Exploring the Therapeutic Potential of Elastase Inhibition in Age-Related Macular Degeneration in Mouse and Human
Abstract
1. Introduction
2. Results
2.1. AMD Pathology Increases Retinal Elastase Activity in Mouse Models of Wet AMD
2.2. Cultured RPE Cells Derived from Human-AMD Patients or Mouse Eyes with Wet AMD Pathology Exhibit Elevated Elastase Activity
2.3. Altered Elastin Turnover and Antibody Levels in Mouse Models of CNV
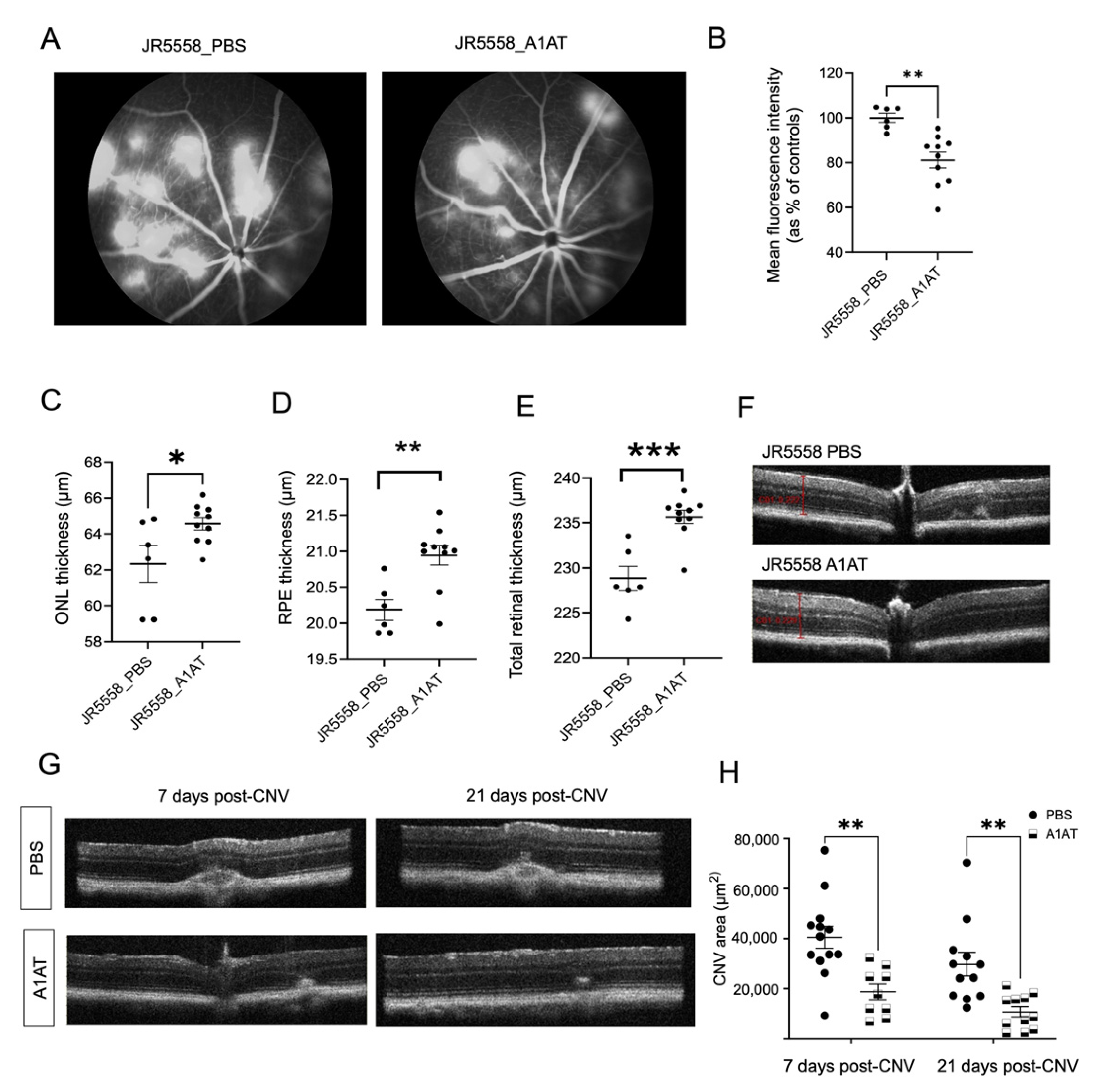
2.4. A1AT, an Elastase Inhibitor, Ameliorates Pathology in Wet AMD Mouse Models
2.5. Increased Elastase Activity Is Associated with Increased VEGF Levels and Decreased RPE Monolayer Integrity
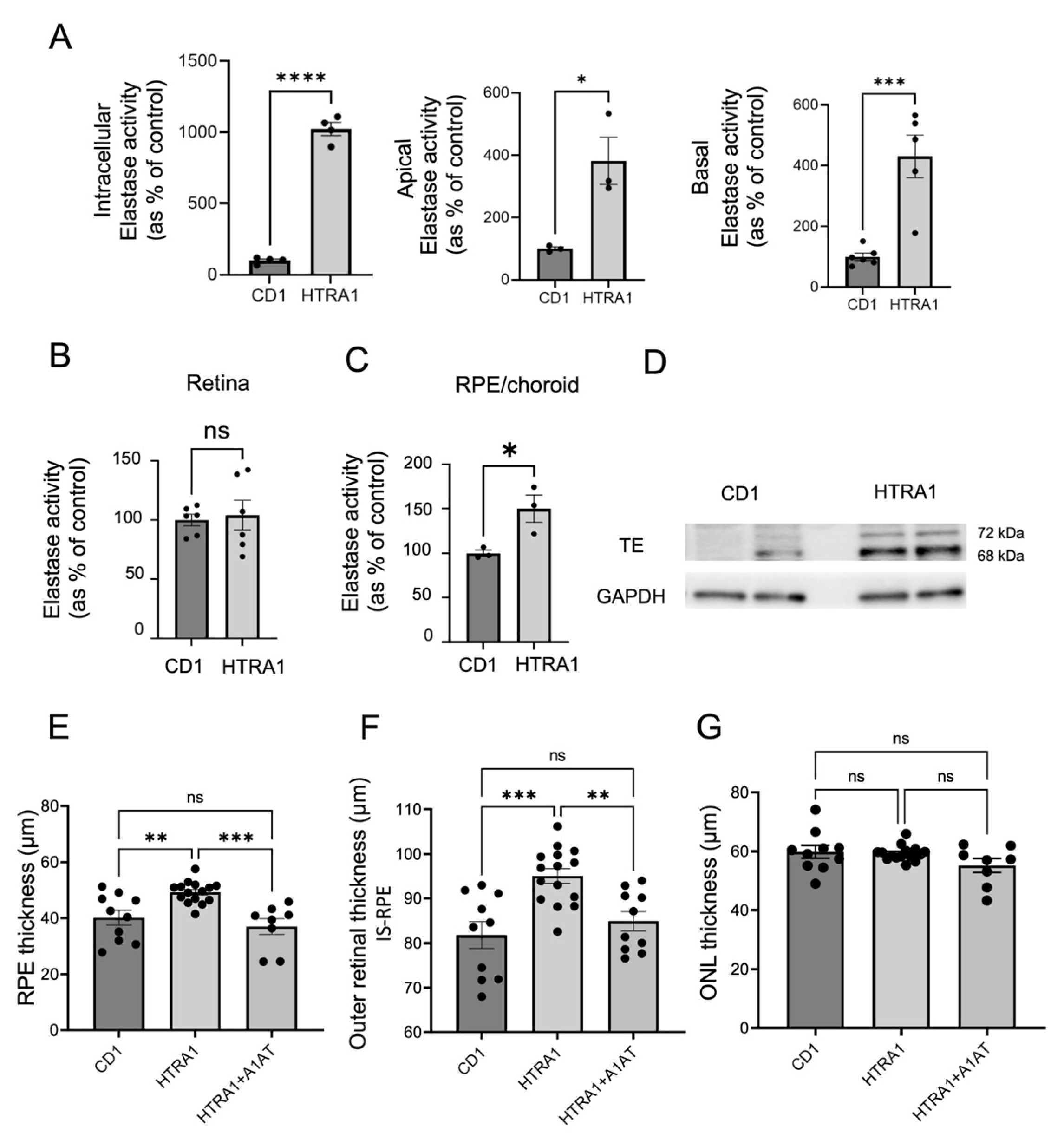
2.6. A1AT Reduces RPE-BrM and Outer Retinal Thickening in HTRA1 Overexpressing Transgenic Mice
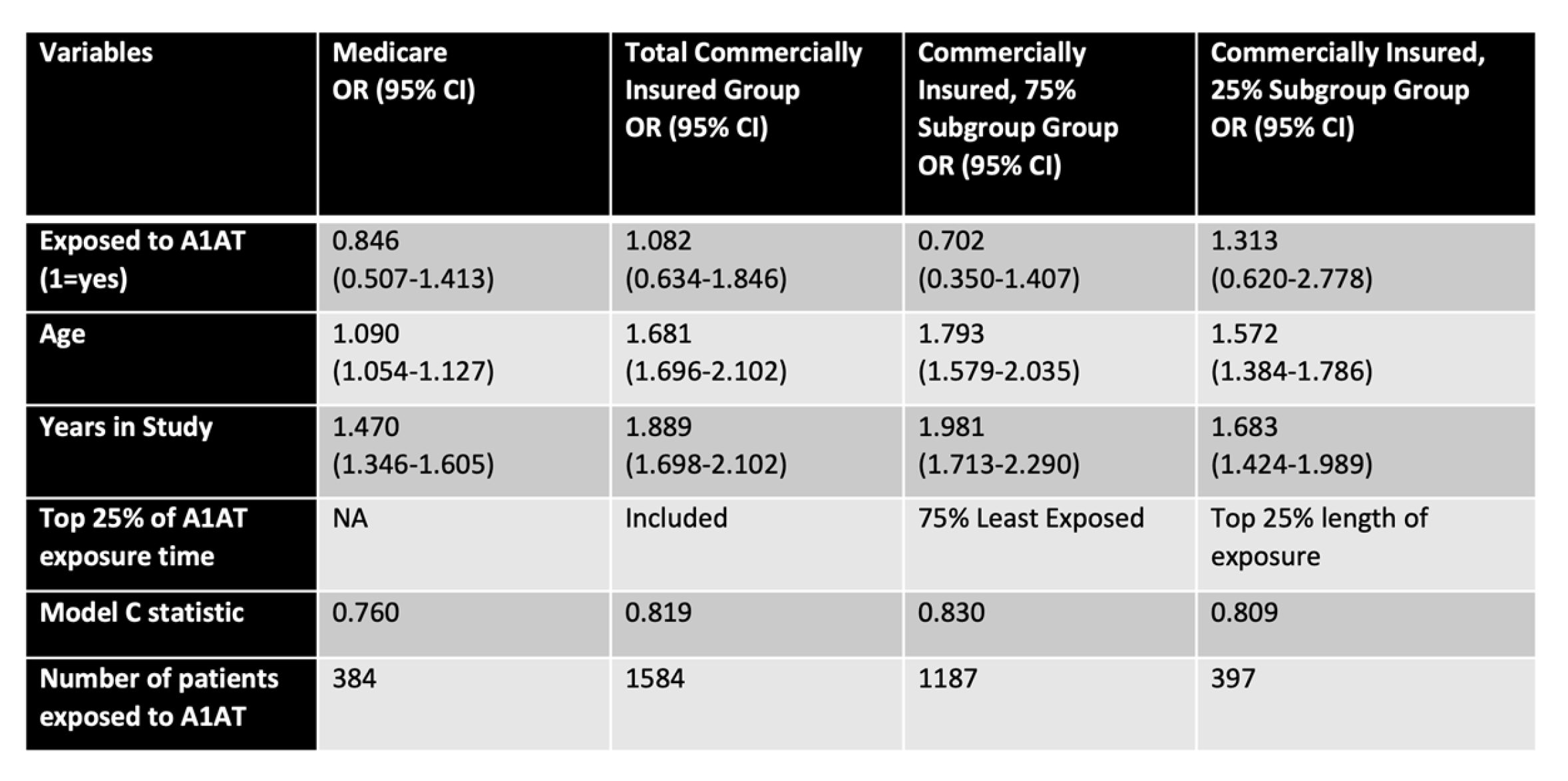
2.7. Preliminary Study on Emphysema Patients, Examining an Association between A1AT Use and AMD Risk
3. Discussion
3.1. Elastase Activation and Its Downstream Effects
3.2. Elastase Activity and A1AT in Human Cells and Patients
3.3. Conclusions
4. Materials and Methods
4.1. Animals and In Vivo Procedures
4.2. RPE Cultures
4.3. Elastase Activity Assay
4.4. VEGF ELISA
4.5. C3a and C5a ELISA
4.6. Western Blotting
4.7. Real-time PCR
4.8. Human Data Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, G.R.; Owsley, C.; Curcio, C.A. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res. Rev. 2002, 1, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Woodell, A.; Rohrer, B. A mechanistic review of cigarette smoke and age-related macular degeneration. Adv. Exp. Med. Biol. 2014, 801, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review. Clin. Ther. 2021, 43, 1792–1818. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Annamalai, B.; Nicholson, C.; Parsons, N.; Stephenson, S.; Atkinson, C.; Jones, B.; Rohrer, B. Immunization Against Oxidized Elastin Exacerbates Structural and Functional Damage in Mouse Model of Smoke-Induced Ocular Injury. Investig. Ophthalmol. Vis. Sci. 2020, 61, 45. [Google Scholar] [CrossRef]
- Chong, N.H.; Keonin, J.; Luthert, P.J.; Frennesson, C.I.; Weingeist, D.M.; Wolf, R.L.; Mullins, R.F.; Hageman, G.S. Decreased thickness and integrity of the macular elastic layer of Bruch’s membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am. J. Pathol. 2005, 166, 241–251. [Google Scholar] [CrossRef]
