Extracellular Release of Citrullinated Vimentin Directly Acts on Osteoclasts to Promote Bone Resorption in a Mouse Model of Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Citrullination of Vimentin
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of CV
2.5. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.6. Pit Formation Assay
2.7. Measurement of C-Terminal Telopeptide of Type I Collagen (CTX-1)
2.8. Quantitative Polymerase Chain Reaction (qPCR)
2.9. PCR Array
2.10. Western Blot
2.11. cAMP Assay
2.12. Periodontal Disease Model
2.13. Measurement of Alveolar Bone Resorption
2.14. Measurement of Vimentin and CV in Mouse GCF
2.15. Histological Analysis
2.16. Statistical Analysis
3. Results
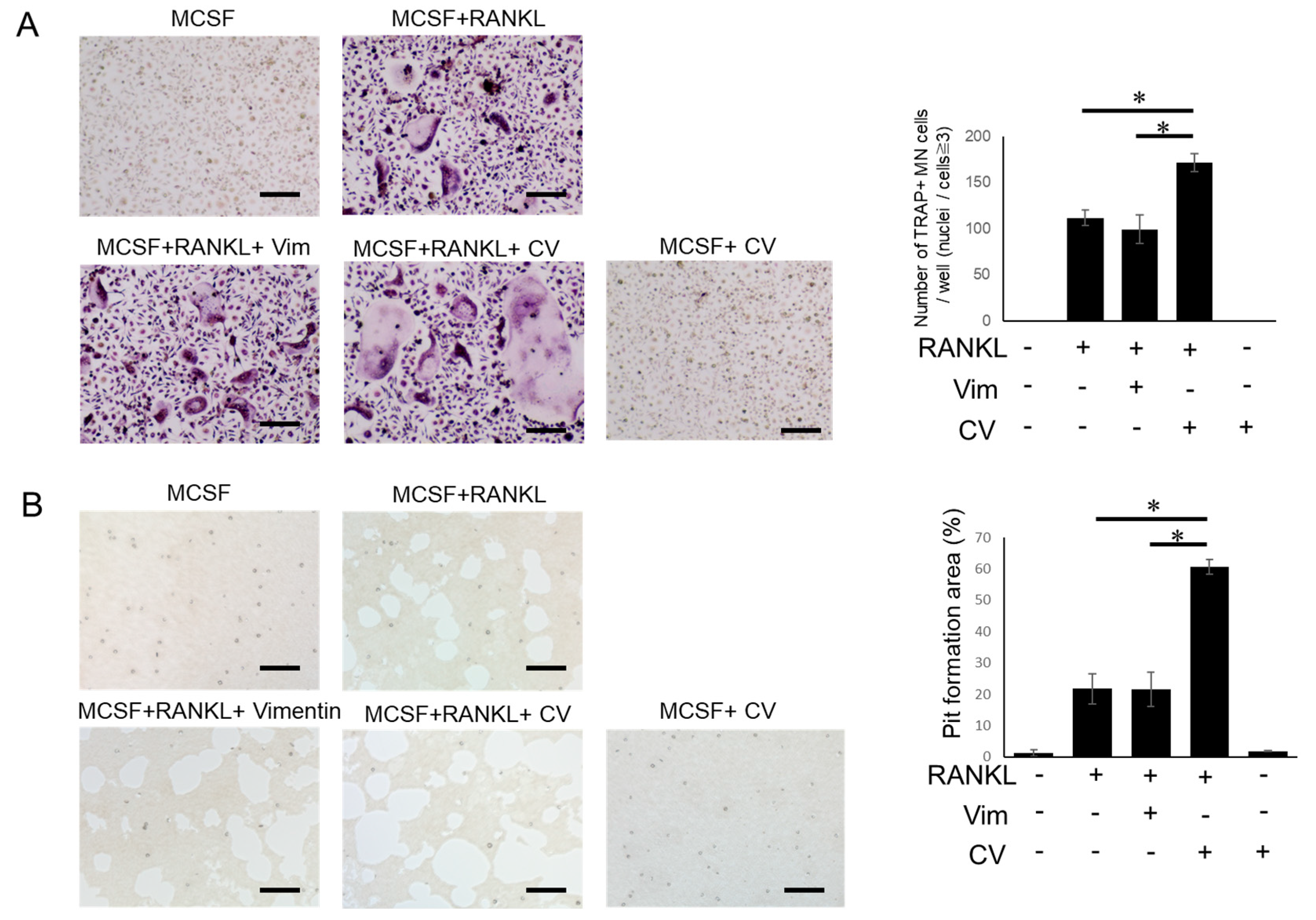
3.1. CV Promotes Osteoclast Differentiation
3.2. PAD-Dependent Citrullinated Vimentin Produced by OC Precursors Is Engaged in the Promotion of RANKL-Induced Osteoclastogenesis
3.3. Anti-Vimentin mAb-Mediated Blocking Can Inhibit the Pro-OC-Genesis Effect by CV
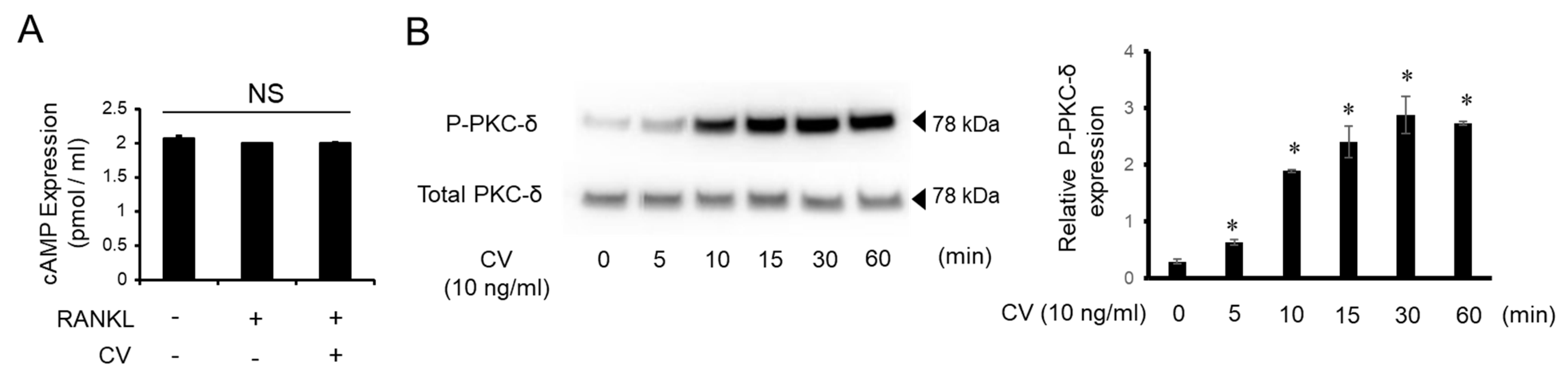
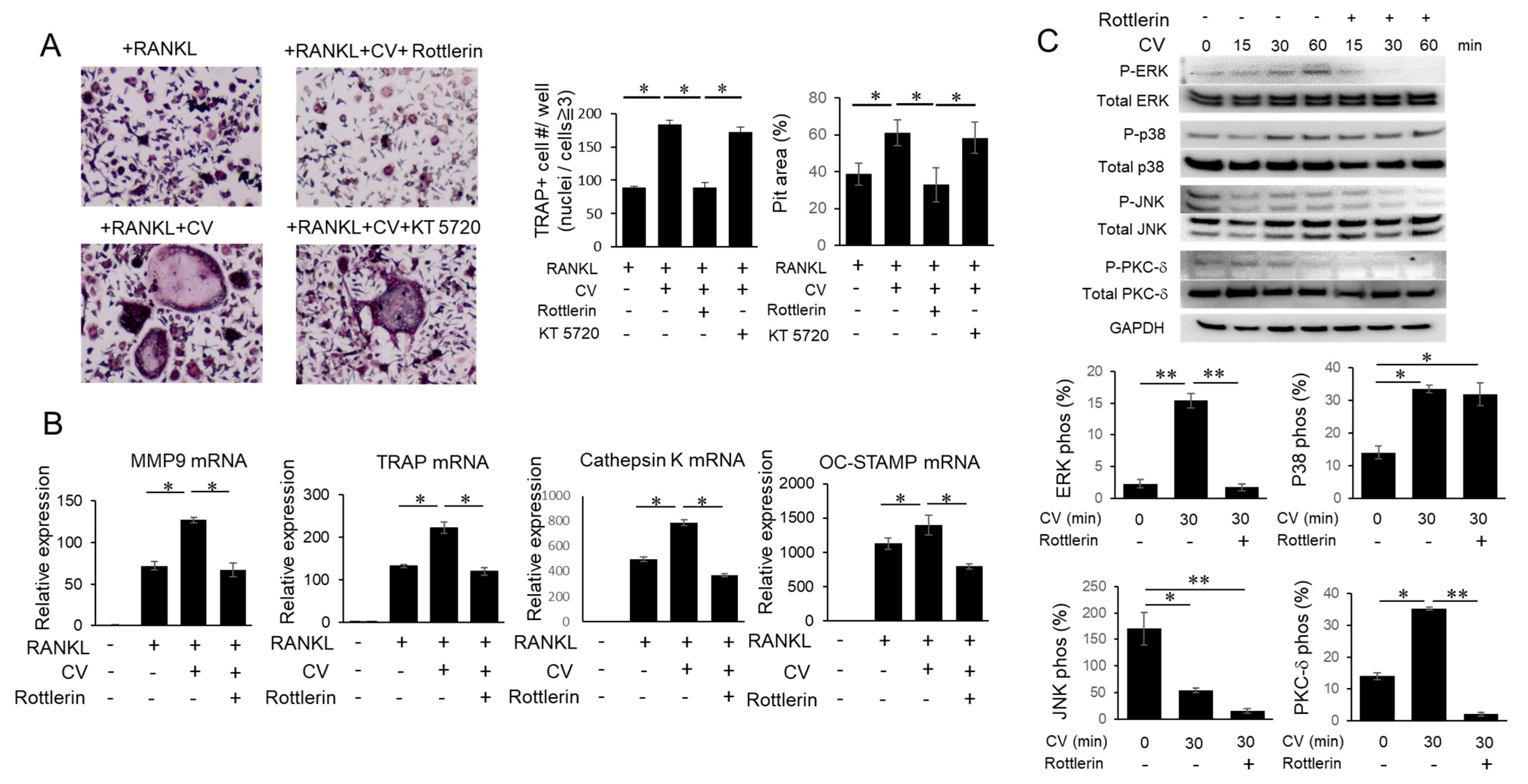
3.4. CV Stimulates OC Precursors via the PKC-δ Pathway
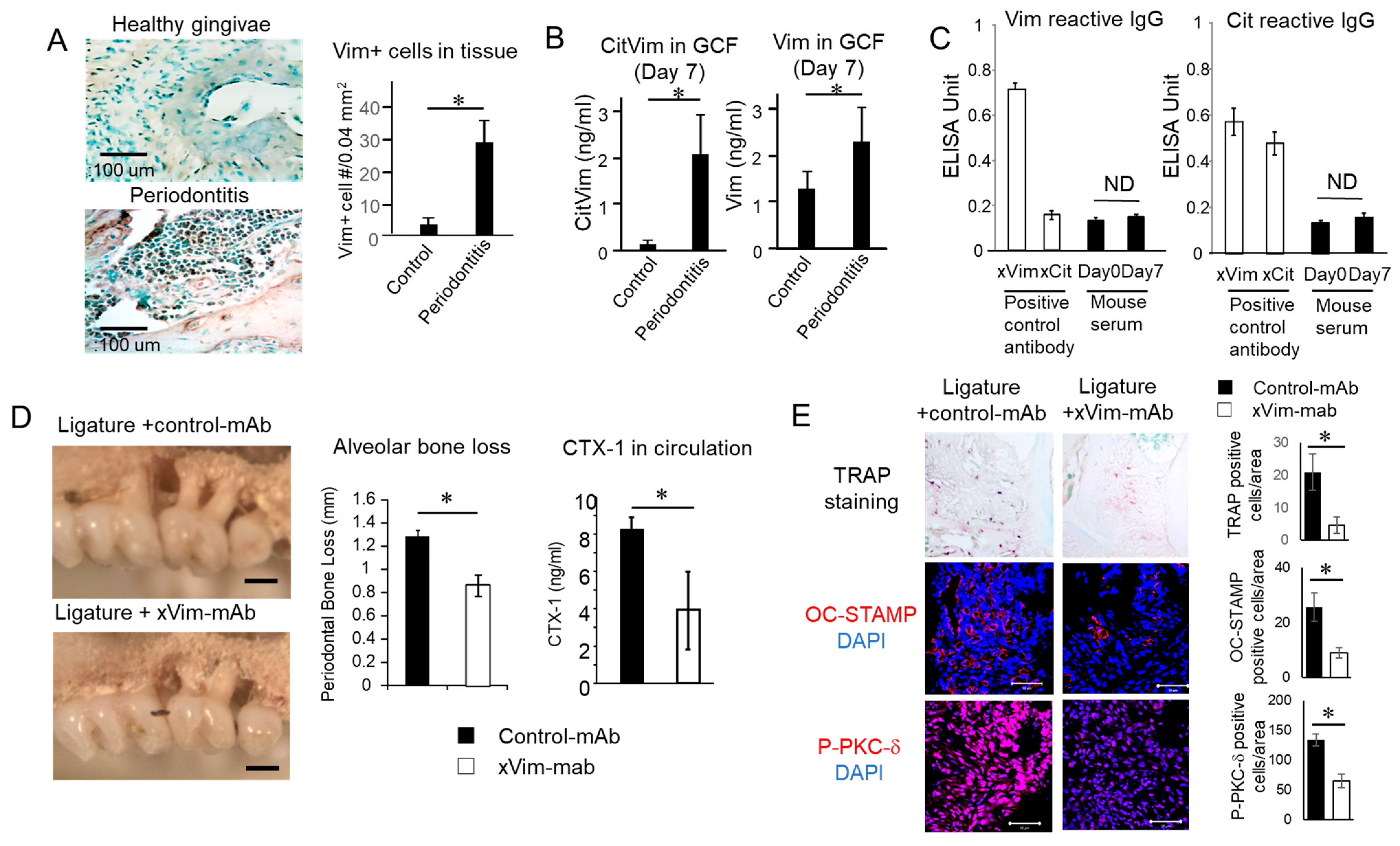
3.5. Vimentin and CV Detected in Periodontitis Is Induced in Mice
3.6. mAb-Based Local Neutralization of Vimentin Suppressed Bone Resorption Is Induced in a Mouse Model of Periodontitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Page, R.C.; Offenbacher, S.; Schroeder, H.E.; Seymour, G.J.; Kornman, K.S. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontology 2000 1997, 14, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J. Importance of the host response in the periodontium. J. Clin. Periodontol. 1991, 18, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Movila, A.; Suzuki, M.; Kajiya, M.; Wisitrasameewong, W.; Kayal, R.; Hirshfeld, J.; Al-Dharrab, A.; Savitri, I.J.; Mira, A.; et al. A novel method of sampling gingival crevicular fluid from a mouse model of periodontitis. J. Immunol. Methods 2016, 438, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Gravallese, E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef]
- Söderström, K.; Stein, E.; Colmenero, P.; Purath, U.; Müller-Ladner, U.; de Matos, C.T.; Tarner, I.H.; Robinson, W.H.; Engleman, E.G. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc. Natl. Acad. Sci. USA 2010, 107, 13028–13033. [Google Scholar] [CrossRef]
