Change in Eosinophil Count in Patients with Heart Failure Treated with Anakinra
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Laboratory Data
2.3. Cardiorespiratory Fitness, Cardiac Function, and Quality of Life
2.4. Objective of the Analysis
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. 2020, 15, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Akuthota, P.; Wang, H.B.; Spencer, L.A.; Weller, P.F. Immunoregulatory roles of eosinophils: A new look at a familiar cell. Clin. Exp. Allergy 2008, 38, 1254–1263. [Google Scholar] [CrossRef]
- Alkhalil, M.; Kearney, A.; Hegarty, M.; Stewart, C.; Devlin, P.; Owens, C.G.; Spence, M.S. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting with Acute Myocardial Infarction. Am. J. Med. 2019, 132, e827–e834. [Google Scholar] [CrossRef]
- Shah, A.D.; Denaxas, S.; Nicholas, O.; Hingorani, A.D.; Hemingway, H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A CALIBER cohort study. Open Heart 2016, 3, e000477. [Google Scholar] [CrossRef]
- Swedish Orphan Biovitrum AB (publ). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf (accessed on 9 April 2023).
- Del Buono, M.G.; Damonte, J.I.; Trankle, C.R.; Kadariya, D.; Carbone, S.; Thomas, G.; Turlington, J.; Markley, R.; Canada, J.M.; Biondi-Zoccai, G.G.; et al. Effect of interleukin-1 blockade with anakinra on leukocyte count in patients with ST-segment elevation acute myocardial infarction. Sci. Rep. 2022, 12, 1254. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef]
