fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Donnez, J.; Dolmans, M.-M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? Int. J. Mol. Sci. 2021, 22, 7138. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A Novel and Compact Review on the Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Moustakli, E.; Zachariou, A.; Tsirka, G.; Tsiampali, C.; Palapela, N.; Sofikitis, N.; Dimitriadis, F. The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants 2023, 12, 1490. [Google Scholar] [CrossRef] [PubMed]
- Puspita, R.D.; Rizal, D.M.; Syarif, R.A.; Sari, I.P. Role of COX-2 for Successful Embryo Implantation Process: A Mini-Review. Open Access Maced. J. Med. Sci. 2023, 11, 31–37. [Google Scholar] [CrossRef]
- Asgari, R.; Vaisi-Raygani, A. The Association of Inflammation with Reproductive System Disorders of Women. Cent. Asian J. Med. Pharm. Sci. Innov. 2021, 1, 67–73. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Moludi, J.; Kamari, N.; Darbandi, M.; Mostafaei, S.; Moradi, S.; Pasdar, Y.; Najafi, F.; Navabi, J.; Saber, A. Association between Dietary Inflammatory Index and Infertility of Women; Results from RaNCD Cohort Study. Nutr. J. 2023, 22, 35. [Google Scholar] [CrossRef]
- Barabás, K.; Szabó-Meleg, E.; Ábrahám, I.M. Effect of Inflammation on Female Gonadotropin-Releasing Hormone (GnRH) Neurons: Mechanisms and Consequences. Int. J. Mol. Sci. 2020, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic Inflammation Induces Telomere Dysfunction and Accelerates Ageing in Mice. Nat. Commun. 2014, 2, 4172. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef] [PubMed]
- Ansariniya, H.; Yavari, A.; Javaheri, A.; Zare, F. Oxidative Stress-related Effects on Various Aspects of Endometriosis. Am. J. Reprod. Immunol. 2022, 88, e13593. [Google Scholar] [CrossRef]
- Barrionuevo, M.J.; Schwandt, R.A.; Rao, P.S.; Graham, L.B.; Maisel, L.P.; Yeko, T.R. Nitric Oxide (NO) and Interleukin-1beta (IL-1beta) in Follicular Fluid and Their Correlation with Fertilization and Embryo Cleavage. Am. J. Reprod. Immunol. N. Y. N 1989 2000, 44, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T.; Mohamed, M.S.; Aleem, A.A.N.; Sharma, R.K.; Worley, S.E.; Thornton, J.; Agarwal, A. Differential Growth of Human Embryos in Vitro: Role of Reactive Oxygen Species. Fertil. Steril. 2004, 82, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. The Role of Nitric Oxide on Male and Female Reproduction. Malays. J. Med. Sci. 2022, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Ma, L.; Ma, W.G.; Maas, R.L.; Dey, S.K. Hoxa-10 Regulates Uterine Stromal Cell Responsiveness to Progesterone during Implantation and Decidualization in the Mouse. Mol. Endocrinol. Baltim. Md 1999, 13, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Wang, T.-S.; Qin, F.-N.; Huang, Z.; Liang, X.-H.; Gao, F.; Song, Z.; Yang, Z.-M. Differential Regulation of Receptivity in Two Uterine Horns of a Recipient Mouse Following Asynchronous Embryo Transfer. Sci. Rep. 2015, 5, 15897. [Google Scholar] [CrossRef]
- Song, H.; Lim, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Dysregulation of EGF Family of Growth Factors and COX-2 in the Uterus during the Preattachment and Attachment Reactions of the Blastocyst with the Luminal Epithelium Correlates with Implantation Failure in LIF- Deficient Mice. Mol. Endocrinol. 2000, 14, 1147–1161. [Google Scholar] [CrossRef]