- Carson, R.E.; Sayegh, F.S.; Fedi, P.F., Jr. Osteoclastic resorption of alveolar bone affected by periodontitis--correlation of light microscopic and scanning electron microscopic observations. J. Periodontol. 1978, 49, 406–414. [Google Scholar] [CrossRef]
- Schwartz, Z.; Goultschin, J.; Dean, D.D.; Boyan, B.D. Mechanisms of alveolar bone destruction in periodontitis. Periodontology 2000 1997, 14, 158–172. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Taubman, M.A. The protective nature of host responses in periodontal diseases. Periodontology 2000 1994, 5, 112–141. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Lin, D.; Li, L.; Sun, Y.; Wang, W.; Wang, X.; Ye, Y.; Chen, X.; Xu, Y. IL-17 regulates the expressions of RANKL and OPG in human periodontal ligament cells via TRAF6/TBK1-JNK/NF-κB pathways. Immunology 2014, 144, 472–485. [Google Scholar] [CrossRef]
- Fujihara, R.; Usui, M.; Yamamoto, G.; Nishii, K.; Tsukamoto, Y.; Okamatsu, Y.; Sato, T.; Asou, Y.; Nakashima, K.; Yamamoto, M. Tumor necrosis factor-α enhances RANKL expression in gingival epithelial cells via protein kinase A signaling. J. Periodontal Res. 2014, 49, 508–517. [Google Scholar] [CrossRef]
- Taubman, M.A.; Kawai, T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2001, 12, 125–135. [Google Scholar] [CrossRef]
- Han, X.; Kawai, T.; Eastcott, J.W.; Taubman, M.A. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J. Immunol. (Baltim. Md. 1950) 2006, 176, 625–631. [Google Scholar] [CrossRef]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Mizutani, H.; Ishihara, Y.; Izawa, A.; Fujihara, Y.; Kobayashi, S.; Gotou, H.; Okabe, E.; Takeda, H.; Ozawa, Y.; Kamiya, Y.; et al. Lipopolysaccharide of Aggregatibacter actinomycetemcomitans up-regulates inflammatory cytokines, prostaglandin E2 synthesis and osteoclast formation in interleukin-1 receptor antagonist-deficient mice. J. Periodontal Res. 2013, 48, 748–756. [Google Scholar] [CrossRef]
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304. [Google Scholar] [CrossRef]
- Repeke, C.E.; Cardoso, C.R.; Claudino, M.; Silveira, E.M.; Trombone, A.P.; Campanelli, A.P.; Silva, J.S.; Martins, W., Jr.; Garlet, G.P. Non-inflammatory destructive periodontal disease: A clinical, microbiological, immunological and genetic investigation. J. Appl. Oral Sci. Rev. FOB 2012, 20, 113–121. [Google Scholar] [CrossRef]
