Immune Response to Seasonal Influenza Vaccination in Multiple Sclerosis Patients Receiving Cladribine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Vaccination
2.4. Study Endpoints
2.5. Blood Sampling and Processing
2.6. Statistical Analyses
3. Results
3.1. Patients
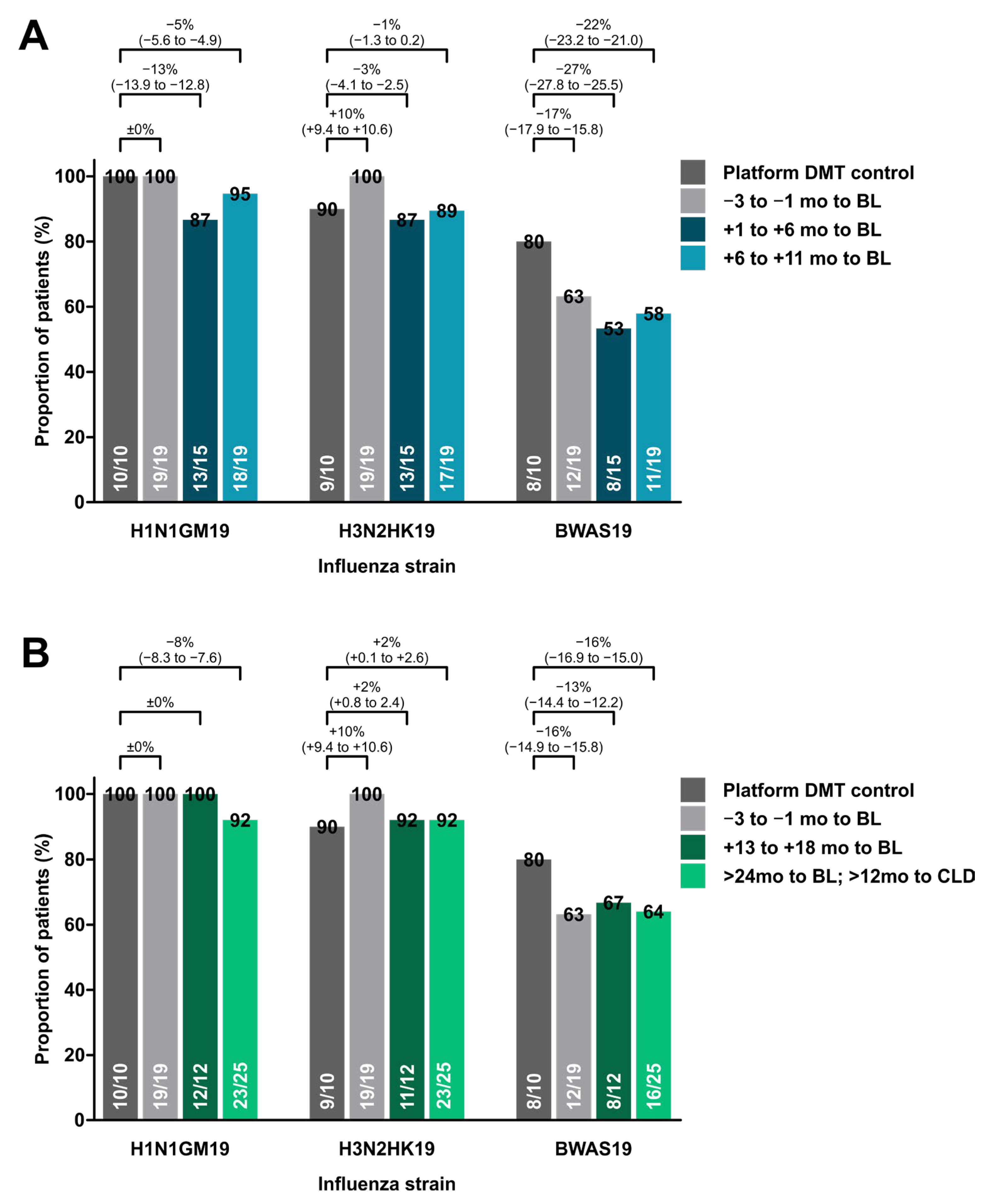
3.2. Response to Influenza Vaccine
3.3. Predictors of Response
3.4. Safety during the Immunization Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunemann, J.D.; Ruck, T.; Muraro, P.A.; Bar-Or, A.; Wiendl, H. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Cladribine Tablets: A Review in Relapsing MS. CNS Drugs 2018, 32, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, L.; Pfeuffer, S.; Huntemann, N.; Schmidt, M.; Su, C.; Skuljec, J.; Aslan, D.; Hackert, J.; Kleinschnitz, K.; Hagenacker, T.; et al. Immunological consequences of cladribine treatment in multiple sclerosis: A real-world study. Mult. Scler. Relat. Disord. 2022, 64, 103931. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Ammoscato, F.; Giovannoni, G.; Baker, D.; Schmierer, K. Cladribine: Mechanisms and mysteries in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1266–1271. [Google Scholar] [CrossRef]
- Comi, G.; Cook, S.; Giovannoni, G.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Galazka, A.; Nolting, A.; Hicking, C.; Dangond, F. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 168–174. [Google Scholar] [CrossRef]
- Farez, M.F.; Correale, J.; Armstrong, M.J.; Rae-Grant, A.; Gloss, D.; Donley, D.; Holler-Managan, Y.; Kachuck, N.J.; Jeffery, D.; Beilman, M.; et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2019, 93, 584–594. [Google Scholar] [CrossRef]
- Olberg, H.K.; Cox, R.J.; Nostbakken, J.K.; Aarseth, J.H.; Vedeler, C.A.; Myhr, K.M. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: An explorative study. Mult. Scler. 2014, 20, 1074–1080. [Google Scholar] [CrossRef]
- Mehling, M.; Fritz, S.; Hafner, P.; Eichin, D.; Yonekawa, T.; Klimkait, T.; Lindberg, R.L.; Kappos, L.; Hess, C. Preserved antigen-specific immune response in patients with multiple sclerosis responding to IFNbeta-therapy. PLoS ONE 2013, 8, e78532. [Google Scholar] [CrossRef]
