Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. EAE Induction
2.3. Estrous Cycle Assessment
2.4. Blood and Tissue Collection
2.5. Steroid Extraction
2.6. Hormone Measurements
2.7. Isolation of RNA, Reverse Transcription, and qPCR
2.8. Protein Isolation and Western Blot
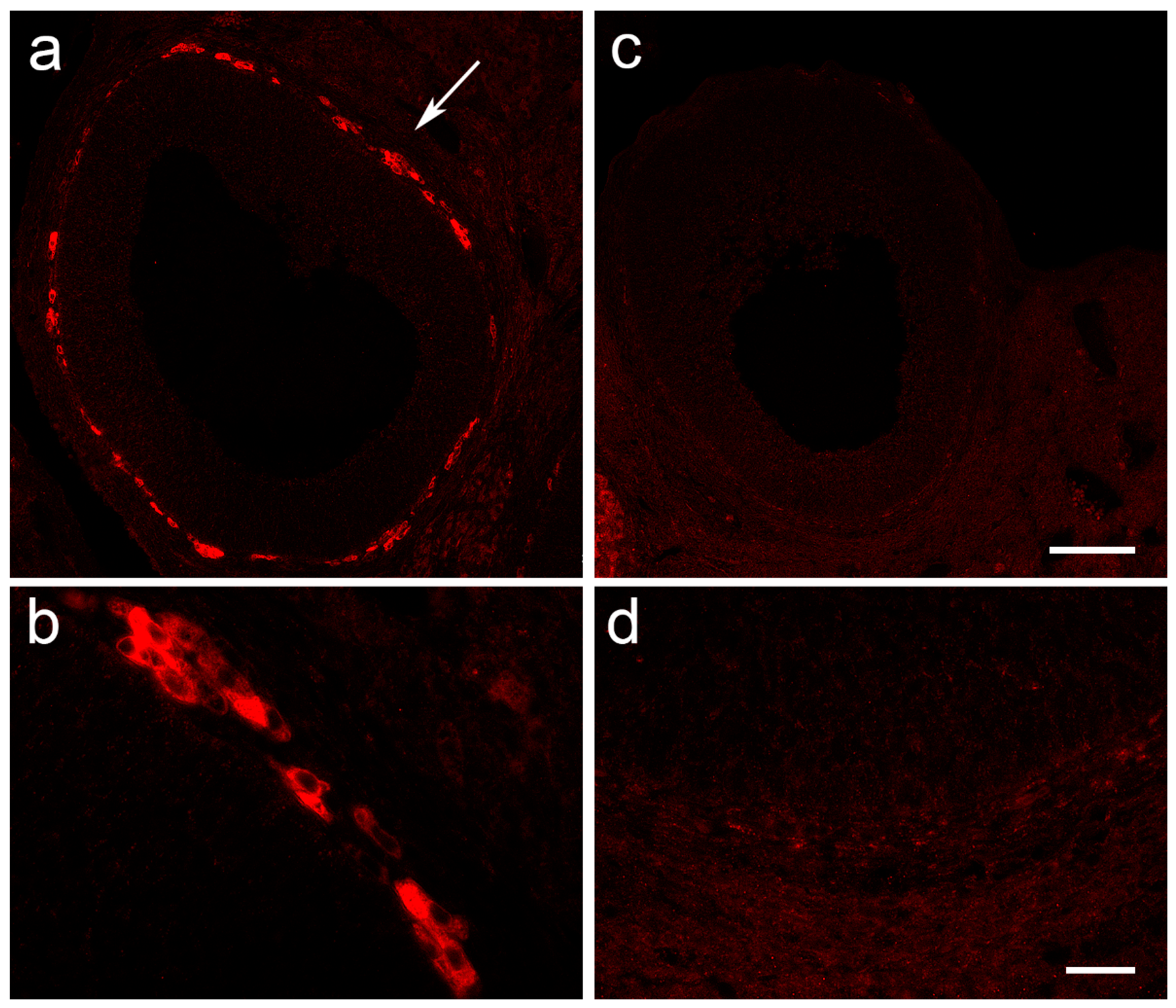
2.9. Histological Staining and Immunofluorescent Labeling of Ovarian Tissue
2.10. Statistical Analysis
3. Results
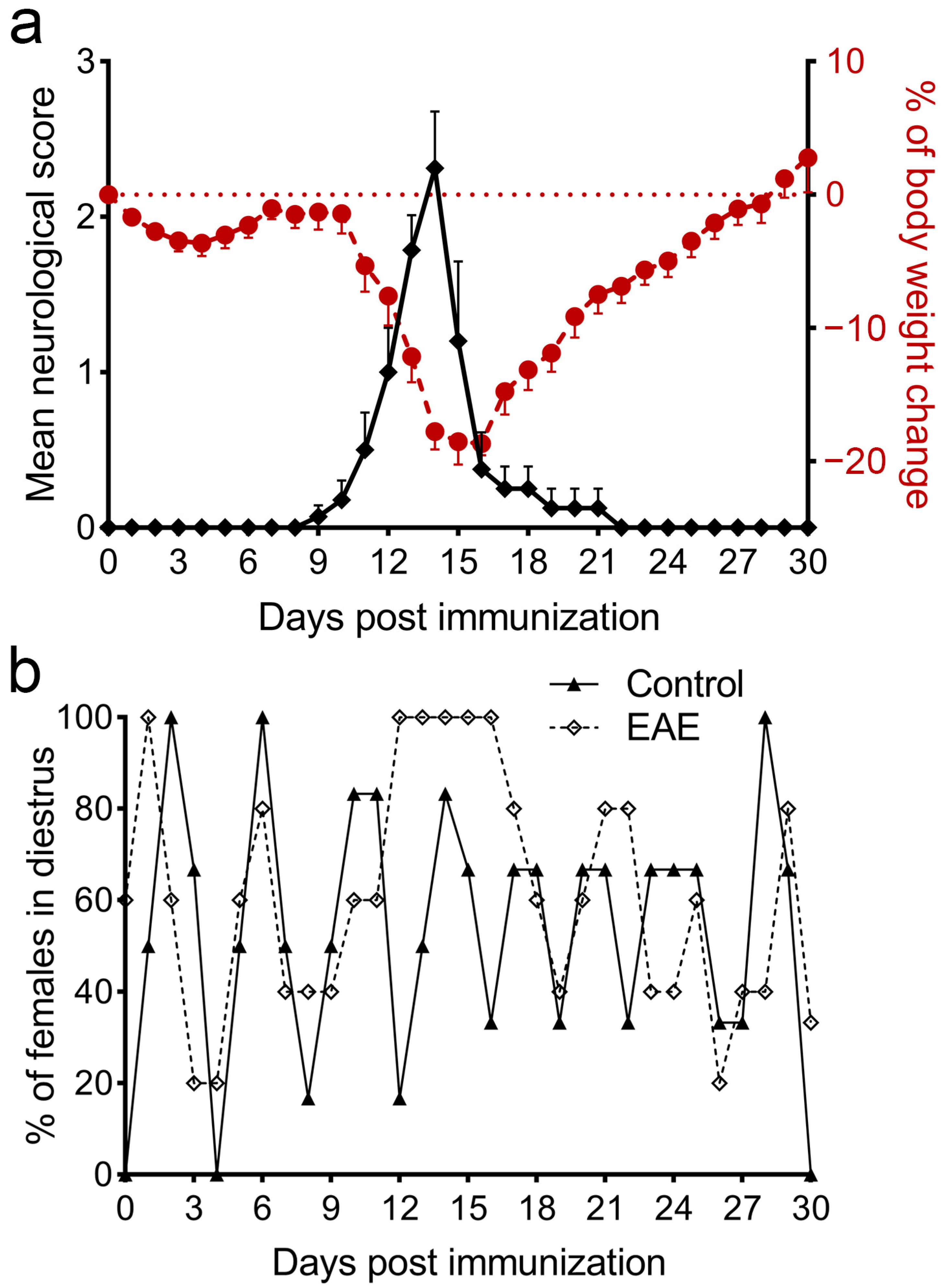
3.1. The Monophasic Course of EAE Is Followed by a Disturbance of the Estrous Cycle
3.2. Ovarian Morphology in the State of Prolonged Diestrus
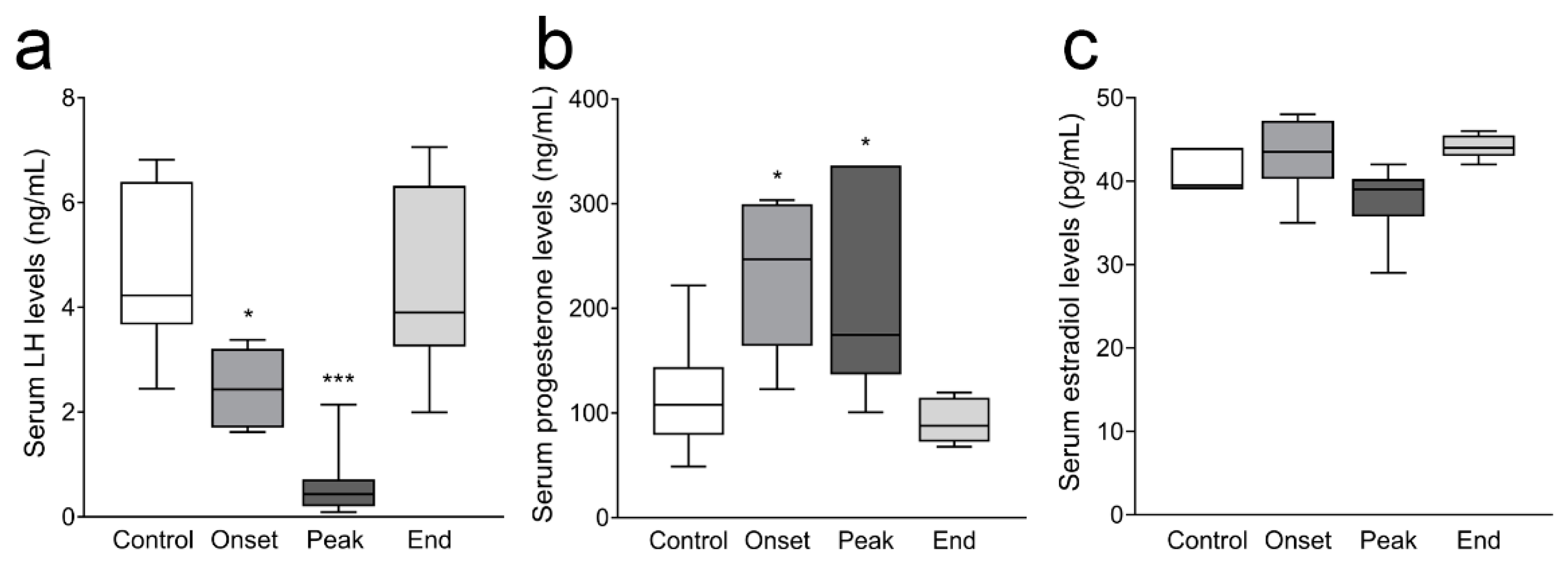
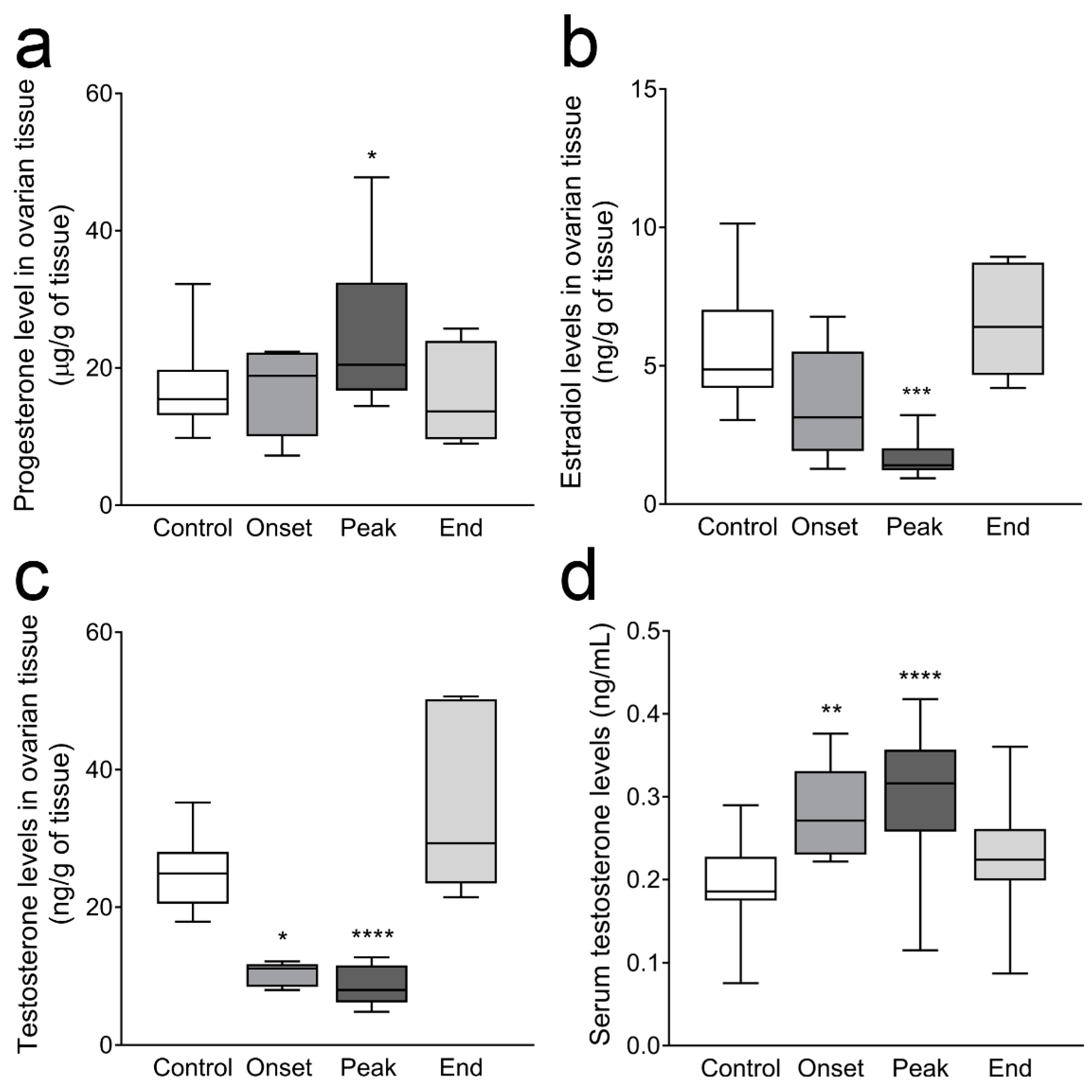
3.3. Ovarian Progesterone and Estradiol Levels and Ovarian and Serum Testosterone Levels
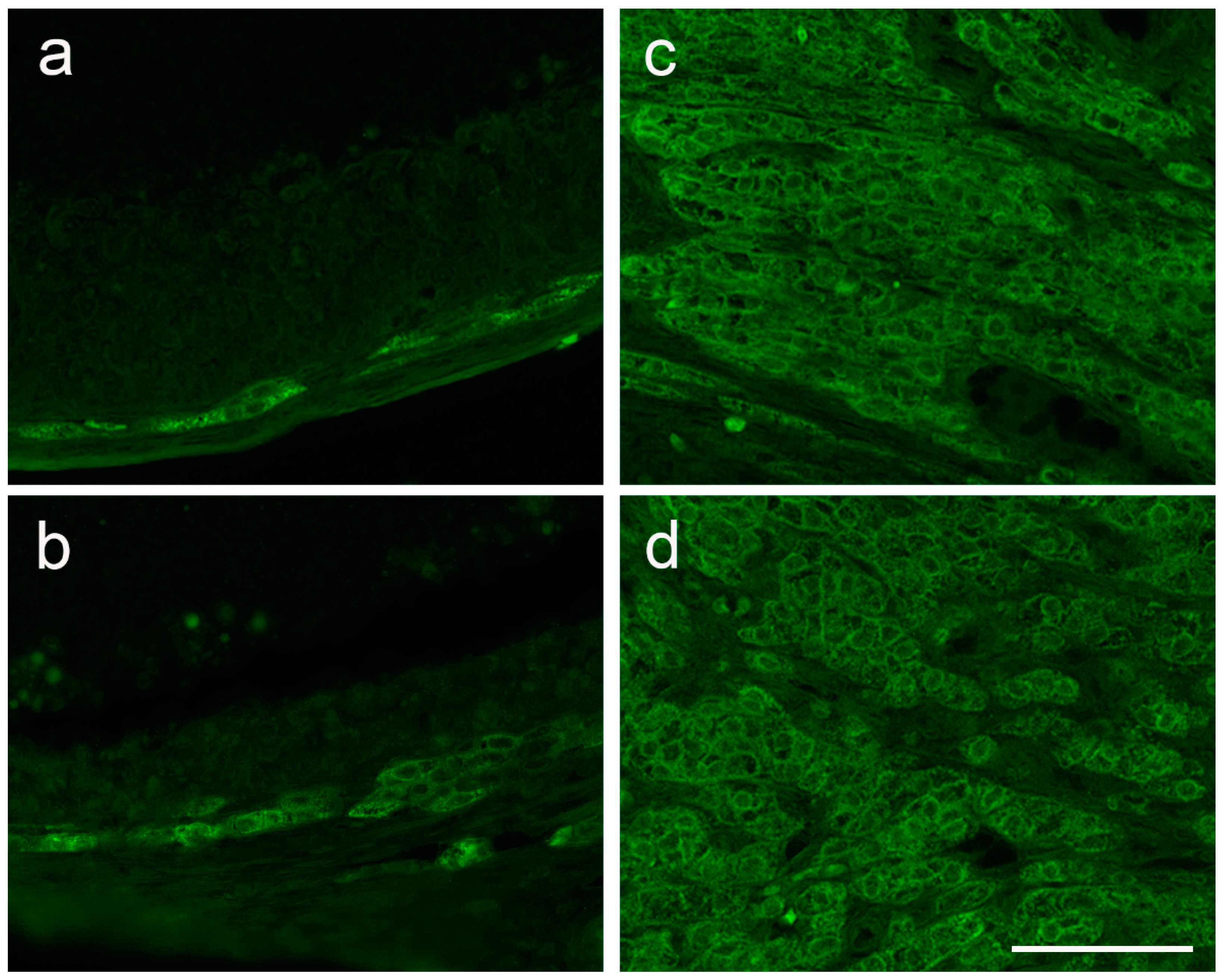
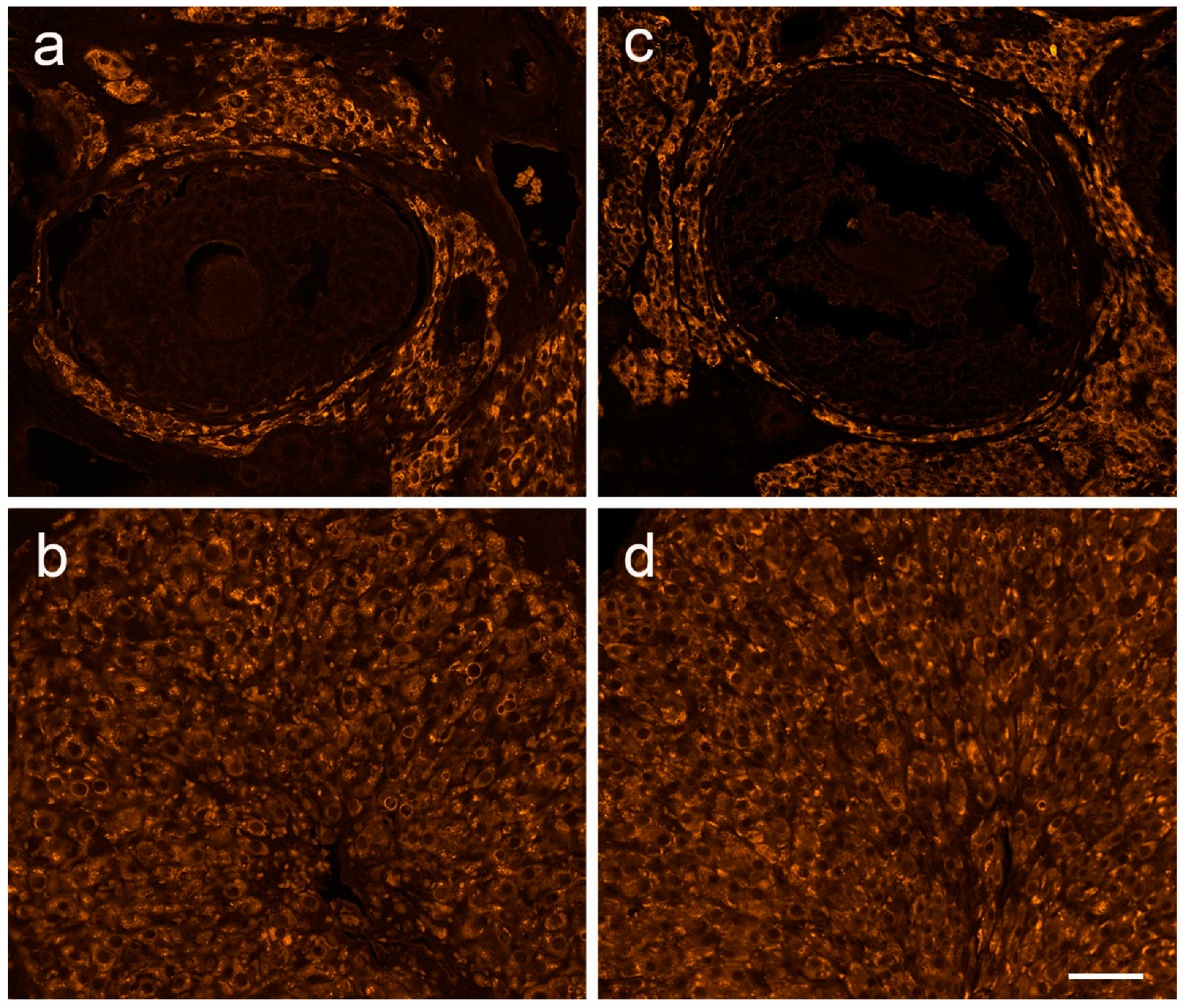
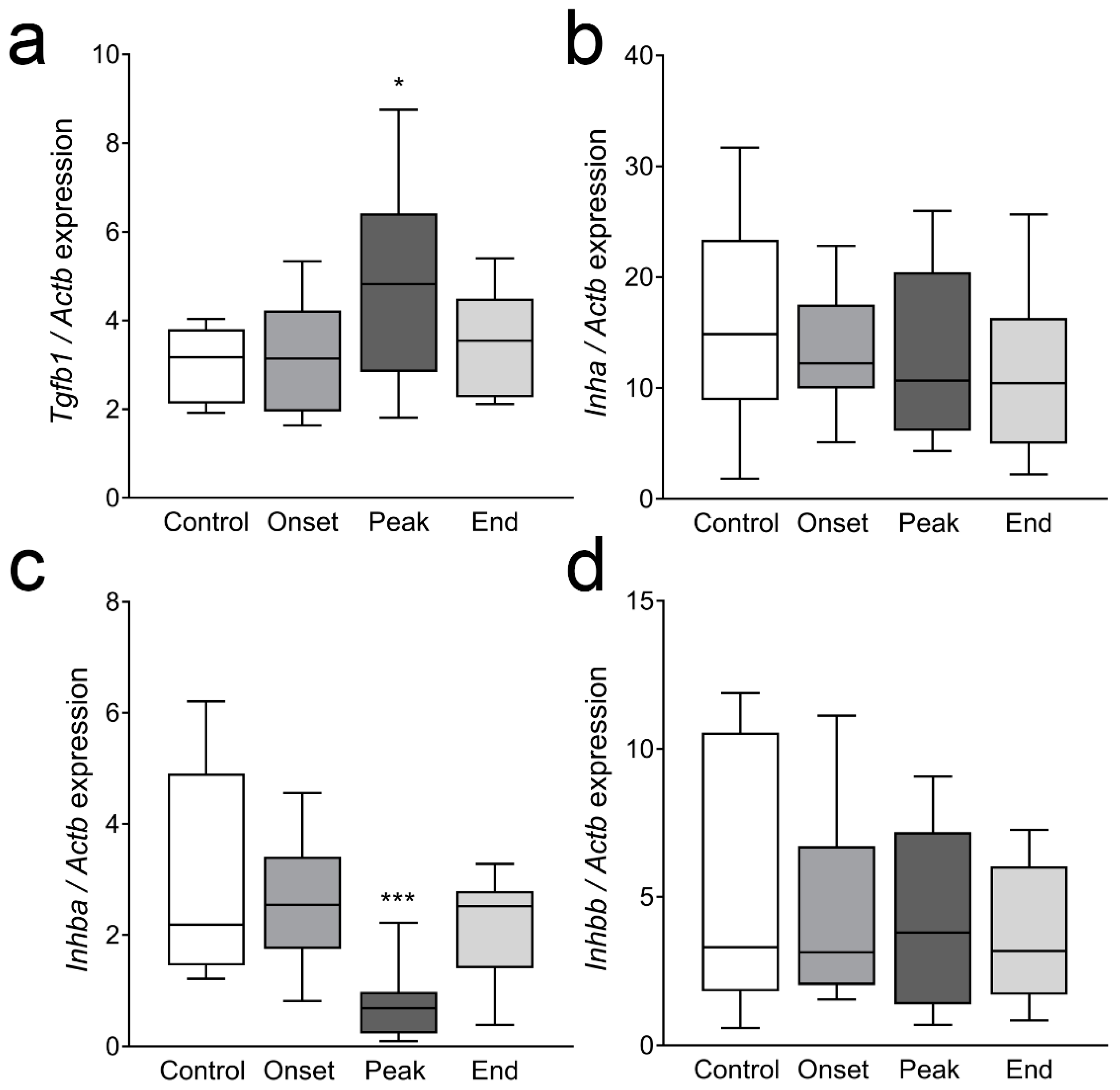
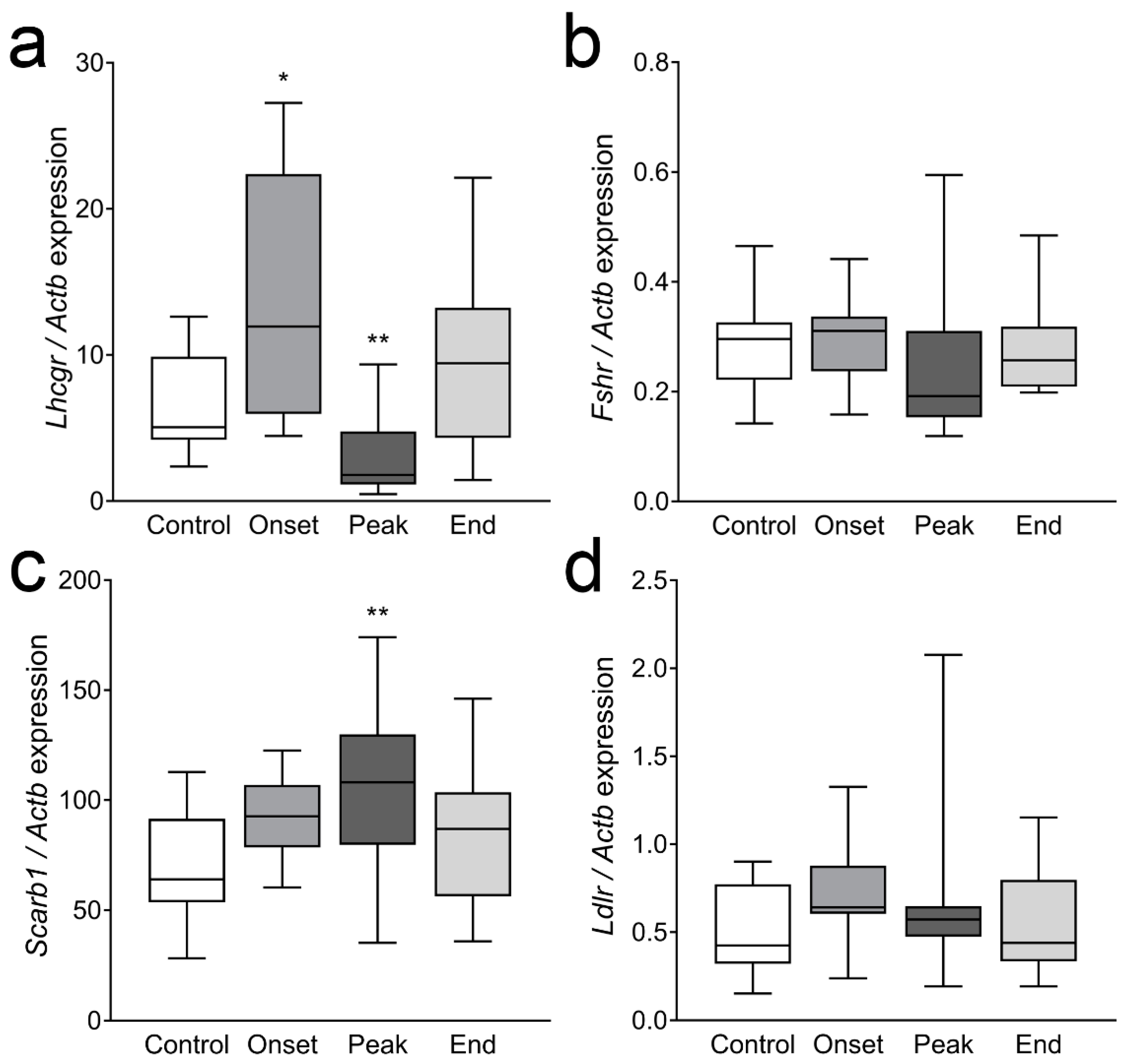
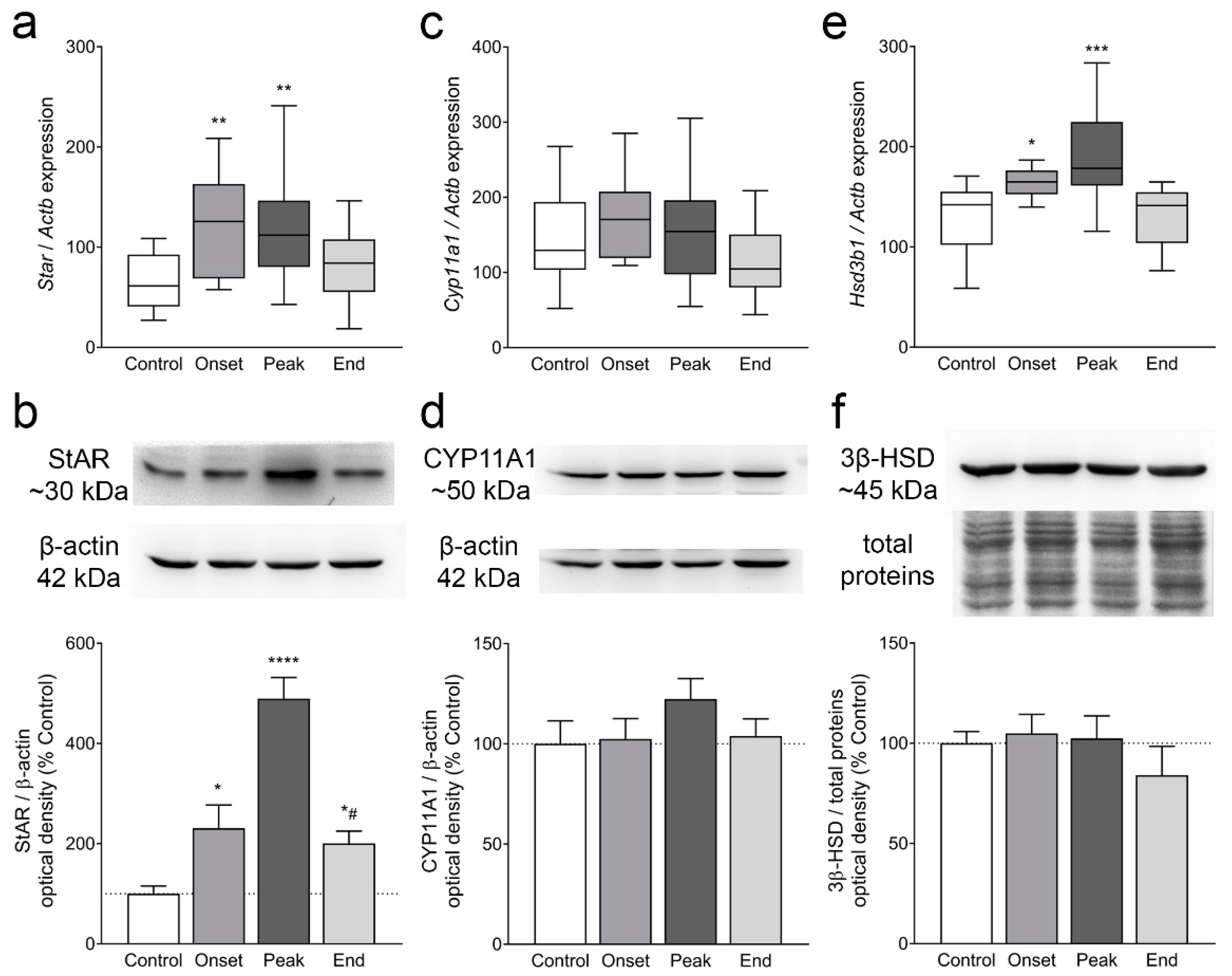
3.4. The Expression of Ovarian Enzymes and Proteins Involved in Sex Hormone Synthesis Is Affected during EAE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Magyari, M.; Koch-Henriksen, N. Quantitative effect of sex on disease activity and disability accumulation in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Sparaco, M.; Bonavita, S. The role of sex hormones in women with multiple sclerosis: From puberty to assisted reproductive techniques. Front. Neuroendocrinol. 2021, 60, 100889. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Langer-Gould, A.; Smith, J.B.; Albers, K.B.; Xiang, A.H.; Wu, J.; Kerezsi, E.H.; McClearnen, K.; Gonzales, E.G.; Leimpeter, A.D.; Van Den Eeden, S.K. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology 2020, 94, e1939–e1949. [Google Scholar] [CrossRef]
- Harness, J.; McCombe, P.A. The effects of pregnancy on myelin basic protein-induced experimental autoimmune encephalomyelitis in Lewis rats: Suppression of clinical disease, modulation of cytokine expression in the spinal cord inflammatory infiltrate and suppression of lymphocyte proliferation by pregnancy sera. Am. J. Reprod. Immunol. 2001, 46, 405–412. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Garren, H.; Slansky, A.; Ruiz, P.J.; Steinman, L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: Evidence for a suppressive pregnancy-related serum factor. J. Immunol. 2002, 169, 1084–1091. [Google Scholar] [CrossRef]
- Fillmore, P.D.; Blankenhorn, E.P.; Zachary, J.F.; Teuscher, C. Adult gonadal hormones selectively regulate sexually dimorphic quantitative traits observed in experimental allergic encephalomyelitis. Am. J. Pathol. 2004, 164, 167–175. [Google Scholar] [CrossRef]
