LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation
Abstract
1. Introduction
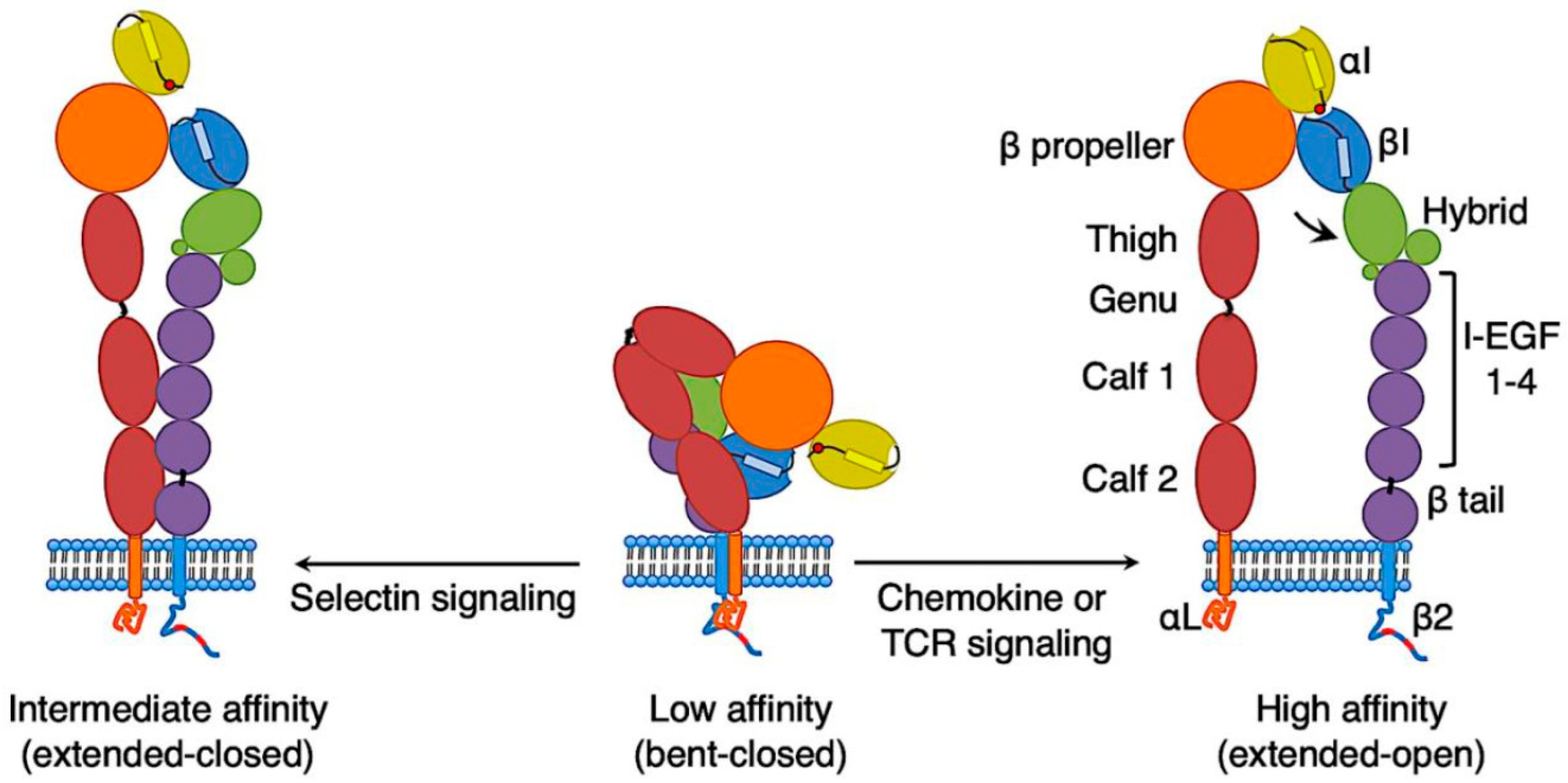
2. Conformations of LFA-1 on T Cells
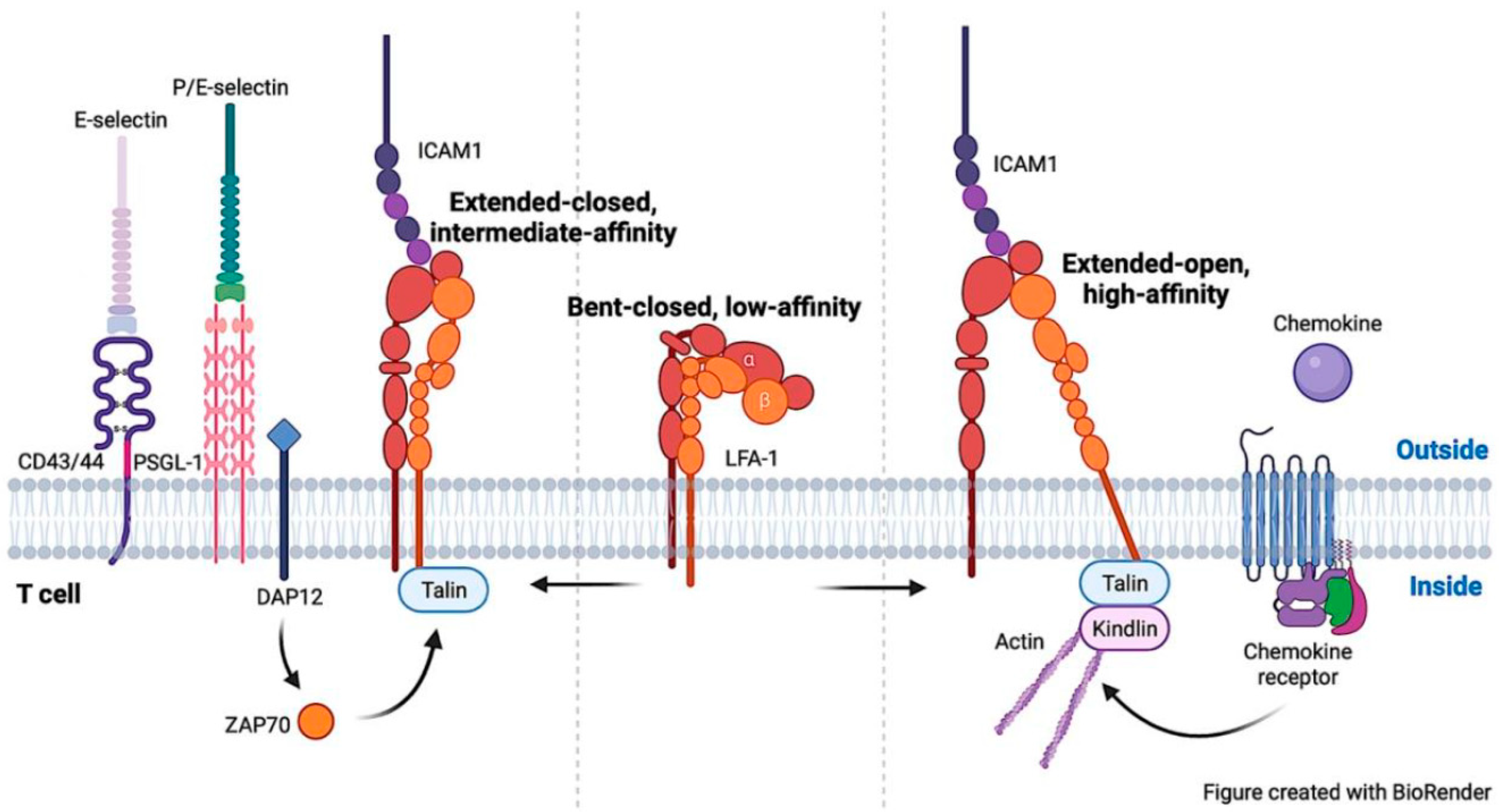
3. Signaling Pathways to Activate LFA-1 on T Cells
4. Regulation of T-Cell Migration by LFA-1
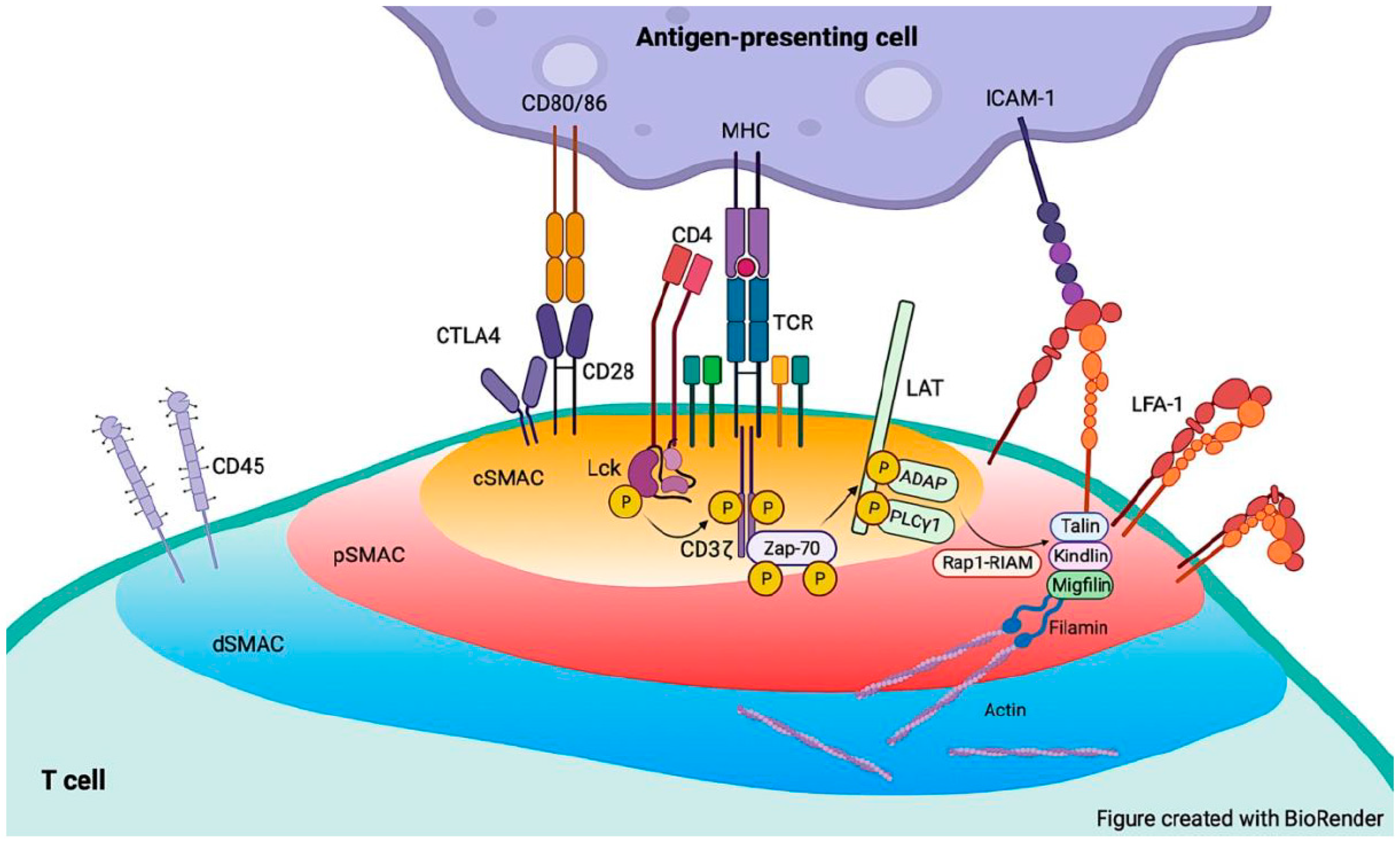
5. LFA-1 Functions in Immunological Synapse Formation
6. Summary and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Salas, A.; Shimaoka, M.; Kogan, A.N.; Harwood, C.; von Andrian, U.H.; Springer, T.A. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity 2004, 20, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Critchley, D.R. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys. 2009, 38, 235–254. [Google Scholar] [CrossRef]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef]
- Schürpf, T.; Springer, T.A. Regulation of integrin affinity on cell surfaces. EMBO J. 2011, 30, 4712–4727. [Google Scholar] [CrossRef]
- Shimaoka, M.; Xiao, T.; Liu, J.H.; Yang, Y.; Dong, Y.; Jun, C.D.; McCormack, A.; Zhang, R.; Joachimiak, A.; Takagi, J.; et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 2003, 112, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; McArdle, S.; Marki, A.; Mikulski, Z.; Gutierrez, E.; Engelhardt, B.; Deutsch, U.; Ginsberg, M.; Groisman, A.; Ley, K. Neutrophil recruitment limited by high-affinity bent beta2 integrin binding ligand in cis. Nat. Commun. 2016, 7, 12658. [Google Scholar] [CrossRef]
- Stanley, P.; Smith, A.; McDowall, A.; Nicol, A.; Zicha, D.; Hogg, N. Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell. EMBO J. 2008, 27, 62–75. [Google Scholar] [CrossRef]
- Shulman, Z.; Shinder, V.; Klein, E.; Grabovsky, V.; Yeger, O.; Geron, E.; Montresor, A.; Bolomini-Vittori, M.; Feigelson, S.W.; Kirchhausen, T.; et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 2009, 30, 384–396. [Google Scholar] [CrossRef]
- Comrie, W.A.; Li, S.; Boyle, S.; Burkhardt, J.K. The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility. J. Cell Biol. 2015, 208, 457–473. [Google Scholar] [CrossRef]
- Shao, B.; Yago, T.; Panicker, S.R.; Zhang, N.; Liu, Z.; McEver, R.P. Th1 Cells Rolling on Selectins Trigger DAP12-Dependent Signals That Activate Integrin alphaLbeta2. J. Immunol. 2020, 204, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Shattil, S.J.; Eto, K.; Tai, V.; Liddington, R.C.; de Pereda, J.M.; Ginsberg, M.H.; Calderwood, D.A. Talin binding to integrin beta tails: A final common step in integrin activation. Science 2003, 302, 103–106. [Google Scholar] [CrossRef]