- Metze, C.; Winkelmann, A.; Loebermann, M.; Hecker, M.; Schweiger, B.; Reisinger, E.C.; Zettl, U.K. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci. Ther. 2019, 25, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Freedman, M.S.; Kremenchutzky, M.; Menguy-Vacheron, F.; Bauer, D.; Jodl, S.; Truffinet, P.; Benamor, M.; Chambers, S.; O’connor, P.W. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 2013, 81, 552–558. [Google Scholar] [CrossRef] [PubMed]
- von Hehn, C.; Howard, J.; Liu, S.; Meka, V.; Pultz, J.; Mehta, D.; Prada, C.; Ray, S.; Edwards, M.R.; Sheikh, S.I. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol.-Neuroimmunol. Neuroinflammation 2018, 5, e409. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef]
- Schmierer, K.; Wiendl, H.; Oreja-Guevara, C.; Centonze, D.; Chudecka, A.; Roy, S.; Boschert, U. Varicella zoster virus and influenza vaccine antibody titres in patients from MAGNIFY-MS who were treated with cladribine tablets for highly active relapsing multiple sclerosis. Mult. Scler. 2022, 28, 2151–2153. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Meyer, S.; Adam, M.; Schweiger, B.; Ilchmann, C.; Eulenburg, C.; Sattinger, E.; Runte, H.; Schlüter, M.; Deuse, T.; Reichenspurner, H.; et al. Antibody response after a single dose of an AS03-adjuvanted split-virion influenza A (H1N1) vaccine in heart transplant recipients. Transplantation 2011, 91, 1031–1035. [Google Scholar] [CrossRef]
- Mohn, N.; Pfeuffer, S.; Ruck, T.; Gross, C.C.; Skripuletz, T.; Klotz, L.; Wiendl, H.; Stangel, M.; Meuth, S.G. Alemtuzumab therapy changes immunoglobulin levels in peripheral blood and CSF. Neurol.-Neuroimmunol. Neuroinflammation 2020, 7, e654. [Google Scholar] [CrossRef]
- Cook, S.; Leist, T.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Hicking, C.; Galazka, A.; Sylvester, E. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult. Scler. Relat. Disord. 2019, 29, 157–167. [Google Scholar] [CrossRef]
- Cook, S.; Vermersch, P.; Comi, G.; Giovannoni, G.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Hamlett, A.; Miret, M.; Weiner, J.; et al. Safety and tolerability of cladribine tablets in multiple sclerosis: The CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult. Scler. 2011, 17, 578–593. [Google Scholar] [CrossRef]
- Olberg, H.K.; Eide, G.E.; Cox, R.J.; Jul-Larsen, Å.; Lartey, S.L.; Vedeler, C.A.; Myhr, K. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur. J. Neurol. 2018, 25, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, S.H.; Huh, S.Y.; Kong, S.-Y.; Choi, Y.J.; Cheong, H.J.; Kim, H.J. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur. J. Neurol. 2013, 20, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012835. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; Inglese, M.; Schiavetti, I.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Da Rin, G.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 2021, 72, 103581. [Google Scholar] [CrossRef]