- Trooster, W.J.; Teelken, A.W.; Gerrits, P.O.; Lijnema, T.H.; Loof, J.G.; Minderhoud, J.M.; Nieuwenhuis, P. The effect of gonadectomy on the clinical course of chronic experimental allergic encephalomyelitis. Clin. Neurol. Neurosurg. 1996, 98, 222–226. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Bonsack, B.; Brinton, R.; Schumacher, M.; Kumar, N.; Lee, J.-Y.; Castelli, V.; Corey, S.; Coats, A.; Sadanandan, N.; et al. Progress in progestin-based therapies for neurological disorders. Neurosci. Biobehav. Rev. 2021, 122, 38–65. [Google Scholar] [CrossRef]
- Caruso, D.; D’Intino, G.; Giatti, S.; Maschi, O.; Pesaresi, M.; Calabrese, D.; Garcia-Segura, L.-M.; Calza, L.; Melcangi, R.C. Sex-dimorphic changes in neuroactive steroid levels after chronic experimental autoimmune encephalomyelitis. J. Neurochem. 2010, 114, 921–932. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Ellestad, K.K.; Maingat, F.; Warren, K.G.; Han, M.H.; Steinman, L.; Baker, G.B.; Power, C. Impaired neurosteroid synthesis in multiple sclerosis. Brain 2011, 134, 2703–2721. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Baker, G.B.; Power, C. Allopregnanolone and neuroinflammation: A focus on multiple sclerosis. Front. Cell. Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.L.; Yong, V.T.; Sharmin, S.; Perucca, P.; Kalincik, T. Seizure risk in multiple sclerosis patients treated with disease-modifying therapy: A systematic review and network meta-analysis. Mult. Scler. 2023, 13524585231151400. [Google Scholar] [CrossRef]
- Lévesque, M.; Biagini, G.; Avoli, M. Neurosteroids and Focal Epileptic Disorders. Int. J. Mol. Sci. 2020, 21, 9391. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Clark, B.J.; Wells, J.; King, S.R.; Stocco, D.M. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 1994, 269, 28314–28322. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Bremer, A.A.; Miller, W.L. Chapter 13—Regulation of Steroidogenesis. In Cellular Endocrinology in Health and Disease; Ulloa-Aguirre, A., Conn, P.M., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 207–227. [Google Scholar]
- Hillier, S.G.; Whitelaw, P.F.; Smyth, C.D. Follicular oestrogen synthesis: The ‘two-cell, two-gonadotrophin’ model revisited. Mol. Cell. Endocrinol. 1994, 100, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, H.; Koch, Y.; Khan, M.I.; Ahrén, K. Effect of follicle-stimulating hormone on cyclic AMP levels in young corpora lutea of the rat. Eur. J. Obstet. Gynecol. Reprod. Biol. 1976, 6, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Uilenbroek, J.T.; Richards, J.S. Ovarian follicular development during the rat estrous cycle: Gonadotropin receptors and follicular responsiveness. Biol. Reprod. 