- St-Louis, I.; Singh, M.; Brasseur, K.; Leblanc, V.; Parent, S.; Asselin, E. Expression of COX-1 and COX-2 in the Endometrium of Cyclic, Pregnant and in a Model of Pseudopregnant Rats and Their Regulation by Sex Steroids. Reprod. Biol. Endocrinol. 2010, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chang, X.; Bai, J.; Chen, Z.-J.; Li, W.-P.; Zhang, C. The Study of Cyclooxygenase 2 in Human Decidua of Preeclampsia. Biol. Reprod. 2016, 95, 56. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, E.; Vázquez-Martínez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbón, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2020, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, R.; Chen, Y.; Fang, Z.; Tang, J.; Yao, J.; Gao, J.; Chen, X.; Shi, X. Prognostic Significance and Mechanisms of CXCL Genes in Clear Cell Renal Cell Carcinoma. Aging 2023, 15, 7974–7996. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.M.; Silvestre, O.F.; Silva, B.F.; Ferreira, C.J.; Lopes, I.; Gomes, A.C.; Espiña, B.; Sárria, M.P. pH-Sensitive Nanoliposomes for Passive and CXCR-4-Mediated Marine Yessotoxin Delivery for Cancer Therapy. Nanomedicine 2022, 17, 717–739. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Lee, A.; Kim, J.; Park, T.J.; Lim, S.B. Transcriptional Heterogeneity of Cellular Senescence in Cancer. Mol. Cells 2022, 45, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Pan, Y.; Shi, G.; Ren, J.; Fan, H.; Dou, H.; Hou, Y. mTOR Regulates NLRP3 Inflammasome Activation via Reactive Oxygen Species in Murine Lupus. Acta Biochim. Biophys. Sin. 2018, 50, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.; Yu, T.; Chu, X. NLRP3 Inflammasome in Endothelial Dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Azlan, A.; Salamonsen, L.A.; Hutchison, J.; Evans, J. Endometrial Inflammasome Activation Accompanies Menstruation and May Have Implications for Systemic Inflammatory Events of the Menstrual Cycle. Hum. Reprod. Oxf. Engl. 2020, 35, 1363–1376. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Ma, J.; Wang, S.; Ma, R.; Ge, X.; Zhao, W.; Xue, T.; Chen, L.; Yao, B. NLRP3 Promotes Endometrial Receptivity by Inducing Epithelial–Mesenchymal Transition of the Endometrial Epithelium. Mol. Hum. Reprod. 2021, 27, gaab056. [Google Scholar] [CrossRef] [PubMed]
- Karizbodagh, M.P.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Implantation Window and Angiogenesis. J. Cell. Biochem. 2017, 118, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Seifer, D.; Arici, A. The Emerging Role of Angiogenic Factor Dysregulation in the Pathogenesis of Polycystic Ovarian Syndrome. Semin. Reprod. Med. 2015, 33, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Don, E.E.; Middelkoop, M.-A.; Hehenkamp, W.J.K.; Mijatovic, V.; Griffioen, A.W.; Huirne, J.A.F. Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7011. [Google Scholar] [CrossRef] [PubMed]
- Mrozikiewicz, A.E.; Kurzawińska, G.; Ożarowski, M.; Walczak, M.; Ożegowska, K.; Jędrzejczak, P. Polymorphic Variants of Genes Encoding Angiogenesis-Related Factors in Infertile Women with Recurrent Implantation Failure. Int. J. Mol. Sci. 2023, 24, 4267. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.A.; Murray, A.A.; Saunders, P.T.K.; Hirani, N.; Carmeliet, P.; Critchley, H.O.D. Hypoxia and Hypoxia Inducible Factor-1α Are Required for Normal Endometrial Repair during Menstruation. Nat. Commun. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, M.-A.; Don, E.E.; Hehenkamp, W.J.K.; Polman, N.J.; Griffioen, A.W.; Huirne, J.A.F. Angiogenesis in Abnormal Uterine Bleeding: A Narrative Review. Hum. Reprod. Update 2023, 29, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Wang, Y.; Lin, C.; Zhang, D.; Chen, J.; Ouyang, L.; Wu, F.; Zhang, J.; Chen, L. Recent Progress on Vascular Endothelial Growth Factor Receptor Inhibitors with Dual Targeting Capabilities for Tumor Therapy. J. Hematol. Oncol. 2022, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.-N. Curcumin: A Therapeutic Strategy in Cancers by Inhibiting the Canonical WNT/β-Catenin Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 323. [Google Scholar] [CrossRef]