- Mewar, D.; Wilson, A.G. Autoantibodies in rheumatoid arthritis: A review. Biomed. Pharmacother. 2006, 60, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Vitkov, L.; Hannig, M.; Minnich, B.; Herrmann, M. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity 2018, 51, 304–309. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, G.A.; Visser, H.; de Jong, B.A.; van den Hoogen, F.H.; Hazes, J.M.; Breedveld, F.C.; van Venrooij, W.J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000, 43, 155–163. [Google Scholar] [CrossRef]
- Mathsson, L.; Mullazehi, M.; Wick, M.C.; Sjöberg, O.; van Vollenhoven, R.; Klareskog, L.; Rönnelid, J. Antibodies against citrullinated vimentin in rheumatoid arthritis: Higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008, 58, 36–45. [Google Scholar] [CrossRef]
- Ebrahimi-Rad, M.; Khatami, S.; Akhbari, H.; Mahmoudzadeh-Niknam, H.; Valadbeigi, S.; Mahmoudi, M.; Jamshidi, A.; Riazi-Rad, F.; Saghiri, R. Evaluation of autoantibodies against vimentin and α-enolase in rheumatoid arthritis patients. Reumatologia 2020, 58, 350–356. [Google Scholar] [CrossRef]
- Barra, L.; Scinocca, M.; Saunders, S.; Bhayana, R.; Rohekar, S.; Racapé, M.; Coles, R.; Cairns, E.; Bell, D.A. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013, 65, 1439–1447. [Google Scholar] [CrossRef]
- Bang, H.; Egerer, K.; Gauliard, A.; Lüthke, K.; Rudolph, P.E.; Fredenhagen, G.; Berg, W.; Feist, E.; Burmester, G.R. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007, 56, 2503–2511. [Google Scholar] [CrossRef]
- Soós, L.; Szekanecz, Z.; Szabó, Z.; Fekete, A.; Zeher, M.; Horváth, I.F.; Dankó, K.; Kapitány, A.; Végvári, A.; Sipka, S.; et al. Clinical evaluation of anti-mutated citrullinated vimentin by ELISA in rheumatoid arthritis. J. Rheumatol. 2007, 34, 1658–1663. [Google Scholar]
- Huang, J.; Zeng, T.; Zhang, X.; Tian, Y.; Wu, Y.; Yu, J.; Pei, Z.; Liu, Y.; Hu, T.; Tan, L. Clinical diagnostic significance of 14-3-3η protein, high-mobility group box-1, anti-cyclic citrullinated peptide antibodies, anti-mutated citrullinated vimentin antibodies and rheumatoid factor in rheumatoid arthritis. Br. J. Biomed. Sci. 2020, 77, 19–23. [Google Scholar] [CrossRef]
- Zhu, T.; Feng, L. Comparison of anti-mutated citrullinated vimentin, anti-cyclic citrullinated peptides, anti-glucose-6-phosphate isomerase and anti-keratin antibodies and rheumatoid factor in the diagnosis of rheumatoid arthritis in Chinese patients. Int. J. Rheum. Dis. 2013, 16, 157–161. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. BioEssays News Rev. Mol. Cell. Dev. Biol. 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Makrygiannakis, D.; af Klint, E.; Lundberg, I.E.; Löfberg, R.; Ulfgren, A.K.; Klareskog, L.; Catrina, A.I. Citrullination is an inflammation-dependent process. Ann. Rheum. Dis. 2006, 65, 1219–1222. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Nakao, T.; Tsuritani, K.; Yamada, T.; Watanabe, N.; Chiba, A.; Robinson, W.H.; Miyake, S. The importance of specific citrullinated clusterin and vimentin found in a multi-coloured bead-based citrulline-peptide array system in rheumatoid arthritis. Clin. Exp. Rheumatol. 2022, 40, 936–944. [Google Scholar] [CrossRef]
- Tabushi, Y.; Nakanishi, T.; Takeuchi, T.; Nakajima, M.; Ueda, K.; Kotani, T.; Makino, S.; Shimizu, A.; Hanafusa, T.; Takubo, T. Detection of citrullinated proteins in synovial fluids derived from patients with rheumatoid arthritis by proteomics-based analysis. Ann. Clin. Biochem. 2008, 45, 413–417. [Google Scholar] [CrossRef]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef]
- Engdahl, C.; Bang, H.; Dietel, K.; Lang, S.C.; Harre, U.; Schett, G. Periarticular Bone Loss in Arthritis Is Induced by Autoantibodies Against Citrullinated Vimentin. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 1681–1691. [Google Scholar] [CrossRef]
- Silva-Boghossian, C.M.; Colombo, A.P.; Tanaka, M.; Rayo, C.; Xiao, Y.; Siqueira, W.L. Quantitative proteomic analysis of gingival crevicular fluid in different periodontal conditions. PLoS ONE 2013, 8, e75898. [Google Scholar] [CrossRef]
- de Pablo, P.; Dietrich, T.; Chapple, I.L.; Milward, M.; Chowdhury, M.; Charles, P.J.; Buckley, C.D.; Venables, P.J. The autoantibody repertoire in periodontitis: A role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Ann. Rheum. Dis. 2014, 73, 580–586. [Google Scholar] [CrossRef]
- Ishigami, A.; Masutomi, H.; Handa, S.; Nakamura, M.; Nakaya, S.; Uchida, Y.; Saito, Y.; Murayama, S.; Jang, B.; Jeon, Y.C.; et al. Mass spectrometric identification of citrullination sites and immunohistochemical detection of citrullinated glial fibrillary acidic protein in Alzheimer’s disease brains. J. Neurosci. Res. 2015, 93, 1664–1674. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyauchi, S.; Tawada, A.; Anada, T.; Suzuki, O. Effect of chondroitin sulfate-E on the osteoclastic differentiation of RAW264 cells. Dent. Mater. J. 2010, 29, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyauchi, S.; Anada, T.; Imaizumi, H.; Suzuki, O. Evaluation of osteoclastic resorption activity using calcium phosphate coating combined with labeled polyanion. Anal. Biochem. 