1979, 20, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.R., Jr. Autoradiographic analysis of gonadotropin binding to rat ovarian tissue sections. Adv. Exp. Med. Biol. 1973, 36, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef]
- Milosevic, A.; Janjic, M.M.; Lavrnja, I.; Savic, D.; Bozic, I.D.; Tesovic, K.; Jakovljevic, M.; Pekovic, S.; Stojilkovic, S.S.; Bjelobaba, I. The sex-specific patterns of changes in hypothalamic-pituitary-gonadal axis during experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2020, 89, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, A.; Bjelobaba, I.; Bozic, I.D.; Lavrnja, I.; Savic, D.; Tesovic, K.; Jakovljevic, M.; Stojilkovic, S.S.; Janjic, M.M. Testicular steroidogenesis is suppressed during experimental autoimmune encephalomyelitis in rats. Sci. Rep. 2021, 11, 8996. [Google Scholar] [CrossRef]
- Janjic, M.M.; Stojkov, N.J.; Bjelic, M.M.; Mihajlovic, A.I.; Andric, S.A.; Kostic, T.S. Transient rise of serum testosterone level after single sildenafil treatment of adult male rats. J. Sex. Med. 2012, 9, 2534–2543. [Google Scholar] [CrossRef]
- Koganti, P.P.; Zhao, A.H.; Selvaraj, V. Exogenous cholesterol acquisition signaling in LH-responsive MA-10 Leydig cells and in adult mice. J. Endocrinol. 2022, 254, 187–199. [Google Scholar] [CrossRef]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef]
- Lara, H.E.; Dissen, G.A.; Leyton, V.; Paredes, A.; Fuenzalida, H.; Fiedler, J.L.; Ojeda, S.R. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 2000, 141, 1059–1072. [Google Scholar] [CrossRef]
- Taketa, Y. Luteal toxicity evaluation in rats. J. Toxicol. Pathol. 2022, 35, 7–17. [Google Scholar] [CrossRef]
- Sato, J.; Hashimoto, S.; Doi, T.; Yamada, N.; Tsuchitani, M. Histological characteristics of the regression of corpora lutea in wistar hannover rats: The comparisons with sprague-dawley rats. J. Toxicol. Pathol. 2014, 27, 107–113. [Google Scholar] [CrossRef]
- Thöne, J.; Kollar, S.; Nousome, D.; Ellrichmann, G.; Kleiter, I.; Gold, R.; Hellwig, K. Serum anti-Müllerian hormone levels in reproductive-age women with relapsing-remitting multiple sclerosis. Mult. Scler. 2015, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, M.; Ros, C.; Martínez-Lapiscina, E.H.; Solà-Valls, N.; Hervàs, M.; Llufriu, S.; La Puma, D.; Casals, E.; Blanco, Y.; Villoslada, P.; et al. Pituitary-ovary axis and ovarian reserve in fertile women with multiple sclerosis: A pilot study. Mult. Scler. 