- Simonson, W.T.; Franco, S.J.; Huttenlocher, A. Talin1 regulates TCR-mediated LFA-1 function. J. Immunol. 2006, 177, 7707–7714. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Moser, M.; Pleines, I.; Varga-Szabo, D.; Monkley, S.; Critchley, D.; Fassler, R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J. Exp. Med. 2007, 204, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.J.; Ades, S.E.; Singer, S.J.; Hynes, R.O. Sequence and domain structure of talin. Nature 1990, 347, 685–689. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Yan, B.; de Pereda, J.M.; Alvarez, B.G.; Fujioka, Y.; Liddington, R.C.; Ginsberg, M.H. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 2002, 277, 21749–21758. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, B.; de Pereda, J.M.; Calderwood, D.A.; Ulmer, T.S.; Critchley, D.; Campbell, I.D.; Ginsberg, M.H.; Liddington, R.C. Structural determinants of integrin recognition by talin. Mol. Cell 2003, 11, 49–58. [Google Scholar] [CrossRef]
- Wegener, K.L.; Partridge, A.W.; Han, J.; Pickford, A.R.; Liddington, R.C.; Ginsberg, M.H.; Campbell, I.D. Structural basis of integrin activation by talin. Cell 2007, 128, 171–182. [Google Scholar] [CrossRef]
- Vinogradova, O.; Velyvis, A.; Velyviene, A.; Hu, B.; Haas, T.; Plow, E.; Qin, J. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell 2002, 110, 587–597. [Google Scholar] [CrossRef]
- Anthis, N.J.; Wegener, K.L.; Ye, F.; Kim, C.; Goult, B.T.; Lowe, E.D.; Vakonakis, I.; Bate, N.; Critchley, D.R.; Ginsberg, M.H.; et al. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009, 28, 3623–3632. [Google Scholar] [CrossRef]
- Kim, C.; Ye, F.; Hu, X.; Ginsberg, M.H. Talin activates integrins by altering the topology of the beta transmembrane domain. J. Cell Biol. 2012, 197, 605–611. [Google Scholar] [CrossRef]
- Saltel, F.; Mortier, E.; Hytonen, V.P.; Jacquier, M.C.; Zimmermann, P.; Vogel, V.; Liu, W.; Wehrle-Haller, B. New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control beta3-integrin clustering. J. Cell Biol. 2009, 187, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.T.; Nygren, P.; Jo, H.; Boesze-Battaglia, K.; Bennett, J.S.; DeGrado, W.F. Affinity of talin-1 for the beta3-integrin cytosolic domain is modulated by its phospholipid bilayer environment. Proc. Natl. Acad. Sci. USA 2012, 109, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.J.; Woods, M.L.; Dmowski, S.A.; Derimanov, G.; Jordan, M.S.; Wu, J.N.; Myung, P.S.; Liu, Q.H.; Pribila, J.T.; Freedman, B.D.; et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 2001, 293, 2263–2265. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, T. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 2005, 5, 546–559. [Google Scholar] [CrossRef]
- Ghandour, H.; Cullere, X.; Alvarez, A.; Luscinskas, F.W.; Mayadas, T.N. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood 2007, 110, 3682–3690. [Google Scholar] [CrossRef]
- Yago, T.; Shao, B.; Miner, J.J.; Yao, L.; Klopocki, A.G.; Maeda, K.; Coggeshall, K.M.; McEver, R.P. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood 2010, 116, 485–494. [Google Scholar] [CrossRef]
- Hidari, K.I.; Weyrich, A.S.; Zimmerman, G.A.; McEver, R.P. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J. Biol. Chem. 1997, 272, 28750–28756. [Google Scholar] [CrossRef]