- Navneet, S.; Rohrer, B. Elastin turnover in ocular diseases: A special focus on age-related macular degeneration. Exp. Eye Res. 2022, 222, 109164. [Google Scholar] [CrossRef]
- Rohrer, B.; Parsons, N.; Annamalai, B.; Nicholson, C.; Obert, E.; Jones, B.W.; Dick, A.D. Peptide-based immunotherapy against oxidized elastin ameliorates pathology in mouse model of smoke-induced ocular injury. Exp. Eye Res. 2021, 212, 108755. [Google Scholar] [CrossRef]
- Kondo, N.; Honda, S.; Ishibashi, K.; Tsukahara, Y.; Negi, A. Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1101–1105. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakayama, T.; Yuzawa, M.; Wang, Z.; Kawamura, A.; Mori, R.; Nakashizuka, H.; Sato, N.; Mizutani, Y. Analysis of candidate genes for age-related macular degeneration subtypes in the Japanese population. Mol. Vis. 2011, 17, 2751–2758. [Google Scholar]
- Yamashiro, K.; Mori, K.; Nakata, I.; Tsuchihashi, T.; Horie-Inoue, K.; Nakanishi, H.; Tsujikawa, A.; Saito, M.; Iida, T.; Yamada, R.; et al. Association of elastin gene polymorphism to age-related macular degeneration and polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8780–8784. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Sakurada, Y.; Miki, A.; Matsumiya, W.; Imoto, I.; Honda, S. The association of elastin gene variants with two angiographic subtypes of polypoidal choroidal vasculopathy. PLoS ONE 2015, 10, e0120643. [Google Scholar] [CrossRef]
- Dewan, A.; Liu, M.; Hartman, S.; Zhang, S.S.; Liu, D.T.; Zhao, C.; Tam, P.O.; Chan, W.M.; Lam, D.S.; Snyder, M.; et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314, 989–992. [Google Scholar] [CrossRef]
- Xu, Y.; Guan, N.; Xu, J.; Yang, X.; Ma, K.; Zhou, H.; Zhang, F.; Snellingen, T.; Jiao, Y.; Liu, X.; et al. Association of CFH, LOC387715, and HTRA1 polymorphisms with exudative age-related macular degeneration in a northern Chinese population. Mol. Vis. 2008, 14, 1373–1381. [Google Scholar]
- Tossetta, G.; Avellini, C.; Licini, C.; Giannubilo, S.R.; Castellucci, M.; Marzioni, D. High temperature requirement A1 and fibronectin: Two possible players in placental tissue remodelling. Eur. J. Histochem. 2016, 60, 2724. [Google Scholar] [CrossRef]
- Vierkotten, S.; Muether, P.S.; Fauser, S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch’s membrane via cleavage of extracellular matrix components. PLoS ONE 2011, 6, e22959. [Google Scholar] [CrossRef]