- Abbate, A.; Wohlford, G.F.; Del Buono, M.G.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Kadariya, D.; Trankle, C.R.; Biondi-Zoccai, G.; Lipinski, M.J.; et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: Results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharm. 2022, 8, 503–510. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Damonte, J.I.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Trankle, C.R.; Kang, L.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Effect of IL-1 Blockade with Anakinra on Heart Failure Outcomes in Patients with Anterior Versus Nonanterior ST Elevation Myocardial Infarction. J. Cardiovasc. Pharm. 2022, 79, 774–780. [Google Scholar] [CrossRef]
- Kaiser, C.; Knight, A.; Nordstrom, D.; Pettersson, T.; Fransson, J.; Florin-Robertsson, E.; Pilstrom, B. Injection-site reactions upon Kineret (anakinra) administration: Experiences and explanations. Rheumatol. Int. 2012, 32, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Vila, A.T.; Puig, L.; Fernandez-Figueras, M.T.; Laiz, A.M.; Vidal, D.; Alomar, A. Adverse cutaneous reactions to anakinra in patients with rheumatoid arthritis: Clinicopathological study of five patients. Br. J. Dermatol. 2005, 153, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.A.; Toldo, S.; Mezzaroma, E.; Azam, T.; Seropian, I.M.; Shah, K.; Canada, J.; Voelkel, N.F.; Dinarello, C.A.; et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE 2012, 7, e33438. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Oddi Erdle, C.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Boineau, R.E.; Higginbotham, M.B.; Lee, K.L.; Mark, D.B.; Califf, R.M.; Cobb, F.R.; Pryor, D.B. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 1989, 64, 651–654. [Google Scholar] [CrossRef]
- Rector, T.S.; Kubo, S.H.; Cohn, J.N. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am. J. Cardiol. 1993, 71, 1106–1107. [Google Scholar] [CrossRef]