2016, 22, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.S.; Henry, R.G.; Cree, B.A.C.; Lambert-Messerlian, G.; Greenblatt, R.M.; Waubant, E.; Cedars, M.I.; Zhu, A.; Bacchetti, P.; Hauser, S.L.; et al. Ovarian aging is associated with gray matter volume and disability in women with MS. Neurology 2018, 90, e254–e260. [Google Scholar] [CrossRef] [PubMed]
- Lamaita, R.; Melo, C.; Laranjeira, C.; Barquero, P.; Gomes, J.; Silva-Filho, A. Multiple Sclerosis in Pregnancy and its Role in Female Fertility: A Systematic Review. JBRA Assist. Reprod. 2021, 25, 493–499. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Hennebold, J.D. Chapter 23Structure, Function, and Regulation of the Corpus Luteum. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1023–1076. [Google Scholar]
- Levine, J.E. Chapter 26—Neuroendocrine Control of the Ovarian Cycle of the Rat. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1199–1257. [Google Scholar]
- Osman, P. Morphometric analysis of follicular dynamics in pregnant and pseudopregnant rats. J. Reprod. Fertil. 1986, 76, 11–22. [Google Scholar] [CrossRef]
- Yu, Y.S.; Sui, H.S.; Han, Z.B.; Li, W.; Luo, M.J.; Tan, J.H. Apoptosis in granulosa cells during follicular atresia: Relationship with steroids and insulin-like growth factors. Cell. Res. 2004, 14, 341–346. [Google Scholar] [CrossRef]
- Welschen, R.; Osman, P.; Dullaart, J.; de Greef, W.J.; Uilenbroek, J.T.; de Jong, F.H. Levels of follicle-stimulating hormone, luteinizing hormone, oestradiol-17 beta and progesterone, and follicular growth in the pseudopregnant rat. J. Endocrinol. 1975, 64, 37–47. [Google Scholar] [CrossRef]
- Braw-Tal, R. Expression of mRNA for follistatin and inhibin/activin subunits during follicular growth and atresia. J. Mol. Endocrinol. 1994, 13, 253–264. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Kumar, T.R.; Bradley, A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 1995, 374, 356–360. [Google Scholar] [CrossRef]
- Chen, Y.J.; Feng, Q.; Liu, Y.X. Expression of the steroidogenic acute regulatory protein and luteinizing hormone receptor and their regulation by tumor necrosis factor alpha in rat corpora lutea. Biol. Reprod. 1999, 60, 419–427. [Google Scholar] [CrossRef]
- Smith, M.S.; Freeman, M.E.; Neill, J.D. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975, 96, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Riskind, P.N.; Massacesi, L.; Doolittle, T.H.; Hauser, S.L. The role of prolactin in autoimmune demyelination: Suppression of experimental allergic encephalomyelitis by bromocriptine. Ann. Neurol. 1991, 29, 542–547. [Google Scholar] [CrossRef]