- Lin, M.K.; Yang, J.; Hsu, C.W.; Gore, A.; Bassuk, A.G.; Brown, L.M.; Colligan, R.; Sengillo, J.D.; Mahajan, V.B.; Tsang, S.H. HTRA1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP1 and TSP1. Aging Cell 2018, 17, e12710. [Google Scholar] [CrossRef]
- Iejima, D.; Itabashi, T.; Kawamura, Y.; Noda, T.; Yuasa, S.; Fukuda, K.; Oka, C.; Iwata, T. HTRA1 (high temperature requirement A serine peptidase 1) gene is transcriptionally regulated by insertion/deletion nucleotides located at the 3’ end of the ARMS2 (age-related maculopathy susceptibility 2) gene in patients with age-related macular degeneration. J. Biol. Chem. 2015, 290, 2784–2797. [Google Scholar] [CrossRef]
- Jones, A.; Kumar, S.; Zhang, N.; Tong, Z.; Yang, J.H.; Watt, C.; Anderson, J.; Amrita; Fillerup, H.; McCloskey, M.; et al. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 14578–14583. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Chong, N.V.; Bailey, T.A. Serum elastin-derived peptides in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3046–3051. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, K.; Patel, N.; Ohbayashi, M.; Chong, V.; Grossniklaus, H.E.; Bird, A.C.; Ono, S.J. Serum autoantibody biomarkers for age-related macular degeneration and possible regulators of neovascularization. Exp. Mol. Pathol. 2012, 92, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S.; Cone, F.E.; Pease, M.E.; Nguyen, T.D.; Myers, K.; Quigley, H.A. The presence and distribution of elastin in the posterior and retrobulbar regions of the mouse eye. Exp. Eye Res. 2010, 90, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Weiland, J.D. Discovery of retinal elastin and its possible role in age-related macular degeneration. Ann. Biomed. Eng. 2014, 42, 678–684. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Pena, J.D.; Selvidge, J.A.; Salvador-Silva, M.; Yang, P. Hydrostatic pressure stimulates synthesis of elastin in cultured optic nerve head astrocytes. Glia 2000, 32, 122–136. [Google Scholar] [CrossRef]
- Zhao, P.; Lieu, T.; Barlow, N.; Sostegni, S.; Haerteis, S.; Korbmacher, C.; Liedtke, W.; Jimenez-Vargas, N.N.; Vanner, S.J.; Bunnett, N.W. Neutrophil Elastase Activates Protease-activated Receptor-2 (PAR2) and Transient Receptor Potential Vanilloid 4 (TRPV4) to Cause Inflammation and Pain. J. Biol. Chem. 2015, 290, 13875–13887. [Google Scholar] [CrossRef]