- Pongdee, T.; Manemann, S.M.; Decker, P.A.; Larson, N.B.; Moon, S.; Killian, J.M.; Liu, H.; Kita, H.; Bielinski, S.J. Rethinking blood eosinophil counts: Epidemiology, associated chronic diseases, and increased risks of cardiovascular disease. J. Allergy Clin. Immunol. Glob. 2022, 1, 233–240. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Liu, T.; Deng, Z.; Fang, W.; Zhang, X.; Li, J.; Huang, Q.; Liu, C.; Wang, Y.; et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 2020, 11, 6396. [Google Scholar] [CrossRef]
- Toor, I.S.; Rückerl, D.; Thomson, A.; Tang, K.; Newby, D.E.; Rossi, A.G.; Allen, J.E.; Grey, G.A. Eosinophils have an essential role in cardiac repair following myocardial infarction. Heart 2017, 103, A152. [Google Scholar] [CrossRef]
- Vural, A.; Aydin, E. The Predictive Value of Eosinophil Indices for Major Cardiovascular Events in Patients with Acute Decompensated HFrEF. Medicina 2022, 58, 1455. [Google Scholar] [CrossRef] [PubMed]
| On-Treatment Analysis (N = 64) | Off-Treatment Analysis (N = 41) | |||||
|---|---|---|---|---|---|---|
| Clinical variables | ||||||
| Age (years) | 55 [51–63] | 54 [51–59] | ||||
| Sex—F (%)/M (%) | 32 (50)/32 (50) | 20 (49)/21 (51) | ||||
| Race—Black (%)/White (%) | 47 (73)/17 (27) | 30 (73)/11 (27) | ||||
| BMI (kg/m2) | 38 [32–44] | 38 [34–44] | ||||
| NYHA class | ||||||
| NYHA class II | 24 (38) | 20 (49) | ||||
| NYHA class III | 40 (63) | 21 (51) | ||||
| CAD | 15 (23) | 9 (22) | ||||
| DM | 38 (59) | 22 (54) | ||||
| HTN | 58 (90) | 35 (85) | ||||
| HLP | 45 (66) | 28 (68) | ||||
| ACEi/ARB | 49 (77) | 32 (78) | ||||
| Beta-blocker | 57 (89) | 37 (90) | ||||
| MRA | 30 (47) | 22 (54) | ||||