- Cummings, R.D. Stuck on sugars—How carbohydrates regulate cell adhesion, recognition, and signaling. Glycoconj. J. 2019, 36, 241–257. [Google Scholar] [CrossRef]
- Yago, T.; Zhang, N.; Zhao, L.; Abrams, C.S.; McEver, R.P. Selectins and chemokines use shared and distinct signals to activate β2 integrins in neutrophils. Blood Adv. 2018, 2, 731–744. [Google Scholar] [CrossRef]
- Haling, J.R.; Monkley, S.J.; Critchley, D.R.; Petrich, B.G. Talin-dependent integrin activation is required for fibrin clot retraction by platelets. Blood 2011, 117, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Petrich, B.G.; Zhang, N.; Liu, Z.; Shao, B.; Ginsberg, M.H.; McEver, R.P. Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J. Exp. Med. 2015, 212, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Lefort, C.T.; Rossaint, J.; Moser, M.; Petrich, B.G.; Zarbock, A.; Monkley, S.J.; Critchley, D.R.; Ginsberg, M.H.; Fässler, R.; Ley, K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 2012, 119, 4275–4282. [Google Scholar] [CrossRef]
- Bezman, N.A.; Lian, L.; Abrams, C.S.; Brass, L.F.; Kahn, M.L.; Jordan, M.S.; Koretzky, G.A. Requirements of SLP76 tyrosines in ITAM and integrin receptor signaling and in platelet function in vivo. J. Exp. Med. 2008, 205, 1775–1788. [Google Scholar] [CrossRef] [PubMed]
- Block, H.; Herter, J.M.; Rossaint, J.; Stadtmann, A.; Kliche, S.; Lowell, C.A.; Zarbock, A. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J. Exp. Med. 2012, 209, 407–421. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef]
- Nordenfelt, P.; Moore, T.I.; Mehta, S.B.; Kalappurakkal, J.M.; Swaminathan, V.; Koga, N.; Lambert, T.J.; Baker, D.; Waters, J.C.; Oldenbourg, R.; et al. Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat. Commun. 2017, 8, 2047. [Google Scholar] [CrossRef]
- Comrie, W.A.; Babich, A.; Burkhardt, J.K. F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J. Cell Biol. 2015, 208, 475–491. [Google Scholar] [CrossRef]
- Alon, R.; Dustin, M.L. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 2007, 26, 17–27. [Google Scholar] [CrossRef]
- Li, J.; Springer, T.A. Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl. Acad. Sci. USA 2017, 114, 4685–4690. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, B.H.; Xiao, T.; Zhang, C.; Nishida, N.; Springer, T.A. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 2008, 32, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Abram, C.L.; Lowell, C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009, 27, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.C.; Bracke, M.; Smith, A.; Davies, D.; Hogg, N. Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J. Immunol. 2002, 168, 6330–6335. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.H.; Kim, S.H.J.; Buffone, A., Jr.; Blumenthal, D.; Huang, B.; Agarwal, S.; Schwartzberg, P.L.; Hammer, D.A.; Burkhardt, J.K. LFA-1 signals to promote actin polymerization and upstream migration in T cells. J. Cell Sci. 2020, 133, jcs248328. [Google Scholar] [CrossRef]