- Rasmussen, J.G.; Riis, S.E.; Frøbert, O.; Yang, S.; Kastrup, J.; Zachar, V.; Simonsen, U.; Fink, T. Activation of protease-activated receptor 2 induces VEGF independently of HIF-1. PLoS ONE 2012, 7, e46087. [Google Scholar] [CrossRef]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Nagai, N.; Lundh von Leithner, P.; Izumi-Nagai, K.; Hosking, B.; Chang, B.; Hurd, R.; Adamson, P.; Adamis, A.P.; Foxton, R.H.; Ng, Y.S.; et al. Spontaneous CNV in a novel mutant mouse is associated with early VEGF-A-driven angiogenesis and late-stage focal edema, neural cell loss, and dysfunction. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3709–3719. [Google Scholar] [CrossRef]
- Qiang, W.; Wei, R.; Chen, Y.; Chen, D. Clinical Pathological Features and Current Animal Models of Type 3 Macular Neovascularization. Front. Neurosci. 2021, 15, 734860. [Google Scholar] [CrossRef]
- Joseph, K.; Kulik, L.; Coughlin, B.; Kunchithapautham, K.; Bandyopadhyay, M.; Thiel, S.; Thielens, N.M.; Holers, V.M.; Rohrer, B. Oxidative Stress Sensitizes RPE Cells to Complement-Mediated Injury in a Natural Antibody-, Lectin Pathway- and Phospholipid Epitope-Dependent Manner. J. Biol. Chem. 2013, 288, 12753–12765. [Google Scholar] [CrossRef]
- Chang, B.; FitzMaurice, B.; Wang, J.; Low, B.E.; Wiles, M.V.; Nishina, P.M. Spontaneous Posterior Segment Vascular Disease Phenotype of a Mouse Model, rnv3, Is Dependent on the Crb1rd8 Allele. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5127–5139. [Google Scholar] [CrossRef]
- Akter, T.; Annamalai, B.; Obert, E.; Simpson, K.N.; Rohrer, B. Dabigatran and Wet AMD, Results From Retinal Pigment Epithelial Cell Monolayers, the Mouse Model of Choroidal Neovascularization, and Patients From the Medicare Data Base. Front. Immunol. 2022, 13, 896274. [Google Scholar] [CrossRef]
- Kurtagic, E.; Jedrychowski, M.P.; Nugent, M.A. Neutrophil elastase cleaves VEGF to generate a VEGF fragment with altered activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L534–L546. [Google Scholar] [CrossRef]
- Bandyopadhyay, M.; Rohrer, B. Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1953–1961. [Google Scholar] [CrossRef]
- Bressler, S.B. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology 2009, 116, S1–S7. [Google Scholar] [CrossRef]
- Le, Y.Z. VEGF production and signaling in Muller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vision Res. 2017, 139, 108–114. [Google Scholar] [CrossRef]
- Ding, X.; Gu, R.; Zhang, M.; Ren, H.; Shu, Q.; Xu, G.; Wu, H. Microglia enhanced the angiogenesis, migration and proliferation of co-cultured RMECs. BMC Ophthalmol. 2018, 18, 249. [Google Scholar] [CrossRef]