- Vallée, A.; Vallée, J.-N.; Le Blanche, A.; Lecarpentier, Y. PPARγ Agonists: Emergent Therapy in Endometriosis. Pharmaceuticals 2021, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.G.; Won, Y.S.; Lee, H.Y.; Kim, Y.I.; Lee, J.-W.; Park, D.C. Increased Expression of Pattern Recognition Receptors and Nitric Oxide Synthase in Patients with Endometriosis. Int. J. Med. Sci. 2013, 10, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic Targeting of the Angiopoietin–TIE Pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, E.A.; Murtomäki, A.; Jha, S.K.; Anisimov, A.; Pink, A.; Zhang, Y.; Stritt, S.; Liaqat, I.; Stanczuk, L.; Alderfer, L.; et al. Lymphangiogenesis Requires Ang2/Tie/PI3K Signaling for VEGFR3 Cell-Surface Expression. J. Clin. Investig. 2022, 132, e155478. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A.-A. The Effects of Resveratrol on the Expression of VEGF, TGF-β, and MMP-9 in Endometrial Stromal Cells of Women with Endometriosis. Sci. Rep. 2021, 11, 6054. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Weng, M.-S.; Chang, J.-H.; Hung, W.-Y.; Yang, Y.-C.; Chien, M.-H. The Interplay of Reactive Oxygen Species and the Epidermal Growth Factor Receptor in Tumor Progression and Drug Resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Shavell, V.I.; Fletcher, N.M.; Abu-Soud, H.M.; Diamond, M.P.; Saed, G.M.; Detti, L. Superoxide Dismutase Levels Are Elevated in the Peri-Implantation Endometrium in Women Undergoing Ovarian Stimulation. Fertil. Steril. 2012, 97, S7. [Google Scholar] [CrossRef]
- Sugino, N.; Karube-Harada, A.; Taketani, T.; Sakata, A.; Nakamura, Y. Withdrawal of Ovarian Steroids Stimulates Prostaglandin F2.ALPHA. Production Through Nuclear Factor-.KAPPA.B Activation via Oxygen Radicals in Human Endometrial Stromal Cells: Potential Relevance to Menstruation. J. Reprod. Dev. 2004, 50, 215–225. [Google Scholar] [CrossRef]
- Riaposova, L.; Kim, S.H.; Hanyaloglu, A.C.; Sykes, L.; MacIntyre, D.A.; Bennett, P.R.; Terzidou, V. Prostaglandin F2α Requires Activation of Calcium-Dependent Signalling to Trigger Inflammation in Human Myometrium. Front. Endocrinol. 2023, 14, 1150125. [Google Scholar] [CrossRef]
- Pretto, C.M.; Gaide Chevronnay, H.P.; Cornet, P.B.; Galant, C.; Delvaux, D.; Courtoy, P.J.; Marbaix, E.; Henriet, P. Production of Interleukin-1α by Human Endometrial Stromal Cells Is Triggered during Menses and Dysfunctional Bleeding and Is Induced in Culture by Epithelial Interleukin-1α Released upon Ovarian Steroids Withdrawal. J. Clin. Endocrinol. Metab. 2008, 93, 4126–4134. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.J.; Adelson, R.P.; Vashistha, H.; Khalili, H.; Nayyar, A.; Puran, R.; Herrera, R.; Chatterjee, P.K.; Lee, A.T.; Truskinovsky, A.M.; et al. Single Cell Analysis of Menstrual Endometrial Tissues Defines Phenotypes Associated with Endometriosis. BMC Med. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, N. Three Oxidative Stress-Related Genes That Associate Endometrial Immune Cells Are Considered as Potential Biomarkers for the Prediction of Unexplained Recurrent Implantation Failure. Front. Immunol. 2022, 13, 902268. [Google Scholar] [CrossRef]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Diaz Sanchez, L.; Sanchez-Aranguren, L.; Wang, K.; Spickett, C.M.; Griffiths, H.R.; Dias, I.H.K. TNF-α-Mediated Endothelial Cell Apoptosis Is Rescued by Hydrogen Sulfide. Antioxidants 2023, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Tschugguel, W.; Schneeberger, C.; Unfried, G.; Bräutigam, G.; Stonek, F.; Wieser, F.; Vytiska-Binstorfer, E.; Czerwenka, K.; Weninger, W.; Kaider, A.; et al. Elevation of Inducible Nitric Oxide Synthase Activity in Human Endometrium during Menstruation. Biol. Reprod. 1999, 60, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Klepcová, Z.; Špaková, I.; Urdzík, P.; Štofilová, J.; Bertková, I.; Kľoc, M.; Rabajdová, M. The Importance of Natural Antioxidants in Female Reproduction. Antioxidants 2023, 12, 907. [Google Scholar] [CrossRef]
- Shan, H.; Luo, R.; Guo, X.; Li, R.; Ye, Z.; Peng, T.; Liu, F.; Yang, Z. Abnormal Endometrial Receptivity and Oxidative Stress in Polycystic Ovary Syndrome. Front. Pharmacol. 2022, 13, 904942. [Google Scholar] [CrossRef]