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.L.; Swift, P.A.; Rajaram, S.; Graves-Jones, A.; Edelman, J.; Burns, F.; et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: An exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 167–179. [Google Scholar] [CrossRef]
- Baker, D.; Herrod, S.S.; Alvarez-Gonzalez, C.; Zalewski, L.; Albor, C.; Schmierer, K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol.-Neuroimmunol. Neuroinflammation 2017, 4, e360. [Google Scholar] [CrossRef]
- Disanto, G.; Sacco, R.; Bernasconi, E.; Martinetti, G.; Keller, F.; Gobbi, C.; Zecca, C. Association of Disease-Modifying Treatment and Anti-CD20 Infusion Timing with Humoral Response to 2 SARS-CoV-2 Vaccines in Patients with Multiple Sclerosis. JAMA Neurol. 2021, 78, 1529–1531. [Google Scholar] [CrossRef]
- Baker, D.; MacDougall, A.; Kang, A.S.; Schmierer, K.; Giovannoni, G.; Dobson, R. CD19 B cell repopulation after ocrelizumab, alemtuzumab and cladribine: Implications for SARS-CoV-2 vaccinations in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103448. [Google Scholar] [CrossRef]
- Wen, F.; Guo, J.; Li, Z.; Huang, S. Sex-specific patterns of gene expression following in-fluenza vaccination. Sci. Rep. 2018, 8, 13517. [Google Scholar] [CrossRef]
- Voigt, E.A.; Ovsyannikova, I.G.; Kennedy, R.B.; Grill, D.E.; Goergen, K.M.; Schaid, D.J.; Poland, G.A. Sex Differences in Older Adults’ Immune Responses to Seasonal Influenza Vaccination. Front. Immunol. 2019, 10, 180. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar]
- Gross, P.A.; Hermogenes, A.W.; Sacks, H.S.; Lau, J.; Levandowski, R.A. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann. Intern. Med. 1995, 123, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Havla, J.; Schwab, N.; Hohlfeld, R.; Barnett, M.; Reddel, S.; Wiendl, H. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419836571. [Google Scholar] [CrossRef] [PubMed]

| −3 to −1 mo to BL (n = 19) | +1 to +6 mo to BL (n = 15) | +6 to +11 mo to BL (n = 19) | +13 to +17 mo to BL (n = 12) | >24 mo to BL; >12 mo to CLD (n = 25) | Platform DMT Control (n = 10) | p | |