- Ghareghani, M.; Ghanbari, A.; Eid, A.; Shaito, A.; Mohamed, W.; Mondello, S.; Zibara, K. Hormones in experimental autoimmune encephalomyelitis (EAE) animal models. Transl. Neurosci. 2021, 12, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Kira, J.-I.; Harada, M.; Yamaguchi, Y.; Shida, N.; Goto, I. Hyperprolactinemia in multiple sclerosis. J. Neurol. Sci. 1991, 102, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L. Intracellular mechanisms triggering gonadotrophin secretion. Rev. Reprod. 1996, 1, 193–202. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.S.; Crawford, J.L.; Taragnat, C.; Nicol, L.; McNeilly, J.R. The differential secretion of FSH and LH: Regulation through genes, feedback and packaging. Reprod. Suppl. 2003, 61, 463–476. [Google Scholar] [CrossRef]
- Das, N.; Kumar, T.R. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J. Mol. Endocrinol. 2018, 60, R131–R155. [Google Scholar] [CrossRef]
- Guven Yorgun, Y.; Ozakbas, S. Effect of hormonal changes on the neurological status in the menstrual cycle of patient with multiple sclerosis. Clin. Neurol. Neurosurg. 2019, 186, 105499. [Google Scholar] [CrossRef]
- Foroughipour, A.; Norbakhsh, V.; Najafabadi, S.H.; Meamar, R. Evaluating sex hormone levels in reproductive age women with multiple sclerosis and their relationship with disease severity. J. Res. Med. Sci. 2012, 17, 882–885. [Google Scholar]
- Oktem, O.; Akin, N.; Bildik, G.; Yakin, K.; Alper, E.; Balaban, B.; Urman, B. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum. Reprod. 2017, 32, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.B.; Welsh, T.H., Jr.; Hsueh, A.J. Regulation of ovarian progestin production by epidermal growth factor in cultured rat granulosa cells. J. Biol. Chem. 1982, 257, 11268–11273. [Google Scholar] [CrossRef] [PubMed]
- Welsh, T.H., Jr.; Zhuang, L.Z.; Hsueh, A.J. Estrogen augmentation of gonadotropin-stimulated progestin biosynthesis in cultured rat granulosa cells. Endocrinology 1983, 112, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Dodson, W.C.; Schomberg, D.W. The effect of transforming growth factor-beta on follicle-stimulating hormone-induced differentiation of cultured rat granulosa cells. Endocrinology 1987, 120, 512–516. [Google Scholar] [CrossRef]
- Lai, W.-A.; Yeh, Y.-T.; Lee, M.-T.; Wu, L.-S.; Ke, F.-C.; Hwang, J.-J. Ovarian granulosa cells utilize scavenger receptor SR-BI to evade cellular cholesterol homeostatic control for steroid synthesis. J. Lipid. Res. 2013, 54, 365–378. [Google Scholar] [CrossRef]