- Kunchithapautham, K.; Rohrer, B. Sublytic membrane-attack-complex (MAC) activation alters regulated rather than constitutive vascular endothelial growth factor (VEGF) secretion in retinal pigment epithelium monolayers. J. Biol. Chem. 2011, 286, 23717–23724. [Google Scholar] [CrossRef]
- Dutra-Oliveira, A.; Monteiro, R.Q.; Mariano-Oliveira, A. Protease-activated receptor-2 (PAR2) mediates VEGF production through the ERK1/2 pathway in human glioblastoma cell lines. Biochem. Biophys. Res. Commun. 2012, 421, 221–227. [Google Scholar] [CrossRef]
- Claesson, R.; Kanasi, E.; Johansson, A.; Kalfas, S. A new cleavage site for elastase within the complement component 3. Apmis 2010, 118, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, E.A.; Rydengård, V.; Nyberg, P.; Nitsche, D.P.; Mörgelin, M.; Malmsten, M.; Björck, L.; Schmidtchen, A. Activation of the complement system generates antibacterial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 16879–16884. [Google Scholar] [CrossRef] [PubMed]
- Ablonczy, Z.; Crosson, C.E. VEGF modulation of retinal pigment epithelium resistance. Exp. Eye Res. 2007, 85, 762–771. [Google Scholar] [CrossRef]
- Lin, J.M.; Wan, L.; Tsai, Y.Y.; Lin, H.J.; Tsai, Y.; Lee, C.C.; Tsai, C.H.; Tsai, F.J.; Tseng, S.H. HTRA1 polymorphism in dry and wet age-related macular degeneration. Retina 2008, 28, 309–313. [Google Scholar] [CrossRef]
- Yang, Z.; Tong, Z.; Chen, Y.; Zeng, J.; Lu, F.; Sun, X.; Zhao, C.; Wang, K.; Davey, L.; Chen, H.; et al. Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet. 2010, 6, e1000836. [Google Scholar] [CrossRef]
- Nakayama, M.; Iejima, D.; Akahori, M.; Kamei, J.; Goto, A.; Iwata, T. Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6514–6523. [Google Scholar] [CrossRef]
- Iejima, D.; Nakayama, M.; Iwata, T. HTRA1 Overexpression Induces the Exudative Form of Age-related Macular Degeneration. J. Stem Cells 2015, 10, 193–203. [Google Scholar]
- Shao, Z.; Friedlander, M.; Hurst, C.G.; Cui, Z.; Pei, D.T.; Evans, L.P.; Juan, A.M.; Tahiri, H.; Duhamel, F.; Chen, J.; et al. Choroid sprouting assay: An ex vivo model of microvascular angiogenesis. PLoS ONE 2013, 8, e69552. [Google Scholar] [CrossRef]
- Churg, A.; Wang, X.; Wang, R.D.; Meixner, S.C.; Pryzdial, E.L.; Wright, J.L. Alpha1-antitrypsin suppresses TNF-alpha and MMP-12 production by cigarette smoke-stimulated macrophages. Am. J. Respir. Cell Mol. Biol. 2007, 37, 144–151. [Google Scholar] [CrossRef]