| Biomarkers | Baseline | On-Treatment | p | On-treatment | Off-Treatment | p |
| WBC (×103 cells/µL) | 6.5 [5.6–8.2] | 5.8 [4.5–6.9] | <0.001 | 5.7 [4.7–7.0] | 6.6 [5.4–8.2] | <0.001 |
| Lymphocytes (×103 cells/µL) | 1.9 [1.6–2.3] | 1.9 [1.6–2.4] | 0.322 | 1.9 [1.6–2.4] | 1.9 [1.6–2.2] | 0.420 |
| Neutrophils (×103 cells/µL) | 4.1 [3.2–5.5] | 2.9 [2.0–4.0] | <0.001 | 2.9 [2.0–4.0] | 4.1 [2.9–5.4] | <0.001 |
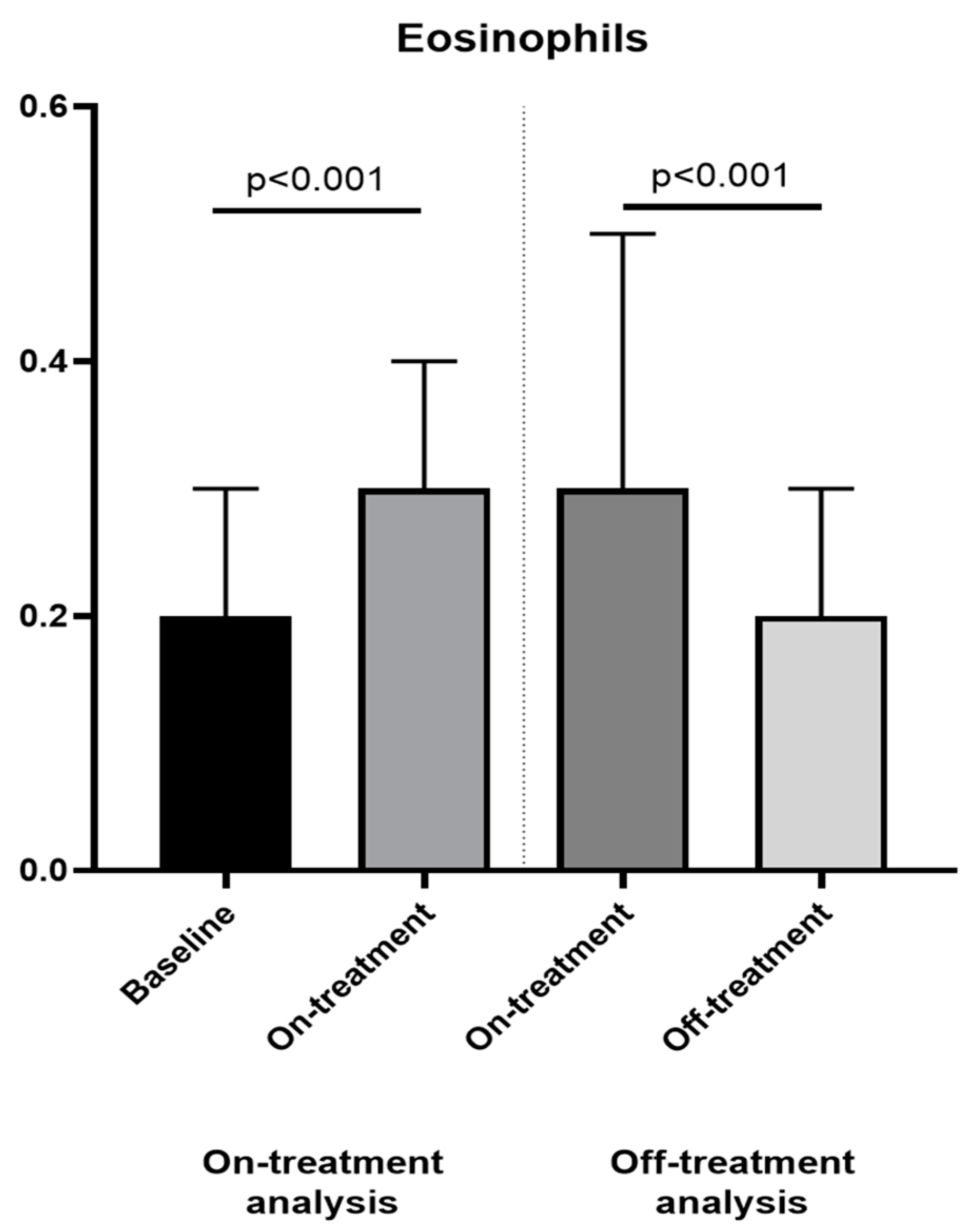
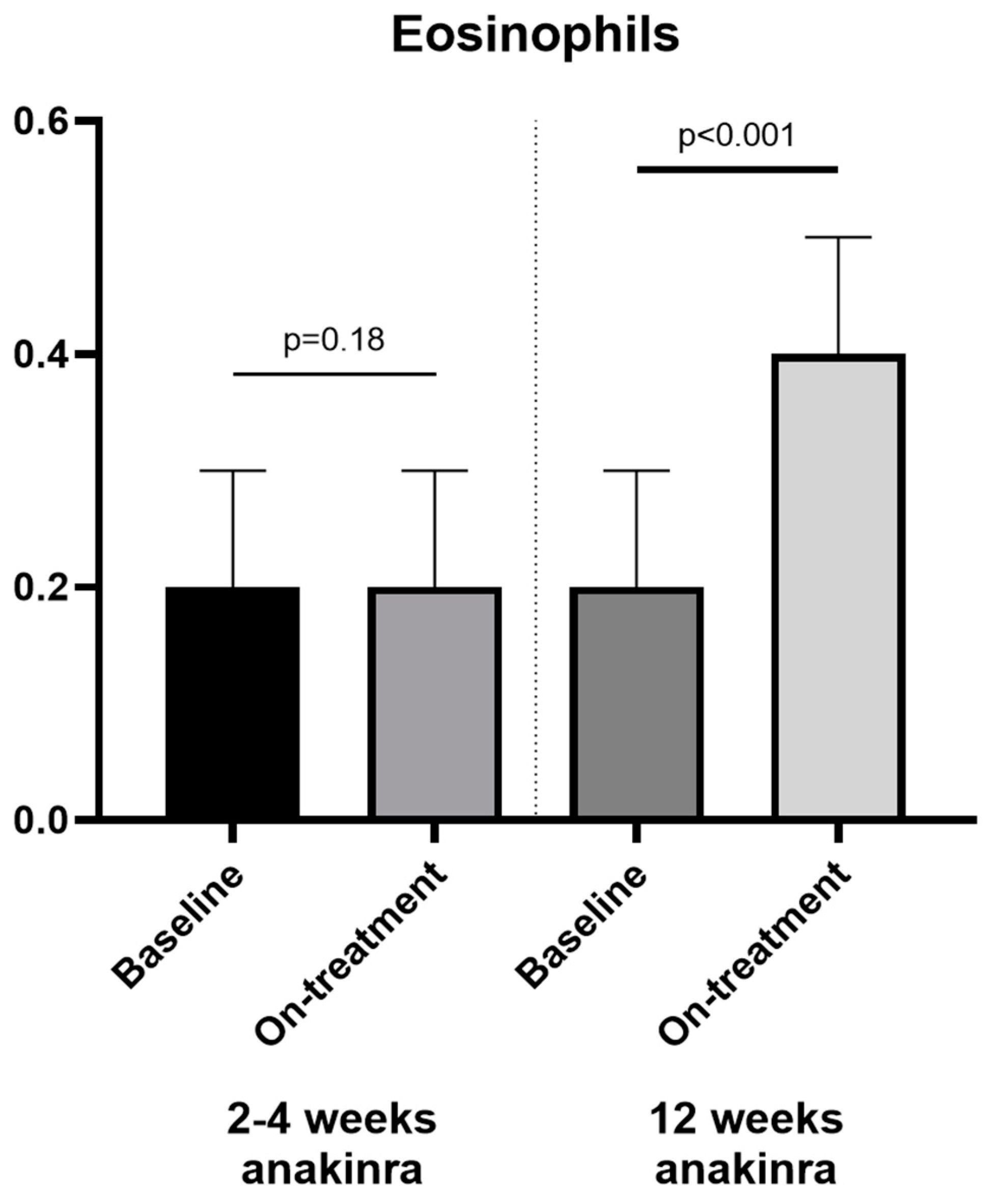
| Eosinophils (×103 cells/µL) | 0.2 [0.1–0.3] | 0.3 [0.1–0.4] | <0.001 | 0.3 [0.2–0.5] | 0.2 [0.1–0.3] | <0.001 |
| Basophils (×103 cells/µL) | 0.1 [0–0.1] | 0 [0–0.1] | <0.028 | 0 [0–0.1] | 0.1 [0–0.1] | 0.285 |
| Neutrophil-to-eosinophil ratio (NER) | 21.0 [14.3–36.8] | 9.7 [6.4–20.9] | <0.001 | 8.0 [5.3–13.0] | 18.5 [10.7–35.0] | <0.001 |
| Leukocyte-to-eosinophil ratio (LER) | 38.0 [21.7–56.0] | 20.5 [12.9–41.6] | <0.001 | 16.0 [11.5–27.7] | 31.0 [19.5–64.0] | <0.001 |
| Variables | C-Reactive Protein | Peak VO2 | VE/VCO2 Slope | Exercise Time | RER | OUES | NT-proBNP | LVEF | E/e′ | DASI Score | MLHFQ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changes in eosinophils | Spearman’s Rho | −0.297 | +0.228 | −0.040 | +0.185 | +0.160 | +0.069 | −0.222 | −0.029 | −0.288 | +0.261 | −0.132 |
| p value | 0.002 | 0.020 | 0.690 | 0.059 | 0.11 | 0.849 | 0.041 | 0.795 | 0.011 | 0.015 | 0.227 |
| Patients with Injection Site Reactions (N = 8) | Patients without Injection Site Reactions (N = 56) | p Value | |
|---|---|---|---|
| Variables | |||
| Age (years) | 51 [45–59] | 55 [52–64] | 0.10 |
| Sex—F (%)/M (%) | 4 (50)/4 (50) | 28 (50)/28 (50) | 1.00 |
| Race—Black (%)/White (%) | 4 (50)/4 (50) | 393 (70)/17 (30) | 0.11 |