2011, 410, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Ruiz-Torruella, M.; Yamamoto, K.; Yamaguchi, T.; Heidari, A.; Pierrelus, R.; Leon, E.; Shindo, S.; Rawas-Qalaji, M.; Pastore, M.R.; et al. Locally Secreted Semaphorin 4D Is Engaged in Both Pathogenic Bone Resorption and Retarded Bone Regeneration in a Ligature-Induced Mouse Model of Periodontitis. Int. J. Mol. Sci. 2022, 23, 5630. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Ruiz-Torruella, M.; Ikeda, A.; Shindo, S.; Movila, A.; Mawardi, H.; Albassam, A.; Kayal, R.A.; Al-Dharrab, A.A.; Egashira, K.; et al. OC-STAMP promotes osteoclast fusion for pathogenic bone resorption in periodontitis via up-regulation of permissive fusogen CD9. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4016–4030. [Google Scholar] [CrossRef]
- Zhao, C.; Irie, N.; Takada, Y.; Shimoda, K.; Miyamoto, T.; Nishiwaki, T.; Suda, T.; Matsuo, K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006, 4, 111–121. [Google Scholar] [CrossRef]
- Blair, H.C. How the osteoclast degrades bone. BioEssays News Rev. Mol. Cell. Dev. Biol. 1998, 20, 837–846. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113 Pt 3, 377–381. [Google Scholar] [CrossRef]
- Fardellone, P.; Séjourné, A.; Paccou, J.; Goëb, V. Bone remodelling markers in rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 484280. [Google Scholar] [CrossRef]
- Vasikaran, S.D.; Miura, M.; Pikner, R.; Bhattoa, H.P.; Cavalier, E. Practical Considerations for the Clinical Application of Bone Turnover Markers in Osteoporosis. Calcif. Tissue Int. 2023, 112, 148–157. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Könönen, E.; Huumonen, S.; Tervahartiala, T.; Pussinen, P.J.; Suominen, A.L.; Sorsa, T. Salivary type I collagen degradation end-products and related matrix metalloproteinases in periodontitis. J. Clin. Periodontol. 2013, 40, 18–25. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Calenic, B.; Mocanu, B.; Didilescu, A.; Mohora, M.; Spinu, T.; Greabu, M. Salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol. Scand. 2014, 72, 42–47. [Google Scholar] [CrossRef]
- Khor, E.C.; Abel, T.; Tickner, J.; Chim, S.M.; Wang, C.; Cheng, T.; Ng, B.; Ng, P.Y.; Teguh, D.A.; Kenny, J.; et al. Loss of protein kinase C-δ protects against LPS-induced osteolysis owing to an intrinsic defect in osteoclastic bone resorption. PLoS ONE 2013, 8, e70815. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Liu, C.; Grizzle, W.E.; Yu, S.; Zhang, S.; Barnes, S.; Koopman, W.J.; Mountz, J.D.; Kimberly, R.P.; et al. Cleavage of p53-vimentin complex enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of rheumatoid arthritis synovial fibroblasts. Am. J. Pathol. 2005, 167, 705–719. [Google Scholar] [CrossRef]