- Li, X.; Mu, J.; Lin, Y.; Zhao, J.; Meng, X. Combination of Cyanidin-3-O-Glucoside and Cisplatin Induces Oxidative Stress and Apoptosis in HeLa Cells by Reducing Activity of Endogenous Antioxidants, Increasing Bax/Bcl-2 mRNA Expression Ratio, and Downregulating Nrf2 Expression. J. Food Biochem. 2021, 45, e13806. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Ferrando, M.; Inglés, M.; Gambini, J.; Lopez-Grueso, R.; Edo, R.; Mas-Bargues, C.; Pellicer, A.; Viña, J. Estrogen Replacement Therapy Induces Antioxidant and Longevity-Related Genes in Women after Medically Induced Menopause. Oxid. Med. Cell. Longev. 2021, 2021, 8101615. [Google Scholar] [CrossRef] [PubMed]
- Lismont, C.; Revenco, I.; Fransen, M. Peroxisomal Hydrogen Peroxide Metabolism and Signaling in Health and Disease. Int. J. Mol. Sci. 2019, 20, 3673. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-J.; Zhang, X.; Abudukeyoumu, A.; Lai, Z.-Z.; Hou, D.-Y.; Wu, J.-N.; Tao, X.; Li, M.-Q.; Zhu, X.-Y.; Xie, F. Active Estrogen–Succinate Metabolism Promotes Heme Accumulation and Increases the Proliferative and Invasive Potential of Endometrial Cancer Cells. Biomolecules 2023, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Vasavda, C.; Kothari, R.; Malla, A.P.; Tokhunts, R.; Lin, A.; Ji, M.; Ricco, C.; Xu, R.; Saavedra, H.G.; Sbodio, J.I.; et al. Bilirubin Links Heme Metabolism to Neuroprotection by Scavenging Superoxide. Cell Chem. Biol. 2019, 26, 1450–1460.e7. [Google Scholar] [CrossRef]
- Wyatt, J.; Fernando, S.M.; Powell, S.G.; Hill, C.J.; Arshad, I.; Probert, C.; Ahmed, S.; Hapangama, D.K. The Role of Iron in the Pathogenesis of Endometriosis: A Systematic Review. Hum. Reprod. Open 2023, 2023, hoad033. [Google Scholar] [CrossRef]
- Hecht, J.L.; Janikova, M.; Choudhury, R.; Liu, F.; Canesin, G.; Janovicova, L.; Csizmadia, E.; Jorgensen, E.M.; Esselen, K.M.; Celec, P.; et al. Labile Heme and Heme Oxygenase-1 Maintain Tumor-Permissive Niche for Endometriosis-Associated Ovarian Cancer. Cancers 2022, 14, 2242. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, W.; Li, Y.; Xu, H.; Han, H.; Li, P.; Zhang, D.-X. Keap1-Targeting microRNA-941 Protects Endometrial Cells from Oxygen and Glucose Deprivation-Re-Oxygenation via Activation of Nrf2 Signaling. Cell Commun. Signal. 2020, 18, 32. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Z.; Chen, Y.; Jiang, S.; Wu, Z.; Ding, B.; Yang, Y.; Jin, Z.; Tang, H. An Overview of the Posttranslational Modifications and Related Molecular Mechanisms in Diabetic Nephropathy. Front. Cell Dev. Biol. 2021, 9, 630401. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.S.; McIntosh, D.J.; Ingram, S.M.; Tillery, L.; Motley, E.D.; Arinze, I.J.; Misra, S. SUMO-Modification of Human Nrf2 at K110 and K533 Regulates Its Nucleocytoplasmic Localization, Stability and Transcriptional Activity. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2021, 55, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Nam, L.B.; Keum, Y.-S. Binding Partners of NRF2: Functions and Regulatory Mechanisms. Arch. Biochem. Biophys. 2019, 678, 108184. [Google Scholar] [CrossRef]
- Lee, J.A.; Kwon, Y.-W.; Kim, H.R.; Shin, N.; Son, H.J.; Cheong, C.S.; Kim, D.J.; Hwang, O. A Novel Pyrazolo[3,4-d]Pyrimidine Induces Heme Oxygenase-1 and Exerts Anti-Inflammatory and Neuroprotective Effects. Mol. Cells 2022, 45, 134–147. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Heidari, Z.; Chrisman, I.M.; Nemetchek, M.D.; Novick, S.J.; Blayo, A.-L.; Patton, T.; Mendes, D.E.; Diaz, P.; Kamenecka, T.M.; Griffin, P.R.; et al. Definition of Functionally and Structurally Distinct Repressive States in the Nuclear Receptor PPARγ. Nat. Commun. 2019, 10, 5825. [Google Scholar] [CrossRef]