- Shao, B.; Yago, T.; Coghill, P.A.; Klopocki, A.G.; Mehta-D’souza, P.; Schmidtke, D.W.; Rodgers, W.; McEver, R.P. Signal-dependent slow leukocyte rolling does not require cytoskeletal anchorage of P-selectin glycoprotein ligand-1 (PSGL-1) or integrin αLβ2. J. Biol. Chem. 2012, 287, 19585–19598. [Google Scholar] [CrossRef]
- Ye, F.; Petrich, B.G.; Anekal, P.; Lefort, C.T.; Kasirer-Friede, A.; Shattil, S.J.; Ruppert, R.; Moser, M.; Fässler, R.; Ginsberg, M.H. The mechanism of kindlin-mediated activation of integrin αIIbβ3. Curr. Biol. 2013, 23, 2288–2295. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Obermajer, N.; Pecar-Fonović, U.; Karaoglanovic-Carmona, A.; Kos, J. Cathepsin X cleaves the beta2 cytoplasmic tail of LFA-1 inducing the intermediate affinity form of LFA-1 and alpha-actinin-1 binding. Eur. J. Immunol. 2009, 39, 3217–3227. [Google Scholar] [CrossRef]
- Gahmberg, C.G.; Grönholm, M. How integrin phosphorylations regulate cell adhesion and signaling. Trends Biochem. Sci. 2022, 47, 265–278. [Google Scholar] [CrossRef]
- Gupta, S.; Chit, J.C.; Feng, C.; Bhunia, A.; Tan, S.M.; Bhattacharjya, S. An Alternative Phosphorylation Switch in Integrin β2 (CD18) Tail for Dok1 Binding. Sci. Rep. 2015, 5, 11630. [Google Scholar] [CrossRef]
- Anthis, N.J.; Haling, J.R.; Oxley, C.L.; Memo, M.; Wegener, K.L.; Lim, C.J.; Ginsberg, M.H.; Campbell, I.D. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J. Biol. Chem. 2009, 284, 36700–36710. [Google Scholar] [CrossRef]
- Nurmi, S.M.; Autero, M.; Raunio, A.K.; Gahmberg, C.G.; Fagerholm, S.C. Phosphorylation of the LFA-1 integrin beta2-chain on Thr-758 leads to adhesion, Rac-1/Cdc42 activation, and stimulation of CD69 expression in human T cells. J. Biol. Chem. 2007, 282, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Takala, H.; Nurminen, E.; Nurmi, S.M.; Aatonen, M.; Strandin, T.; Takatalo, M.; Kiema, T.; Gahmberg, C.G.; Ylänne, J.; Fagerholm, S.C. Beta2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood 2008, 112, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, S.; Morrice, N.; Gahmberg, C.G.; Cohen, P. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem. 2002, 277, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Madhavan, S.; Rolova, T.; Viazmina, L.; Grönholm, M.; Gahmberg, C.G. Phosphorylation of the α-chain in the integrin LFA-1 enables β2-chain phosphorylation and α-actinin binding required for cell adhesion. J. Biol. Chem. 2018, 293, 12318–12330. [Google Scholar] [CrossRef]
- Zarbock, A.; Abram, C.L.; Hundt, M.; Altman, A.; Lowell, C.A.; Ley, K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J. Exp. Med. 2008, 205, 2339–2347. [Google Scholar] [CrossRef]
- Humphrey, M.B.; Lanier, L.L.; Nakamura, M.C. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol. Rev. 2005, 208, 50–65. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Lanier, L.L. Inhibition of immune responses by ITAM-bearing receptors. Sci. STKE 2006, 2006, re1. [Google Scholar] [CrossRef]