|---|---|---|---|---|---|---|---|
| Age, yrs, median (IQR) | 40 (31–53) | 34 (29–52) | 41 (35–50) | 49 (34–53) | 41 (32–51) | 53 (31–61) | 0.462 * |
| Male sex, No. (%) | 7 (37) | 4 (27) | 8 (42) | 3 (25) | 9 (36) | 5 (50) | 0.797 # |
| MS duration, mo, median (IQR) | 80 (8–160) | 13 (7–63) | 48 (10–187) | 137 (15–231) | 68 (25–172) | 78 (37–180) | 0.075 * |
| Annualized relapse rate, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0 (0–0.5) | 0.031 * |
| EDSS, median (IQR) | 2.5 (2–4.5) | 2 (1–4) | 2 (1–3.5) | 3.5 (1.5–4.5) | 2.5 (2–4) | 1.5 (1.5–2) | 0.130 * |
| Number of previous DMT, median (IQR) | 1 (0–3) | 1 (0–1) | 1 (0–2) | 1 (0–2) | 2 (1–3) | 1 (0–1) | 0.071 * |
| Last previous DMT, No. (%) | 0.201 # | ||||||
| Naïve | 5 (26) | 7 (47) | 11 (58) | 7 (58) | 6 (24) | 1 (10) | |
| Platform | 5 (26) | 2 (13) | 2 (11) | 1 (8) | 8 (32) | 5 (50) | |
| DMF/TERI | 7 (37) | 6 (40) | 4 (21) | 3 (25) | 8 (32) | 4 (40) | |
| Active | 2 (11) | 0 (0) | 2 (11) | 1 (8) | 3 (12) | 0 (0) | |
| Vaccine used, No. (%) | 0.116 # | ||||||
| Influsplit® | 9 (47) | 3 (20) | 9 (47) | 6 (50) | 16 (64) | 7 (70) | |
| Flucelvax® | 4 (21) | 6 (40) | 2 (11) | 2 (17) | 3 (12) | 0 (0) | |
| Influvac® | 4 (21) | 4 (27) | 8 (42) | 4 (33) | 6 (24) | 3 (30) | |
| Vaxigrip® | 2 (11) | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Smoker status, No. (%) | 8 (42) | 7 (47) | 5 (26) | 2 (17) | 11 (44) | 3 (30) | 0.464 # |
| A. | Platform DMT Control | Overall CLD COHORT | ||||||
| strain | pre (n = 10) | post (n = 10) | pre (n = 85) | post (n = 90) | ||||
| H1N1GM19 | 76.4 (35.5–164.3) | 264.5 (170.5–410.3) | 80.2 (46.0–141.3) | 178.7 (113.4–284.6) | ||||
| H3N2HK19 | 252.4 (87.1–731.1) | 552.4 (209.2–1459.0) | 172.2 (83.0–374.1) | 397.5 (192.5–849.1) | ||||
| BWAS19 | 15.2 (9.0–25.9) | 93.6 (42.0–208.3) | 16.8 (11.5–24.7) | 56.8 (31.3–105.2) | ||||
| B | Platform DMT Control | Cohort 1: −3 to −1 mo to BL | Cohort 2: +1 to +6 mo to BL | Cohort 3: +6 to +11 mo to BL | ||||
| strain | pre (n = 10) | post (n = 10) | pre (n = 16) | post (n = 19) | pre (n = 14) | post (n = 15) | pre (n = 19) | post (n = 19) |
| H1N1GM19 | 76.4 (35.5–164.3) | 264.5 (170.5–410.3) | 77.1 (45.1–131.9) | 230.2 (147.2–360.0) | 59.3 (38.4–91.6) | 129.6 (63.8–263.3) | 78.5 (44.9–137.2) | 110.5 (77.8–156.9) |
| H3N2HK19 | 252.4 (87.1–731.1) | 552.4 (209.2–1459.0) | 164.7 (92.2–294.3) | 443.8 (265.2–742.6) | 144.9 (75.2–279.3) | 302.0 (127.6–714.9) | 149.2 (67.3–330.6) | 347.0 (187.0–644.1) |