- Stocco, C.; Callegari, E.; Gibori, G. Opposite effect of prolactin and prostaglandin F2α on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology 2001, 142, 4158–4161. [Google Scholar] [CrossRef]
- Minegishi, T.; Tsuchiya, M.; Hirakawa, T.; Abe, K.; Inoue, K.; Mizutani, T.; Miyamoto, K. Expression of steroidogenic acute regulatory protein (StAR) in rat granulosa cells. Life Sci. 2000, 67, 1015–1024. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Hsiao, P.-W.; Lee, M.-T.; Mason, J.I.; Ke, F.-C.; Hwang, J.-J. Interplay of PI3K and cAMP/PKA signaling, and rapamycin-hypersensitivity in TGFβ1 enhancement of FSH-stimulated steroidogenesis in rat ovarian granulosa cells. J. Endocrinol. 2007, 192, 405–419. [Google Scholar] [CrossRef]
- Eimerl, S.; Orly, J. Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: Differential responses of cytochrome P450 side-chain cleavage, steroidogenic acute regulatory protein, and 3β-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biol. Reprod. 2002, 67, 900–910. [Google Scholar] [CrossRef]
- Ghoumari, A.M.; Abi Ghanem, C.; Asbelaoui, N.; Schumacher, M.; Hussain, R. Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. Int. J. Mol. Sci. 2020, 21, 3163. [Google Scholar] [CrossRef]
- Kempe, P.; Eklund, D.; Hallin, A.; Hammar, M.; Olsson, T.; Brynhildsen, J.; Ernerudh, J. Immune profile in relation to sex steroid cyclicity in healthy women and women with multiple sclerosis. J. Reprod. Immunol. 2018, 126, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, V.; Onesti, E.; Mainero, C.; Giugni, E.; Paolillo, A.; Salvetti, M.; Nicoletti, F.; Pozzilli, C. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J. Neurol. Neurosurg. Psychiatry 2005, 76, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.L.; Carlone, D.L.; Robker, R.L.; Richards, J.S. Expression of aromatase in the ovary: Down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids 1997, 62, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Collongues, N.; Patte-Mensah, C.; De Seze, J.; Mensah-Nyagan, A.-G.; Derfuss, T. Testosterone and estrogen in multiple sclerosis: From pathophysiology to therapeutics. Expert. Rev. Neurother. 2018, 18, 515–522. [Google Scholar] [CrossRef] [PubMed]
- You, L. Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem. Biol. Interact. 2004, 147, 233–246. [Google Scholar] [CrossRef]
- Ghaffarinia, A.; Jalili, C.; Mostafaie, A.; Parvaneh, S.; Pakravan, N. Liver Damage and Mortality in a Male Lewis Rat of Experimental Autoimmune Encephalomyelitis. Iran. J. Pathol. 2015, 10, 9–16. [Google Scholar]