- Takada, I.; Makishima, M. Peroxisome Proliferator-Activated Receptor Agonists and Antagonists: A Patent Review (2014-Present). Expert Opin. Ther. Pat. 2020, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reisenbichler, H.; Eckl, P.M. Genotoxic Effects of Selected Peroxisome Proliferators. Mutat. Res. 1993, 286, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.; Rose, M.L.; Bojes, H.K.; Thurman, R.G. Novel Role of Oxidants in the Molecular Mechanism of Action of Peroxisome Proliferators. Antioxid. Redox Signal. 2000, 2, 607–621. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-Regulation of the Inflammatory Response by Peroxisome Proliferator-Activated Receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Badr, M.Z. PPARs: History and Advances. In Peroxisome Proliferator-Activated Receptors (PPARs): Methods and Protocols; Badr, M.Z., Youssef, J.A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 1–6. ISBN 978-1-62703-155-4. [Google Scholar]
- Hu, W.; Jiang, C.; Kim, M.; Xiao, Y.; Richter, H.J.; Guan, D.; Zhu, K.; Krusen, B.M.; Roberts, A.N.; Miller, J.; et al. Isoform-Specific Functions of PPARγ in Gene Regulation and Metabolism. Genes Dev. 2022, 36, 300–312. [Google Scholar] [CrossRef]
- Ballav, S.; Biswas, B.; Sahu, V.K.; Ranjan, A.; Basu, S. PPAR-γ Partial Agonists in Disease-Fate Decision with Special Reference to Cancer. Cells 2022, 11, 3215. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer. Cells 2022, 11, 2432. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. PPARs and Angiogenesis—Implications in Pathology. Int. J. Mol. Sci. 2020, 21, 5723. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The Role of Peroxisome Proliferator-Activated Receptors (PPAR) in Immune Responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Rastinejad, F. Retinoic Acid Receptor Structures: The Journey from Single Domains to Full-Length Complex. J. Mol. Endocrinol. 2022, 69, T25–T36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shen, T.; Chitranshi, N.; Gupta, V.; Basavarajappa, D.; Sarkar, S.; Mirzaei, M.; You, Y.; Krezel, W.; Graham, S.L.; et al. Retinoid X Receptor: Cellular and Biochemical Roles of Nuclear Receptor with a Focus on Neuropathological Involvement. Mol. Neurobiol. 2022, 59, 2027–2050. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, J.; Byun, J.; Park, J.Y.; Yamamoto, T.; Schesing, K.; Tian, B.; Sadoshima, J.; Oka, S. An Ideal PPAR Response Element Bound to and Activated by PPARα. PLoS ONE 2015, 10, e0134996. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.V.; Tchepourina, E.; Korsunskaya, I.M.; Geppe, N.A.; Chebysheva, S.N.; Soboleva, A.G.; Mezentsev, A. The Role of Transcription Factor PPAR-γ in the Pathogenesis of Psoriasis, Skin Cells, and Immune Cells. Int. J. Mol. Sci. 2022, 23, 9708. [Google Scholar] [CrossRef] [PubMed]
- Dharap, A.; Pokrzywa, C.; Murali, S.; Kaimal, B.; Vemuganti, R. Mutual Induction of Transcription Factor PPARγ and microRNAs miR-145 and miR-329. J. Neurochem. 2015, 135, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Xu, S.; Zhu, Y.; Zheng, X.; Lu, Y.; Tu, J.; He, Y.; Jin, L.; Li, Y. PPARγ Transcription Deficiency Exacerbates High-Fat Diet-Induced Adipocyte Hypertrophy and Insulin Resistance in Mice. Front. Pharmacol. 2020, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Nemetchek, M.D.; Chrisman, I.M.; Rayl, M.L.; Voss, A.H.; Hughes, T.S. A Structural Mechanism of Nuclear Receptor Biased Agonism. Proc. Natl. Acad. Sci. USA 2022, 119, e2215333119. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Fleckenstein, F.N.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Preeclampsia. Cells 2023, 12, 647. [Google Scholar] [CrossRef]