- Eckes, B.; Dogic, D.; Colucci-Guyon, E.; Wang, N.; Maniotis, A.; Ingber, D.; Merckling, A.; Langa, F.; Aumailley, M.; Delouvée, A.; et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 1998, 111 Pt 13, 1897–1907. [Google Scholar] [CrossRef]
- Nieminen, M.; Henttinen, T.; Merinen, M.; Marttila-Ichihara, F.; Eriksson, J.E.; Jalkanen, S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006, 8, 156–162. [Google Scholar] [CrossRef]
- Cheng, F.; Eriksson, J.E. Intermediate Filaments and the Regulation of Cell Motility during Regeneration and Wound Healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef]
- Fan, L.Y.; He, D.Y.; Wang, Q.; Zong, M.; Zhang, H.; Yang, L.; Sun, L.S. Citrullinated vimentin stimulates proliferation, pro-inflammatory cytokine secretion, and PADI4 and RANKL expression of fibroblast-like synoviocytes in rheumatoid arthritis. Scand. J. Rheumatol. 2012, 41, 354–358. [Google Scholar] [CrossRef]
- Zhu, J.N.; Nie, L.Y.; Lu, X.Y.; Wu, H.X. Meta-analysis: Compared with anti-CCP and rheumatoid factor, could anti-MCV be the next biomarker in the rheumatoid arthritis classification criteria? Clin. Chem. Lab. Med. 2019, 57, 1668–1679. [Google Scholar] [CrossRef]
- Puszczewicz, M.; Iwaszkiewicz, C. Role of anti-citrullinated protein antibodies in diagnosis and prognosis of rheumatoid arthritis. Arch. Med. Sci. AMS 2011, 7, 189–194. [Google Scholar] [CrossRef]
- de Oliveira Ferreira, R.; de Brito Silva, R.; Magno, M.B.; Carvalho Almeida, A.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. Does periodontitis represent a risk factor for rheumatoid arthritis? A systematic review and meta-analysis. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19858514. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Z.; Li, Y.; Han, Y.; Zhou, Y.; Cao, X. Rheumatoid arthritis risk in periodontitis patients: A systematic review and meta-analysis. Jt. Bone Spine 2020, 87, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Rönnelid, J.; Lundberg, K.; Padyukov, L.; Alfredsson, L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 2008, 26, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Balsa, A.; Cabezón, A.; Orozco, G.; Cobo, T.; Miranda-Carus, E.; López-Nevot, M.A.; Vicario, J.L.; Martín-Mola, E.; Martín, J.; Pascual-Salcedo, D. Influence of HLA DRB1 alleles in the susceptibility of rheumatoid arthritis and the regulation of antibodies against citrullinated proteins and rheumatoid factor. Arthritis Res. Ther. 2010, 12, R62. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G. Auto-antibodies and autoreactive T-cells in rheumatoid arthritis: Pathogenetic players and diagnostic tools. Clin. Rev. Allergy Immunol. 2007, 32, 23–36. [Google Scholar] [CrossRef]