- Anuka, E.; Gal, M.; Stocco, D.M.; Orly, J. Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Mol. Cell. Endocrinol. 2013, 371, 47–61. [Google Scholar] [CrossRef]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Li, J.; Daly, E.; Campioli, E.; Wabitsch, M.; Papadopoulos, V. De novo synthesis of steroids and oxysterols in adipocytes. J. Biol. Chem. 2014, 289, 747–764. [Google Scholar] [CrossRef]
- Wagner, I.V.; Savchuk, I.; Sahlin, L.; Kulle, A.; Klöting, N.; Dietrich, A.; Holterhus, P.-M.; Dötsch, J.; Blüher, M.; Söder, O. De Novo and Depot-Specific Androgen Production in Human Adipose Tissue: A Source of Hyperandrogenism in Women with Obesity. Obes. Facts 2022, 15, 281–291. [Google Scholar] [CrossRef]
- Byeon, H.R.; Lee, S.-H. Expression of Steroidogenesis-related Genes in Rat Adipose Tissues. Dev. Reprod. 2016, 20, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Sahlin, L.; Savchuk, I.; Klöting, N.; Svechnikov, K.; Söder, O. Adipose Tissue is a Potential Source of Hyperandrogenism in Obese Female Rats. Obesity 2018, 26, 1161–1167. [Google Scholar] [CrossRef]
- Bernardes, D.; Oliveira-Lima, O.C.; da Silva, T.V.; Juliano, M.A.; dos Santos, D.M.; Carvalho-Tavares, J. Metabolic Alterations in Experimental Autoimmune Encephalomyelitis in Mice: Effects of Prior Physical Exercise. Neurophysiology 2016, 48, 117–121. [Google Scholar] [CrossRef]
- Schuh, K.; Häussler, S.; Sadri, H.; Prehn, C.; Lintelmann, J.; Adamski, J.; Koch, C.; Frieten, D.; Ghaffari, M.H.; Dusel, G.; et al. Blood and adipose tissue steroid metabolomics and mRNA expression of steroidogenic enzymes in periparturient dairy cows differing in body condition. Sci. Rep. 2022, 12, 2297. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence | Accession Number |
|---|---|---|
| Actb | forward: AGATTACTGCCCTGGCTCCT reverse: ACATCTGCTGGAAGGTGGAC | NM_031144.3 |
| Cyp11a1 | forward: ACTTCCTGAGGGAGAACGGC reverse: TCCATGTTGCCCAGCTTCTC | NM_017286.3 |
| Cyp17a1 | forward: GTTTCTCCCCAGACGTGGTC reverse: GGTCCGACAAGAGGCTTTGA | NM_012753.2 |
| Cyp19a1 | forward: CTCAACCTCACCACGGATGT reverse: GGCTCTCTGGATGGATGCTC | NM_017085.2 |
| Fshr | forward: CTACACATTGACAGCCATCACCCTA reverse: GGCAAAAGTCCAGCCCAATACC | NM_199237.1 |
| Hsd17b1 | forward: ATGAGTTGAACGCTGTGGGT reverse: GGCACAGTACACTTCGTGGA | NM_012851.2 |
| Hsd3b1 | forward: GACAGGAGCAGGAGGGTTTGTGG reverse: CTCCTTCTAACATTGTCACCTTGGCCT | NM_001007719.3 |
| Inha | forward: GCCTGAGACCACTCCTTTCC reverse: AAGGAGATGTTGAGGGCAGC | NM_012590.2 |
| Inhba | forward: CTGGCAAAACAGAAGGGACC reverse: GCATCCTGGCAGCAAAAGTC | NM_017128.2 |
| Inhbb | forward: TCCTAGTGCCCTGCTGAGAT reverse: ACCCACAGGGACAACTTCTG | NM_080771.1 |