| BMI (kg/m2) | 36 [35–41] | 38 [32–45] | 0.45 |
| NYHA class | |||
| NYHA class II | 4 (50) | 20 (36) | 0.43 |
| NYHA class III | 4 (50) | 36 (64) | 0.43 |
| CAD | 2 (25) | 13 (23) | 0.91 |
| DM | 6 (75) | 32 (57) | 0.34 |
| HTN | 6 (75) | 52 (93) | 0.11 |
| HLP | 4 (50) | 41 (73) | 0.18 |
| ACEi/ARB | 7 (88) | 42 (75) | 0.44 |
| Beta-blocker | 8 (100) | 49 (88) | 0.29 |
| MRA | 6 (75) | 24 (43) | 0.09 |
| CPX | |||
| Peak VO2 (mLO2·kg−1·min−1) | |||
| - Baseline | 16.8 [13.2–19.4] | 14.0 [11.6–16.5] | 0.16 |
| - On-treatment | 18.5 [14.5–25.2] | 14.9 [12.3–17.2] | 0.044 |
| - Off-treatment | 15.0 [13.7–25.5] | 14.8 [11.4–17.5] | 0.19 |
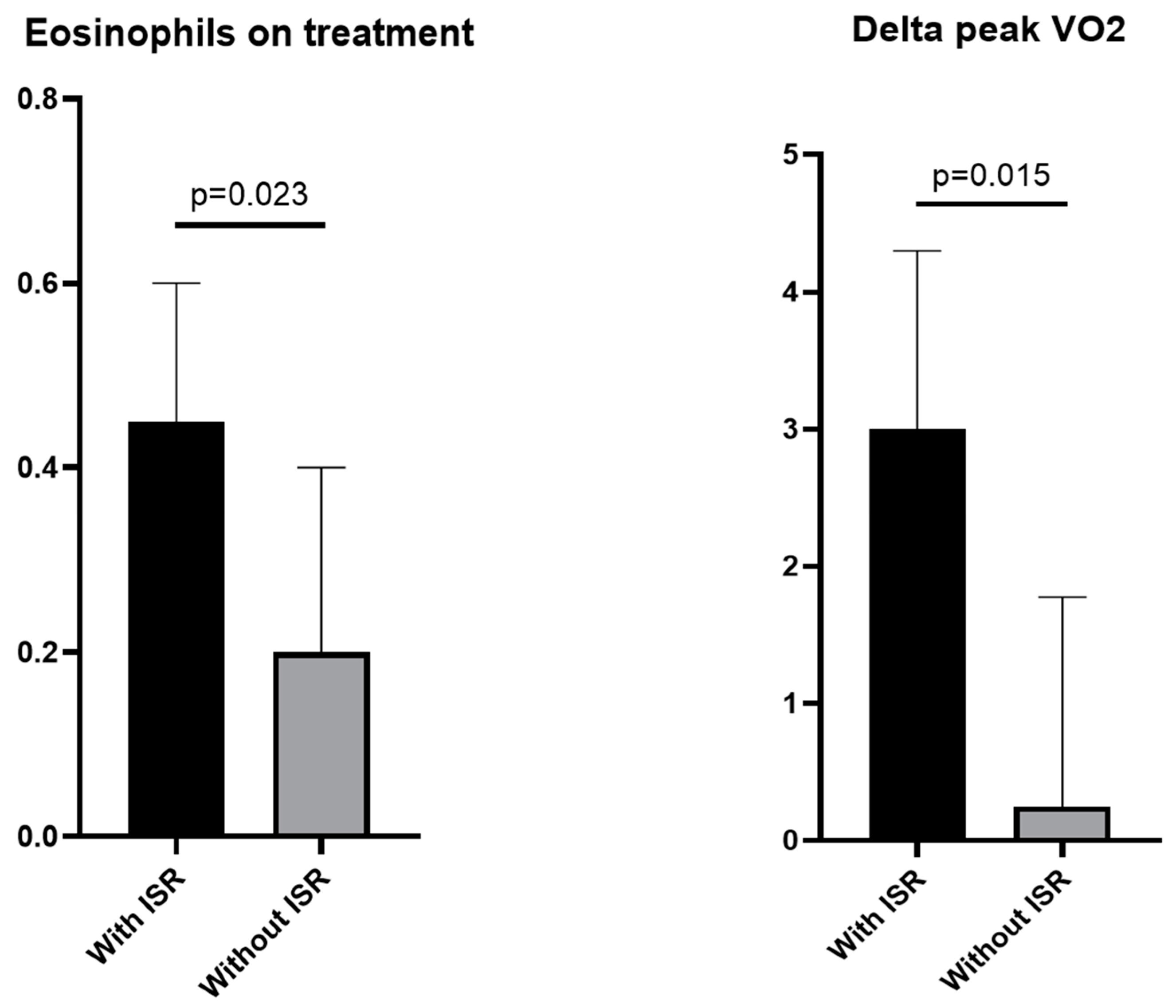
| - Delta baseline-on treatment | 3.0 [0.9–4.3] | 0.3 [−0.6–1.8] | 0.015 |
| - Delta on-off treatment | −1.5 [−2.1–0.7] | −0.3 [−2.1–0.6] | 0.62 |
| Exercise time (seconds) | |||
| - Baseline | 595 [505–725] | 455 [290–548] | 0.007 |
| - On-treatment | 650 [490–830] | 500 [370–583] | 0.016 |
| - Off-treatment | 730 [580–870] | 510 [375–630] | 0.026 |
| Biomarkers | |||
| CRP (mg/L) | |||
| - Baseline | 2.8 [2.3–13.3] | 6.5 [3.6–15.5] | 0.12 |
| - On-treatment | 1.3 [0.5–7.2] | 1.4 [0.9–3.7] | 0.81 |
| - Off-treatment | 2.1 [0.6–9.7] | 6.8 [2.9–13.6] | 0.11 |
| NT-proBNP (pg/mL) | |||
| - Baseline | 74 [13–219] | 634 [200–1774] | 0.002 |
| - On-treatment | 81 [13–120] | 445 [95–1187] | 0.003 |
| - Off-treatment | 57 [20–120] | 383 [121–1094] | 0.001 |
| WBC (×103 cells/µL) | |||
| - Baseline | 6.1 [5.2–7.8] | 6.6 [5.6–8.3] | 0.50 |
| - On-treatment | 6.1 [4.4–7.4] | 5.6 [4.5–6.7] | 0.44 |
| - Off-treatment | 6.2 [5.4–7.3] | 6.9 [5.4–8.3] | 0.48 |
| Neutrophils (×103 cells/µL) | |||
| - Baseline | 3.5 [2.5–5.1] | 4.4 [3.2–5.7] | 0.27 |