- Huang, H.; Campbell, S.C.; Nelius, T.; Bedford, D.F.; Veliceasa, D.; Bouck, N.P.; Volpert, O.V. Alpha1-antitrypsin inhibits angiogenesis and tumor growth. Int. J. Cancer 2004, 112, 1042–1048. [Google Scholar] [CrossRef]
- Jonigk, D.; Al-Omari, M.; Maegel, L.; Müller, M.; Izykowski, N.; Hong, J.; Hong, K.; Kim, S.H.; Dorsch, M.; Mahadeva, R.; et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA 2013, 110, 15007–15012. [Google Scholar] [CrossRef]
- Koulmanda, M.; Bhasin, M.; Fan, Z.; Hanidziar, D.; Goel, N.; Putheti, P.; Movahedi, B.; Libermann, T.A.; Strom, T.B. Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc. Natl. Acad. Sci. USA 2012, 109, 15443–15448. [Google Scholar] [CrossRef]
- Ortiz, G.; Lopez, E.S.; Salica, J.P.; Potilinski, C.; Fernández Acquier, M.; Chuluyan, E.; Gallo, J.E. Alpha-1-antitrypsin ameliorates inflammation and neurodegeneration in the diabetic mouse retina. Exp. Eye Res. 2018, 174, 29–39. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Zhu, X.; Sun, X.; Liu, Y.; Cheng, B.; Li, M.; Liu, Y.; He, C.; Liu, X. Alpha-1 Antitrypsin Attenuates M1 Microglia-Mediated Neuroinflammation in Retinal Degeneration. Front. Immunol. 2018, 9, 1202. [Google Scholar] [CrossRef]
- Janciauskiene, S.; Wrenger, S.; Immenschuh, S.; Olejnicka, B.; Greulich, T.; Welte, T.; Chorostowska-Wynimko, J. The Multifaceted Effects of Alpha1-Antitrypsin on Neutrophil Functions. Front. Pharmacol. 2018, 9, 341. [Google Scholar] [CrossRef]
- Liu, H.; Lessieur, E.M.; Saadane, A.; Lindstrom, S.I.; Taylor, P.R.; Kern, T.S. Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy. Diabetologia 2019, 62, 2365–2374. [Google Scholar] [CrossRef]
- Yi, C.; Liu, J.; Deng, W.; Luo, C.; Qi, J.; Chen, M.; Xu, H. Macrophage elastase (MMP12) critically contributes to the development of subretinal fibrosis. J. Neuroinflammation 2022, 19, 78. [Google Scholar] [CrossRef]
- Hasegawa, E.; Sweigard, H.; Husain, D.; Olivares, A.M.; Chang, B.; Smith, K.E.; Birsner, A.E.; D’Amato, R.J.; Michaud, N.A.; Han, Y.; et al. Characterization of a spontaneous retinal neovascular mouse model. PLoS ONE 2014, 9, e106507. [Google Scholar] [CrossRef]