- Scally, S.W.; Law, S.C.; Ting, Y.T.; Heemst, J.V.; Sokolove, J.; Deutsch, A.J.; Bridie Clemens, E.; Moustakas, A.K.; Papadopoulos, G.K.; van der Woude, D.; et al. Molecular basis for increased susceptibility of Indigenous North Americans to seropositive rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1915–1923. [Google Scholar] [CrossRef]
- Reichert, S.; Jurianz, E.; Natalie, P.; Schlumberger, W.; Dähnrich, C.; Johannsen, N.; Altermann, W.; Schlaf, G.; Keyßer, G.; Schaefer, C.; et al. Is periodontitis a prognostic factor in order to indicate antibodies against citrullinated peptides in patients with rheumatoid arthritis? Clin. Exp. Rheumatol. 2020, 38, 227–238. [Google Scholar] [CrossRef]
- Le Goff, B.; Berthelot, J.M.; Maugars, Y.; Heymann, D. Osteoclasts in RA: Diverse origins and functions. Jt. Bone Spine 2013, 80, 586–591. [Google Scholar] [CrossRef]
- Sørensen, M.G.; Karsdal, M.A.; Dziegiel, M.H.; Boutin, J.A.; Nosjean, O.; Henriksen, K. Screening of protein kinase inhibitors identifies PKC inhibitors as inhibitors of osteoclastic acid secretion and bone resorption. BMC Musculoskelet. Disord. 2010, 11, 250. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.; Shin, H.I.; Jeong, D. Specific targeting of PKCδ suppresses osteoclast differentiation by accelerating proteolysis of membrane-bound macrophage colony-stimulating factor receptor. Sci. Rep. 2019, 9, 7044. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, W.; Jiang, J.; Wang, Y.; Guo, Y.; Yang, R.; Chang, Y.; Zhao, B.; Wang, Z.; Zhang, J.; et al. Peiminine Suppresses RANKL-Induced Osteoclastogenesis by Inhibiting the NFATc1, ERK, and NF-κB Signaling Pathways. Front. Endocrinol. 2021, 12, 736863. [Google Scholar] [CrossRef]
- Miyazaki, T.; Katagiri, H.; Kanegae, Y.; Takayanagi, H.; Sawada, Y.; Yamamoto, A.; Pando, M.P.; Asano, T.; Verma, I.M.; Oda, H.; et al. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J. Cell Biol. 2000, 148, 333–342. [Google Scholar] [CrossRef]
- Black, J.D.; Affandi, T.; Black, A.R.; Reyland, M.E. PKCα and PKCδ: Friends and Rivals. J. Biol. Chem. 2022, 298, 102194. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Radstake, T.R.; van der Heijden, A.; van Mansum, M.A.; Dieteren, C.; de Rooij, D.J.; Barrera, P.; Zendman, A.J.; van Venrooij, W.J. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann. Rheum. Dis. 2004, 63, 373–381. [Google Scholar] [CrossRef]