- Jang, Y.; Park, Y.-K.; Lee, J.-E.; Wan, D.; Tran, N.; Gavrilova, O.; Ge, K. MED1 Is a Lipogenesis Coactivator Required for Postnatal Adipose Expansion. Genes Dev. 2021, 35, 713. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Tamura, I.; Fujimura, T.; Doi-Tanaka, Y.; Shirafuta, Y.; Mihara, Y.; Maekawa, R.; Taketani, T.; Sato, S.; Tamura, H.; et al. Transcriptional Coactivator PGC-1α Contributes to Decidualization by Forming a Histone-Modifying Complex with C/EBPβ and P300. J. Biol. Chem. 2022, 298, 101874. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Fan, R. PPARα and NCOR/SMRT Corepressor Network in Liver Metabolic Regulation. FASEB J. 2020, 34, 8796–8809. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Maurya, S.K.; Pruzinsky, E.; Batmanov, K.; Xiao, Y.; Sulon, S.M.; Sakamoto, T.; Wang, Y.; Lai, L.; McDaid, K.S.; et al. RIP140 Deficiency Enhances Cardiac Fuel Metabolism and Protects Mice from Heart Failure. J. Clin. Investig. 2023, 133, e162309. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S. The Role of Histone Deacetylase 3 Complex in Nuclear Hormone Receptor Action. Int. J. Mol. Sci. 2021, 22, 9138. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, W.; Yin, L.; Shi, Z.; Luan, J.; Chen, L.; Liu, L. The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes. Biomolecules 2022, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Johri, S.; Sagar, S.; Mundhada, A.; Agrawal, A.; Ray, P.; Chakraborty, K. Cardiolipin-Mediated PPARγ S112 Phosphorylation Impairs IL-10 Production and Inflammation Resolution during Bacterial Pneumonia. Cell Rep. 2021, 34, 108736. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, F.; Wang, R.; He, J. Lysine 222 in PPAR Γ1 Functions as the Key Site of MuRF2-Mediated Ubiquitination Modification. Sci. Rep. 2023, 13, 1999. [Google Scholar] [CrossRef]
- Watanabe, M.; Takahashi, H.; Saeki, Y.; Ozaki, T.; Itoh, S.; Suzuki, M.; Mizushima, W.; Tanaka, K.; Hatakeyama, S. The E3 Ubiquitin Ligase TRIM23 Regulates Adipocyte Differentiation via Stabilization of the Adipogenic Activator PPARγ. eLife 2015, 4, e05615. [Google Scholar] [CrossRef]
- Lee, K.W.; Kwak, S.H.; Koo, Y.D.; Cho, Y.-K.; Lee, H.M.; Jung, H.S.; Cho, Y.M.; Park, Y.J.; Chung, S.S.; Park, K.S. F-Box Only Protein 9 Is an E3 Ubiquitin Ligase of PPARγ. Exp. Mol. Med. 2016, 48, e234. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, K.W.; Lee, E.-W.; Jang, W.-S.; Seo, J.; Shin, S.; Hwang, K.-A.; Song, J. Suppression of PPARγ through MKRN1-Mediated Ubiquitination and Degradation Prevents Adipocyte Differentiation. Cell Death Differ. 2014, 21, 594–603. [Google Scholar] [CrossRef]
- Huang, J.; Tabbi-Anneni, I.; Gunda, V.; Wang, L. Transcription Factor Nrf2 Regulates SHP and Lipogenic Gene Expression in Hepatic Lipid Metabolism. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G1211–G1221. [Google Scholar] [CrossRef] [PubMed]
- Polvani, S.; Tarocchi, M.; Galli, A. PPAR and Oxidative Stress: Con() Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef]
- Reddy, R.C.; Standiford, T.J. Nrf2 and PPARγ. Am. J. Respir. Crit. Care Med. 2010, 182, 134–135. [Google Scholar] [CrossRef]
- Lee, C. Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell. Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- De Nuccio, C.; Bernardo, A.; Troiano, C.; Brignone, M.S.; Falchi, M.; Greco, A.; Rosini, M.; Basagni, F.; Lanni, C.; Serafini, M.M.; et al. NRF2 and PPAR-γ Pathways in Oligodendrocyte Progenitors: Focus on ROS Protection, Mitochondrial Biogenesis and Promotion of Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 7216. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, Z.; Jin, H.; Zhuang, M.; Wang, T.; Su, C.; Lei, Y.; Zou, J.; Zhong, B. Cyclooxygenase-2 up-Regulates Vascular Endothelial Growth Factor via a Protein Kinase C Pathway in Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2011, 30, 6. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; O’Connor, J.P.; He, M.; Tripathi, T.; Harrison, L.E. Phosphorylation of PPARγ via Active ERK1/2 Leads to Its Physical Association with P65 and Inhibition of NF-κβ. J. Cell. Biochem. 2003, 90, 732–744. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Z.; Liu, Z.; Wei, M.; Xue, L.; Vlantis, A.C.; Zhang, Y.; Chan, J.Y.K.; van Hasselt, C.A.; Zeng, X.; et al. Differential Effects of Estrogen Receptor Alpha and Beta on Endogenous Ligands of Peroxisome Proliferator-Activated Receptor Gamma in Papillary Thyroid Cancer. Front. Endocrinol. 2021, 12, 708248. [Google Scholar] [CrossRef]