- Hwang, J.R.; Byeon, Y.; Kim, D.; Park, S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef]
- Krummel, M.F.; Bartumeus, F.; Gerard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 2016, 16, 193–201. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Matsumoto, M.; Shigeta, A.; Furukawa, Y.; Tanaka, T.; Miyasaka, M.; Hirata, T. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J. Immunol. 2007, 178, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Luscinskas, F.W.; Ding, H.; Lichtman, A.H. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J. Exp. Med. 1995, 181, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Nolz, J.C.; Starbeck-Miller, G.R.; Harty, J.T. Naive, effector and memory CD8 T-cell trafficking: Parallels and distinctions. Immunotherapy 2011, 3, 1223–1233. [Google Scholar] [CrossRef]
- Walling, B.L.; Kim, M. LFA-1 in T Cell Migration and Differentiation. Front. Immunol. 2018, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. A molecular trio in relapse and remission in multiple sclerosis. Nat. Rev. Immunol. 2009, 9, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Hordijk, P.L.; Sixt, M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 2010, 11, 366–378. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Zheng, Y.; Pan, Y.; Zhang, K.; Chen, J. Distinct chemokine signaling regulates integrin ligand specificity to dictate tissue-specific lymphocyte homing. Dev. Cell 2014, 30, 61–70. [Google Scholar] [CrossRef]
- Porter, J.C.; Hall, A. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J. 2009, 23, 492–502. [Google Scholar] [CrossRef]
- Ohmatsu, H.; Kadono, T.; Sugaya, M.; Tomita, M.; Kai, H.; Miyagaki, T.; Saeki, H.; Tamaki, K.; Steeber, D.A.; Tedder, T.F.; et al. α4β7 Integrin is essential for contact hypersensitivity by regulating migration of T cells to skin. J. Allergy Clin. Immunol. 2010, 126, 1267–1276. [Google Scholar] [CrossRef]
- Hyun, Y.M.; Sumagin, R.; Sarangi, P.P.; Lomakina, E.; Overstreet, M.G.; Baker, C.M.; Fowell, D.J.; Waugh, R.E.; Sarelius, I.H.; Kim, M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J. Exp. Med. 2012, 209, 1349–1362. [Google Scholar] [CrossRef]
- Carman, C.V.; Sage, P.T.; Sciuto, T.E.; de la Fuente, M.A.; Geha, R.S.; Ochs, H.D.; Dvorak, H.F.; Dvorak, A.M.; Springer, T.A. Transcellular diapedesis is initiated by invasive podosomes. Immunity 2007, 26, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, G.; Weber, K.S.; Zernecke, A.; Schröder, A.; Weber, C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.; Cope, A.P.; Kemper, C.; Alon, R.; Köchl, R. LFA-1 in T cell priming, differentiation, and effector functions. Trends Immunol. 2021, 42, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Liu, Z.; Ahamed, J.; McEver, R.P. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood 2018, 132, 1426–1437. [Google Scholar] [CrossRef]