- Foulquier, C.; Sebbag, M.; Clavel, C.; Chapuy-Regaud, S.; Al Badine, R.; Méchin, M.C.; Vincent, C.; Nachat, R.; Yamada, M.; Takahara, H.; et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007, 56, 3541–3553. [Google Scholar] [CrossRef]
- Harvey, G.P.; Fitzsimmons, T.R.; Dhamarpatni, A.A.; Marchant, C.; Haynes, D.R.; Bartold, P.M. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J. Periodontal Res. 2013, 48, 252–261. [Google Scholar] [CrossRef]
- Engström, M.; Eriksson, K.; Lee, L.; Hermansson, M.; Johansson, A.; Nicholas, A.P.; Gerasimcik, N.; Lundberg, K.; Klareskog, L.; Catrina, A.I.; et al. Increased citrullination and expression of peptidylarginine deiminases independently of P. gingivalis and A. actinomycetemcomitans in gingival tissue of patients with periodontitis. J. Transl. Med. 2018, 16, 214. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shindo, S.; Pierrelus, R.; Ikeda, A.; Nakamura, S.; Heidari, A.; Pastore, M.R.; Leon, E.; Ruiz, S.; Chheda, H.; Khatiwala, R.; et al. Extracellular Release of Citrullinated Vimentin Directly Acts on Osteoclasts to Promote Bone Resorption in a Mouse Model of Periodontitis. Cells 2023, 12, 1109. https://doi.org/10.3390/cells12081109
Shindo S, Pierrelus R, Ikeda A, Nakamura S, Heidari A, Pastore MR, Leon E, Ruiz S, Chheda H, Khatiwala R, et al. Extracellular Release of Citrullinated Vimentin Directly Acts on Osteoclasts to Promote Bone Resorption in a Mouse Model of Periodontitis. Cells. 2023; 12(8):1109. https://doi.org/10.3390/cells12081109
Chicago/Turabian StyleShindo, Satoru, Roodelyne Pierrelus, Atsushi Ikeda, Shin Nakamura, Alireza Heidari, Maria Rita Pastore, Elizabeth Leon, Sunniva Ruiz, Harsh Chheda, Rhea Khatiwala, and et al. 2023. "Extracellular Release of Citrullinated Vimentin Directly Acts on Osteoclasts to Promote Bone Resorption in a Mouse Model of Periodontitis" Cells 12, no. 8: 1109. https://doi.org/10.3390/cells12081109
APA StyleShindo, S., Pierrelus, R., Ikeda, A., Nakamura, S., Heidari, A., Pastore, M. R., Leon, E., Ruiz, S., Chheda, H., Khatiwala, R., Kumagai, T., Tolson, G., Elderbashy, I., Ouhara, K., Han, X., Hernandez, M., Vardar-Sengul, S., Shiba, H., & Kawai, T. (2023). Extracellular Release of Citrullinated Vimentin Directly Acts on Osteoclasts to Promote Bone Resorption in a Mouse Model of Periodontitis. Cells, 12(8), 1109. https://doi.org/10.3390/cells12081109