| Lhcgr | forward: TATGCTCGGAGGATGGCTCT reverse: AGCACAGATGACGACGAAGG | NM_012978.1 |
| Scarb1 | forward: GCTTCTGGTGCCCATCATTTAC reverse: AGCTTGGCTTCTTGCAGTACC | NM_031541.1 |
| Star | forward: AGCAAGGAGAGGAAGCTATGC reverse: GGCACCACCTTACTTAGCACT | NM_031558.3 |
| Tgfb1 | forward: CTGCTGACCCCCACTGATAC reverse: AGCCCTGTATTCCGTCTCCT | NM_021578.2 |
| Antibody | Source and Type | Manufacturer | Dilution |
|---|---|---|---|
| β-actin | Mouse, monoclonal | Sigma, A5316 | 1:5000 WB |
| StAR | Rabbit, polyclonal | Gift from Prof. Vimal Selvaraj [28] | 1:500 WB |
| CYP11A1 | Rabbit, polyclonal | Protein Tech, 13363-1-AP | 1:2000 WB |
| 3β-HSD | Mouse, monoclonal | Santa Cruz, sc-515120 | 1:500 WB 1:50 IF |
| LHR | Rabbit, polyclonal | Abcam, ab125214 | 1:200 IF |
| CYP17A1 | Rabbit, polyclonal | Protein Tech, 14447-1-AP | 1:500 IF |
| Anti-mouse IgG, HRP-conjugated | Donkey, polyclonal | Santa Cruz, sc-2314 | 1:5000 WB |
| Anti-rabbit IgG, HRP-conjugated | Donkey, polyclonal | Santa Cruz, sc-2313 | 1:5000 WB |
| Anti-mouse IgG, Alexa Fluor 555-conjugated | Goat, polyclonal | Life technologies, A21424 | 1:400 IF |
| Anti-rabbit IgG, Alexa Fluor 555-conjugated | Goat, polyclonal | Life technologies, A21429 | 1:400 IF |
| Anti-rabbit IgG, Alexa Fluor 488-conjugated | Goat, polyclonal | Invitrogen, A11008 | 1:400 IF |
| Ovarian Weight | Control | Onset | Peak | End |
|---|---|---|---|---|
| Absolute (mg) | 48 (41–52) | 46 (42–50) | 36 (27–40) * | 49 (46–51) |
| Relative to body weight (mg/g) | 0.28 (0.24–0.30) | 0.30 (0.26–0.33) | 0.28 (0.22–0.30) | 0.29 (0.28–0.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosevic, A.; Lavrnja, I.; Savic, D.; Milosevic, K.; Skuljec, J.; Bjelobaba, I.; Janjic, M.M. Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis. Cells 2023, 12, 1045. https://doi.org/10.3390/cells12071045
Milosevic A, Lavrnja I, Savic D, Milosevic K, Skuljec J, Bjelobaba I, Janjic MM. Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis. Cells. 2023; 12(7):1045. https://doi.org/10.3390/cells12071045
Chicago/Turabian StyleMilosevic, Ana, Irena Lavrnja, Danijela Savic, Katarina Milosevic, Jelena Skuljec, Ivana Bjelobaba, and Marija M. Janjic. 2023. "Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis" Cells 12, no. 7: 1045. https://doi.org/10.3390/cells12071045
APA StyleMilosevic, A., Lavrnja, I., Savic, D., Milosevic, K., Skuljec, J., Bjelobaba, I., & Janjic, M. M. (2023). Rat Ovarian Function Is Impaired during Experimental Autoimmune Encephalomyelitis. Cells, 12(7), 1045. https://doi.org/10.3390/cells12071045