- Servant, G.; Weiner, O.D.; Herzmark, P.; Balla, T.; Sedat, J.W.; Bourne, H.R. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000, 287, 1037–1040. [Google Scholar] [CrossRef]
- Borisy, G.G.; Svitkina, T.M. Actin machinery: Pushing the envelope. Curr. Opin. Cell Biol. 2000, 12, 104–112. [Google Scholar] [CrossRef]
- Rossy, J.; Schlicht, D.; Engelhardt, B.; Niggli, V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS ONE 2009, 4, e5403. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef]
- Hornung, A.; Sbarrato, T.; Garcia-Seyda, N.; Aoun, L.; Luo, X.; Biarnes-Pelicot, M.; Theodoly, O.; Valignat, M.P. A Bistable Mechanism Mediated by Integrins Controls Mechanotaxis of Leukocytes. Biophys. J. 2020, 118, 565–577. [Google Scholar] [CrossRef]
- Grönholm, M.; Jahan, F.; Bryushkova, E.A.; Madhavan, S.; Aglialoro, F.; Soto Hinojosa, L.; Uotila, L.M.; Gahmberg, C.G. LFA-1 integrin antibodies inhibit leukocyte α4β1-mediated adhesion by intracellular signaling. Blood 2016, 128, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.M.; Liu, S.; Woodside, D.G.; Han, J.; Schlaepfer, D.D.; Ginsberg, M.H. Paxillin binding to the alpha 4 integrin subunit stimulates LFA-1 (integrin alpha L beta 2)-dependent T cell migration by augmenting the activation of focal adhesion kinase/proline-rich tyrosine kinase-2. J. Immunol. 2003, 170, 5912–5918. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 2008, 221, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.S.; Dustin, M.L. Making the T cell receptor go the distance: A topological view of T cell activation. Immunity 1997, 6, 361–369. [Google Scholar] [CrossRef]
- Shimaoka, M.; Kim, M.; Cohen, E.H.; Yang, W.; Astrof, N.; Peer, D.; Salas, A.; Ferrand, A.; Springer, T.A. AL-57, a ligand-mimetic antibody to integrin LFA-1, reveals chemokine-induced affinity up-regulation in lymphocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 13991–13996. [Google Scholar] [CrossRef]
- Shakhar, G.; Lindquist, R.L.; Skokos, D.; Dudziak, D.; Huang, J.H.; Nussenzweig, M.C.; Dustin, M.L. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat. Immunol. 2005, 6, 707–714. [Google Scholar] [CrossRef]
- Celli, S.; Garcia, Z.; Bousso, P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J. Exp. Med. 2005, 202, 1271–1278. [Google Scholar] [CrossRef]
- Bajénoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef]
- Wei, X.; Tromberg, B.J.; Cahalan, M.D. Mapping the sensitivity of T cells with an optical trap: Polarity and minimal number of receptors for Ca2+ signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 8471–8476. [Google Scholar] [CrossRef]
- Bachmann, M.F.; McKall-Faienza, K.; Schmits, R.; Bouchard, D.; Beach, J.; Speiser, D.E.; Mak, T.W.; Ohashi, P.S. Distinct roles for LFA-1 and CD28 during activation of naive T cells: Adhesion versus costimulation. Immunity 1997, 7, 549–557. [Google Scholar] [CrossRef]