| - On-treatment | 2.9 [1.9–5.1] | 2.9 [2.0–4.0] | 0.75 |
| - Off-treatment | 3.7 [2.7–4.3] | 4.4 [3.1–5.7] | 0.34 |
| Eosinophils (×103 cells/µL) | |||
| - Baseline | 0.3 [0.1–0.4] | 0.2 [0.1–0.3] | 0.21 |
| - On-treatment | 0.5 [0.3–0.6] | 0.2 [0.1–0.4] | 0.023 |
| - Off-treatment | 0.2 [0.2–0.4] | 0.2 [0.1–0.3] | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golino, M.; Moroni, F.; Del Buono, M.G.; Canada, J.M.; Talasaz, A.H.; Piñel, S.; Mbualungu, J.; Vecchiè, A.; Ho, A.-C.; Thomas, G.K.; et al. Change in Eosinophil Count in Patients with Heart Failure Treated with Anakinra. Cells 2023, 12, 1129. https://doi.org/10.3390/cells12081129
Golino M, Moroni F, Del Buono MG, Canada JM, Talasaz AH, Piñel S, Mbualungu J, Vecchiè A, Ho A-C, Thomas GK, et al. Change in Eosinophil Count in Patients with Heart Failure Treated with Anakinra. Cells. 2023; 12(8):1129. https://doi.org/10.3390/cells12081129
Chicago/Turabian StyleGolino, Michele, Francesco Moroni, Marco Giuseppe Del Buono, Justin M. Canada, Azita H. Talasaz, Sebastian Piñel, James Mbualungu, Alessandra Vecchiè, Ai-Chen (Jane) Ho, Georgia K. Thomas, and et al. 2023. "Change in Eosinophil Count in Patients with Heart Failure Treated with Anakinra" Cells 12, no. 8: 1129. https://doi.org/10.3390/cells12081129
APA StyleGolino, M., Moroni, F., Del Buono, M. G., Canada, J. M., Talasaz, A. H., Piñel, S., Mbualungu, J., Vecchiè, A., Ho, A.-C., Thomas, G. K., Carbone, S., Billingsley, H. E., Turlington, J., Markley, R., Trankle, C., De Ponti, R., Van Tassell, B., & Abbate, A. (2023). Change in Eosinophil Count in Patients with Heart Failure Treated with Anakinra. Cells, 12(8), 1129. https://doi.org/10.3390/cells12081129