- Patel, J.A.; Patel, A.J.; Banker, J.M.; Shah, S.I.; Banker, M. Effect of Endometrial Thickness and Duration of Estrogen Supplementation on In Vitro Fertilization-Intracytoplasmic Sperm Injection Outcomes in Fresh Ovum/Embryo Donation Cycles. J. Hum. Reprod. Sci. 2021, 14, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.M.; Kim, T.H.; Shin, J.-H.; Jeong, J.-W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.B.; Dorsa, D.M. Estrogen Activation of Cyclic Adenosine 5′-Monophosphate Response Element-Mediated Transcription Requires the Extracellularly Regulated Kinase/Mitogen-Activated Protein Kinase Pathway. Endocrinology 2003, 144, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, T.H.; Oh, S.J.; Yoo, J.-Y.; Akira, S.; Ku, B.J.; Lydon, J.P.; Jeong, J.-W. Signal Transducer and Activator of Transcription-3 (Stat3) Plays a Critical Role in Implantation via Progesterone Receptor in Uterus. FASEB J. 2013, 27, 2553–2563. [Google Scholar] [CrossRef] [PubMed]
- Leehy, K.A.; Truong, T.H.; Mauro, L.J.; Lange, C.A. Progesterone Receptors (PR) Mediate STAT Actions: PR and Prolactin Receptor Signaling Crosstalk in Breast Cancer Models. J. Steroid Biochem. Mol. Biol. 2018, 176, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Fabi, F.; Grenier, K.; Parent, S.; Adam, P.; Tardif, L.; Leblanc, V.; Asselin, E. Regulation of the PI3K/Akt Pathway during Decidualization of Endometrial Stromal Cells. PLoS ONE 2017, 12, e0177387. [Google Scholar] [CrossRef]
- Catalano, R.D.; Johnson, M.H.; Campbell, E.A.; Charnock-Jones, D.S.; Smith, S.K.; Sharkey, A.M. Inhibition of Stat3 Activation in the Endometrium Prevents Implantation: A Nonsteroidal Approach to Contraception. Proc. Natl. Acad. Sci. 2005, 102, 8585–8590. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-H.; Liu, X.-K.; Tian, X.-T.; Liu, F.; Yao, X.-J.; Dong, J.-F. PPARG: A Promising Therapeutic Target in Breast Cancer and Regulation by Natural Drugs. PPAR Res. 2023, 2023, e4481354. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Lim, J.W.; Kim, H. Lutein Inhibits IL-6 Expression by Inducing PPAR-γ Activation and SOCS3 Expression in Cerulein-stimulated Pancreatic Acinar Cells. Mol. Med. Rep. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Hu, L.; Chen, H.; Zhang, X.; Feng, Z.; Zhang, H.; Meng, Q. Rosiglitazone Ameliorates Radiation-Induced Intestinal Inflammation in Rats by Inhibiting NLRP3 Inflammasome and TNF-α Production. J. Radiat. Res. (Tokyo) 2020, 61, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Wu, C.-H.; Lin, T.-C.; Cheng, Y.-N.; Chang, C.-S.; Lee, K.-T.; Tsai, P.-J.; Tsai, Y.-S. Inhibitory Effect of PPARγ on NLRP3 Inflammasome Activation. Theranostics 2021, 11, 2424–2441. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; van Hasselt, A.; Vlantis, A.C.; Ng, E.K.W.; Liu, S.Y.W.; Fan, M.D.; Ng, S.K.; Chan, A.B.W.; Liu, Z.; Li, X.; et al. The Cross-Talk between Estrogen Receptor and Peroxisome Proliferator-Activated Receptor Gamma in Thyroid Cancer. Cancer 2014, 120, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, S.; Zhong, G.; Kong, W.; Wang, Y. Agonist of PPAR-γ Reduced Epithelial-Mesenchymal Transition in Eosinophilic Chronic Rhinosinusitis with Nasal Polyps via Inhibition of High Mobility Group Box1. Int. J. Med. Sci. 2019, 16, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, W.; Wang, H.; Zhu, Y.; Lei, C. PPARγ Activation Serves as Therapeutic Strategy against Bladder Cancer via Inhibiting PI3K-Akt Signaling Pathway. BMC Cancer 2019, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Ahn, J.H.; Cheon, H.G. Anti-Angiogenic Action of PPARγ Ligand in Human Umbilical Vein Endothelial Cells Is Mediated by PTEN Upregulation and VEGFR-2 Downregulation. Mol. Cell. Biochem. 2011, 358, 375–385. [Google Scholar] [CrossRef]