- Scholer, A.; Hugues, S.; Boissonnas, A.; Fetler, L.; Amigorena, S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity 2008, 28, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.; Bushnell, T.; Miller, J. LFA-1-mediated T cell costimulation through increased localization of TCR/class II complexes to the central supramolecular activation cluster and exclusion of CD45 from the immunological synapse. J. Immunol. 2007, 179, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.K.; Dustin, M.L. Stimulation of naïve T-cell adhesion and immunological synapse formation by chemokine-dependent and -independent mechanisms. Immunology 2002, 106, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, T.; Kobayashi, W.; Sakata-Sogawa, K.; Takamatsu, M.; Hashimoto-Tane, A.; Dustin, M.L.; Tokunaga, M.; Saito, T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity 2008, 29, 589–601. [Google Scholar] [CrossRef]
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Monks, C.R.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef]
- Sims, T.N.; Soos, T.J.; Xenias, H.S.; Dubin-Thaler, B.; Hofman, J.M.; Waite, J.C.; Cameron, T.O.; Thomas, V.K.; Varma, R.; Wiggins, C.H.; et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell 2007, 129, 773–785. [Google Scholar] [CrossRef]
- Roy, N.H.; Burkhardt, J.K. The Actin Cytoskeleton: A Mechanical Intermediate for Signal Integration at the Immunological Synapse. Front. Cell Dev. Biol. 2018, 6, 116. [Google Scholar] [CrossRef]
- Li, D.; Molldrem, J.J.; Ma, Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J. Biol. Chem. 2009, 284, 21001–21010. [Google Scholar] [CrossRef]
- Ma, V.P.; Hu, Y.; Kellner, A.V.; Brockman, J.M.; Velusamy, A.; Blanchard, A.T.; Evavold, B.D.; Alon, R.; Salaita, K. The magnitude of LFA-1/ICAM-1 forces fine-tune TCR-triggered T cell activation. Sci. Adv. 2022, 8, eabg4485. [Google Scholar] [CrossRef]
- Sampath, R.; Gallagher, P.J.; Pavalko, F.M. Cytoskeletal interactions with the leukocyte integrin beta2 cytoplasmic tail. Activation-dependent regulation of associations with talin and alpha-actinin. J. Biol. Chem. 1998, 273, 33588–33594. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A.; Dustin, M.L. Integrin inside-out signaling and the immunological synapse. Curr. Opin. Cell Biol. 2012, 24, 107–115. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Le Floc’h, A.; Boutet, M.; Vergnon, I.; Schmitt, A.; Mami-Chouaib, F. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 2013, 73, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Anikeeva, N.; Somersalo, K.; Sims, T.N.; Thomas, V.K.; Dustin, M.L.; Sykulev, Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 6437–6442. [Google Scholar] [CrossRef]
- Davignon, D.; Martz, E.; Reynolds, T.; Kürzinger, K.; Springer, T.A. Lymphocyte function-associated antigen 1 (LFA-1): A surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc. Natl. Acad. Sci. USA 1981, 78, 4535–4539. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013, 5, 177ra138. [Google Scholar] [CrossRef]