- Acton, J.H.; Smith, R.T.; Hood, D.C.; Greenstein, V.C. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7618–7624. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Parrulli, S.; Monteduro, D.; Cereda, M.G.; Nguyen, V.; Staurenghi, G.; Cheung, C.M.G.; Gillies, M.; Teo, K.Y.C. Outer Retinal Layer Thickening Predicts the Onset of Exudative Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2021, 231, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Skeie, J.M.; Hernandez, J.; Hinek, A.; Mullins, R.F. Molecular responses of choroidal endothelial cells to elastin derived peptides through the elastin-binding protein (GLB1). Matrix Biol. 2012, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C.; Morrison, M.A.; Ji, F.; Xu, H.; Reinecke, J.B.; Adams, S.M.; Arneberg, T.M.; Janssian, M.; Lee, J.E.; Yuan, Y.; et al. Convergence of linkage, gene expression and association data demonstrates the influence of the RAR-related orphan receptor alpha (RORA) gene on neovascular AMD: A systems biology based approach. Vision Res. 2010, 50, 698–715. [Google Scholar] [CrossRef]
- Tosi, G.M.; Caldi, E.; Neri, G.; Nuti, E.; Marigliani, D.; Baiocchi, S.; Traversi, C.; Cevenini, G.; Tarantello, A.; Fusco, F.; et al. HTRA1 and TGF-β1 Concentrations in the Aqueous Humor of Patients With Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 162–167. [Google Scholar] [CrossRef]
- Tom, I.; Pham, V.C.; Katschke, K.J., Jr.; Li, W.; Liang, W.C.; Gutierrez, J.; Ah Young, A.; Figueroa, I.; Eshghi, S.T.; Lee, C.V.; et al. Development of a therapeutic anti-HtrA1 antibody and the identification of DKK3 as a pharmacodynamic biomarker in geographic atrophy. Proc. Natl. Acad. Sci. USA 2020, 117, 9952–9963. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Schnabolk, G.; Obert, E.; Banda, N.K.; Rohrer, B. Systemic Inflammation by Collagen-Induced Arthritis Affects the Progression of Age-Related Macular Degeneration Differently in Two Mouse Models of the Disease. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Tawfik, A.; Markand, S.; Al-Shabrawey, M.; Mayo, J.N.; Reynolds, J.; Bearden, S.E.; Ganapathy, V.; Smith, S.B. Alterations of retinal vasculature in cystathionine-β-synthase heterozygous mice: A model of mild to moderate hyperhomocysteinemia. Am. J. Pathol. 2014, 184, 2573–2585. [Google Scholar] [CrossRef]
- Gong, J.; Cai, H.; Noggle, S.; Paull, D.; Rizzolo, L.J.; Del Priore, L.V.; Fields, M.A. Stem cell-derived retinal pigment epithelium from patients with age-related macular degeneration exhibit reduced metabolism and matrix interactions. Stem Cells Transl. Med. 2020, 9, 364–376. [Google Scholar] [CrossRef]