- Aljada, A.; O’Connor, L.; Fu, Y.-Y.; Mousa, S.A. PPARγ Ligands, Rosiglitazone and Pioglitazone, Inhibit bFGF- and VEGF-Mediated Angiogenesis. Angiogenesis 2008, 11, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lv, M.; Wang, P.; Guo, C.; Ni, Z.; Bao, H.; Tang, Y.; Cai, H.; Lu, J.; Deng, W.; et al. Sequential Activation of Uterine Epithelial IGF1R by Stromal IGF1 and Embryonic IGF2 Directs Normal Uterine Preparation for Embryo Implantation. J. Mol. Cell Biol. 2021, 13, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Vrettou, K.; Troungos, C.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 9190. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Przybycień, P.; Gąsior-Perczak, D.; Placha, W. Cannabinoids and PPAR Ligands: The Future in Treatment of Polycystic Ovary Syndrome Women with Obesity and Reduced Fertility. Cells 2022, 11, 2569. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewski, K.; Paukszto, Ł.; Kurzyńska, A.; Kunicka, Z.; Jastrzębski, J.P.; Makowczenko, K.G.; Golubska, M.; Bogacka, I. PPARγ Regulates the Expression of Genes Involved in the DNA Damage Response in an Inflamed Endometrium. Sci. Rep. 2022, 12, 4026. [Google Scholar] [CrossRef] [PubMed]
- Vitti, M.; Di Emidio, G.; Di Carlo, M.; Carta, G.; Antonosante, A.; Artini, P.G.; Cimini, A.; Tatone, C.; Benedetti, E. Peroxisome Proliferator-Activated Receptors in Female Reproduction and Fertility. PPAR Res. 2016, 2016, e4612306. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor-Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Matsuda, M.; Miyata, Y.; Fukuhara, A.; Komuro, R.; Shimabukuro, M.; Shimomura, I. Human Catalase Gene Is Regulated by Peroxisome Proliferator Activated Receptor-Gamma through a Response Element Distinct from That of Mouse. Endocr. J. 2010, 57, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.T.; Lakshmi, S.P.; Banno, A.; Reddy, R.C. Role of GPx3 in PPARγ-Induced Protection against COPD-Associated Oxidative Stress. Free Radic. Biol. Med. 2018, 126, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, D.; Zhang, Y.; Xie, X.; Wu, Y.; Li, S.; Li, M. A Novel Hypothesis: Up-Regulation of HO-1 by Activation of PPARγ Inhibits HMGB1-RAGE Signaling Pathway and Ameliorates the Development of ALI/ARDS. J. Thorac. Dis. 2013, 5, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the Human Endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef]
- Santos, D.; Payan-Carreira, R. The role of Superoxid Dismutase in Endometrial Functional Integrity. Available online: https://dspace.uevora.pt/rdpc/handle/10174/25309 (accessed on 5 November 2023).
- Elliot, S.J.; Catanuto, P.; Pereira-Simon, S.; Xia, X.; Pastar, I.; Thaller, S.; Head, C.R.; Stojadinovic, O.; Tomic-Canic, M.; Glassberg, M.K. Catalase, a Therapeutic Target in the Reversal of Estrogen-Mediated Aging. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 947–962. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artimovič, P.; Badovská, Z.; Toporcerová, S.; Špaková, I.; Smolko, L.; Sabolová, G.; Kriváková, E.; Rabajdová, M. Oxidative Stress and the Nrf2/PPARγ Axis in the Endometrium: Insights into Female Fertility. Cells 2024, 13, 1081. https://doi.org/10.3390/cells13131081
Artimovič P, Badovská Z, Toporcerová S, Špaková I, Smolko L, Sabolová G, Kriváková E, Rabajdová M. Oxidative Stress and the Nrf2/PPARγ Axis in the Endometrium: Insights into Female Fertility. Cells. 2024; 13(13):1081. https://doi.org/10.3390/cells13131081
Chicago/Turabian StyleArtimovič, Peter, Zuzana Badovská, Silvia Toporcerová, Ivana Špaková, Lukáš Smolko, Gabriela Sabolová, Eva Kriváková, and Miroslava Rabajdová. 2024. "Oxidative Stress and the Nrf2/PPARγ Axis in the Endometrium: Insights into Female Fertility" Cells 13, no. 13: 1081. https://doi.org/10.3390/cells13131081
APA StyleArtimovič, P., Badovská, Z., Toporcerová, S., Špaková, I., Smolko, L., Sabolová, G., Kriváková, E., & Rabajdová, M. (2024). Oxidative Stress and the Nrf2/PPARγ Axis in the Endometrium: Insights into Female Fertility. Cells, 13(13), 1081. https://doi.org/10.3390/cells13131081