- Li, R.; Ma, C.; Cai, H.; Chen, W. The CAR T-Cell Mechanoimmunology at a Glance. Adv. Sci. 2020, 7, 2002628. [Google Scholar] [CrossRef]
- Davenport, A.J.; Cross, R.S.; Watson, K.A.; Liao, Y.; Shi, W.; Prince, H.M.; Beavis, P.A.; Trapani, J.A.; Kershaw, M.H.; Ritchie, D.S.; et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2068–E2076. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, W.; Wang, B.; Yang, J.; Yang, J.; Yu, Z.; Qin, Z.; Shi, A.; Xu, W.; Zheng, C.; et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 2022, 57, 329–343.e327. [Google Scholar] [CrossRef]
- Ley, K.; Rivera-Nieves, J.; Sandborn, W.J.; Shattil, S. Integrin-based therapeutics: Biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016, 15, 173–183. [Google Scholar] [CrossRef]
- Verma, N.K.; Kelleher, D. Adaptor regulation of LFA-1 signaling in T lymphocyte migration: Potential druggable targets for immunotherapies? Eur. J. Immunol. 2014, 44, 3484–3499. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.H.; Golec, D.P.; Huang, B.; Park, C.; Thomsen, J.H.; Preite, S.; Cannons, J.L.; Garcon, F.; Schrom, E.C.; Courreges, C.J.F.; et al. A CRISPR screen targeting PI3K effectors identifies RASA3 as a negative regulator of LFA-1-mediated adhesion in T cells. Sci. Signal. 2022, 15, eabl9169. [Google Scholar] [CrossRef] [PubMed]
- Paloneva, J.; Kestila, M.; Wu, J.; Salminen, A.; Bohling, T.; Ruotsalainen, V.; Hakola, P.; Bakker, A.B.; Phillips, J.H.; Pekkarinen, P.; et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000, 25, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Hoek, R.M.; Cerwenka, A.; Blom, B.; Lucian, L.; McNeil, T.; Murray, R.; Phillips, L.H.; Sedgwick, J.D.; Lanier, L.L. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 2000, 13, 345–353. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Hickman, A.; Koetsier, J.; Kurtanich, T.; Nielsen, M.C.; Winn, G.; Wang, Y.; Bentebibel, S.E.; Shi, L.; Punt, S.; Williams, L.; et al. LFA-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with CTLA-4 blockade. J. Clin. Investig. 2022, 132, e154152. [Google Scholar] [CrossRef]
- Yanguas, A.; Garasa, S.; Teijeira, Á.; Aubá, C.; Melero, I.; Rouzaut, A. ICAM-1-LFA-1 Dependent CD8+ T-Lymphocyte Aggregation in Tumor Tissue Prevents Recirculation to Draining Lymph Nodes. Front. Immunol. 2018, 9, 2084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Shao, B. LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation. Cells 2023, 12, 1136. https://doi.org/10.3390/cells12081136
Shi H, Shao B. LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation. Cells. 2023; 12(8):1136. https://doi.org/10.3390/cells12081136
Chicago/Turabian StyleShi, Huiping, and Bojing Shao. 2023. "LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation" Cells 12, no. 8: 1136. https://doi.org/10.3390/cells12081136
APA StyleShi, H., & Shao, B. (2023). LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation. Cells, 12(8), 1136. https://doi.org/10.3390/cells12081136