- Sharma, R.; Bose, D.; Montford, J.; Ortolan, D.; Bharti, K. Triphasic developmentally guided protocol to generate retinal pigment epithelium from induced pluripotent stem cells. STAR Protoc. 2022, 3, 101582. [Google Scholar] [CrossRef]
- Gong, J.; Fields, M.A.; Moreira, E.F.; Bowrey, H.E.; Gooz, M.; Ablonczy, Z.; Del Priore, L.V. Differentiation of Human Protein-Induced Pluripotent Stem Cells toward a Retinal Pigment Epithelial Cell Fate. PLoS ONE 2015, 10, e0143272. [Google Scholar] [CrossRef]
- Kunchithapautham, K.; Atkinson, C.; Rohrer, B. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J. Biol. Chem. 2014, 289, 14534–14546. [Google Scholar] [CrossRef]
- Ginzberg, H.H.; Shannon, P.T.; Suzuki, T.; Hong, O.; Vachon, E.; Moraes, T.; Abreu, M.T.; Cherepanov, V.; Wang, X.; Chow, C.W.; et al. Leukocyte elastase induces epithelial apoptosis: Role of mitochondial permeability changes and Akt. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G286–G298. [Google Scholar] [CrossRef]
- Zaidi, S.H.; You, X.M.; Ciura, S.; Husain, M.; Rabinovitch, M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 2002, 105, 516–521. [Google Scholar] [CrossRef]
- Navneet, S.; Cui, X.; Zhao, J.; Wang, J.; Kaidery, N.A.; Thomas, B.; Bollinger, K.E.; Yoon, Y.; Smith, S.B. Excess homocysteine upregulates the NRF2-antioxidant pathway in retinal Müller glial cells. Exp. Eye Res. 2019, 178, 228–237. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navneet, S.; Brandon, C.; Simpson, K.; Rohrer, B. Exploring the Therapeutic Potential of Elastase Inhibition in Age-Related Macular Degeneration in Mouse and Human. Cells 2023, 12, 1308. https://doi.org/10.3390/cells12091308
Navneet S, Brandon C, Simpson K, Rohrer B. Exploring the Therapeutic Potential of Elastase Inhibition in Age-Related Macular Degeneration in Mouse and Human. Cells. 2023; 12(9):1308. https://doi.org/10.3390/cells12091308
Chicago/Turabian StyleNavneet, Soumya, Carlene Brandon, Kit Simpson, and Bärbel Rohrer. 2023. "Exploring the Therapeutic Potential of Elastase Inhibition in Age-Related Macular Degeneration in Mouse and Human" Cells 12, no. 9: 1308. https://doi.org/10.3390/cells12091308
APA StyleNavneet, S., Brandon, C., Simpson, K., & Rohrer, B. (2023). Exploring the Therapeutic Potential of Elastase Inhibition in Age-Related Macular Degeneration in Mouse and Human. Cells, 12(9), 1308. https://doi.org/10.3390/cells12091308