| BWAS19 | 15.2 (9.0–25.9) | 93.6 (42.0–208.3) | 17.7 (11.9–27.0) | 54.2 (29.7–98.4) | 11.6 (9.1–14.8) | 38.9 (19.8–76.3) | 17.4 (13.2–22.8) | 48.6 (28.5–82.7) |
| C | Platform DMT Control | Cohort 1: −3 to −1 mo to BL | Cohort 4: +13 to +17 mo to BL | Cohort 5: >24 mo to BL; >12 mo to CLD | ||||
| strain | pre (n = 10) | post (n = 10) | pre (n = 16) | post (n = 19) | pre (n = 11) | post (n = 12) | pre (n = 25) | post (n = 25) |
| H1N1GM19 | 76.4 (35.5–164.3) | 264.5 (170.5–410.3) | 77.1 (45.1–131.9) | 230.2 (147.2–360.0) | 87.3 (47.1–161.6) | 171.2 (105.7–277.2) | 102.6 (65.2–161.5) | 166.4 (115.4–239.9) |
| H3N2HK19 | 252.4 (87.1–731.1) | 552.4 (209.2–1459.0) | 164.7 (92.2–294.3) | 443.8 (265.2–742.6) | 72.1 (27.4–190.0) | 206.0 (78.0–544.1) | 249.7 (148.6–419.3) | 533.8 (287.9–989.9) |
| BWAS19 | 15.2 (9.0–25.9) | 93.6 (42.0–208.3) | 17.7 (11.9–27.0) | 54.2 (29.7–98.4) | 18.2 (10.9–30.3) | 54.6 (34.2–87.3) | 20.2 (15.1–27.1) | 51.2 (33.5–78.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolfes, L.; Pfeuffer, S.; Skuljec, J.; He, X.; Su, C.; Oezalp, S.-H.; Pawlitzki, M.; Ruck, T.; Korsen, M.; Kleinschnitz, K.; et al. Immune Response to Seasonal Influenza Vaccination in Multiple Sclerosis Patients Receiving Cladribine. Cells 2023, 12, 1243. https://doi.org/10.3390/cells12091243
Rolfes L, Pfeuffer S, Skuljec J, He X, Su C, Oezalp S-H, Pawlitzki M, Ruck T, Korsen M, Kleinschnitz K, et al. Immune Response to Seasonal Influenza Vaccination in Multiple Sclerosis Patients Receiving Cladribine. Cells. 2023; 12(9):1243. https://doi.org/10.3390/cells12091243
Chicago/Turabian StyleRolfes, Leoni, Steffen Pfeuffer, Jelena Skuljec, Xia He, Chuanxin Su, Sinem-Hilal Oezalp, Marc Pawlitzki, Tobias Ruck, Melanie Korsen, Konstanze Kleinschnitz, and et al. 2023. "Immune Response to Seasonal Influenza Vaccination in Multiple Sclerosis Patients Receiving Cladribine" Cells 12, no. 9: 1243. https://doi.org/10.3390/cells12091243
APA StyleRolfes, L., Pfeuffer, S., Skuljec, J., He, X., Su, C., Oezalp, S.-H., Pawlitzki, M., Ruck, T., Korsen, M., Kleinschnitz, K., Aslan, D., Hagenacker, T., Kleinschnitz, C., Meuth, S. G., & Pul, R. (2023). Immune Response to Seasonal Influenza Vaccination in Multiple Sclerosis Patients Receiving Cladribine. Cells, 12(9), 1243. https://doi.org/10.3390/cells12091243









