Notch Signaling Pathway in Tooth Shape Variations throughout Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dental Nomenclature, Imaging, Measurements and Phenotyping
2.3. Statistical Analyses
2.4. RNA Sequencing
2.4.1. Samples Preparation
2.4.2. Library Preparation
2.4.3. Cluster Generation and Sequencing
2.4.4. Data Analysis
2.4.5. Adapter Sequences
2.5. RNA Isolation RT-PCR
2.6. Immunohistochemistry
2.7. 3D Morphing of Tooth Geometries and Computation/PREDICTION of human Jagged1-Mutant M1
3. Results
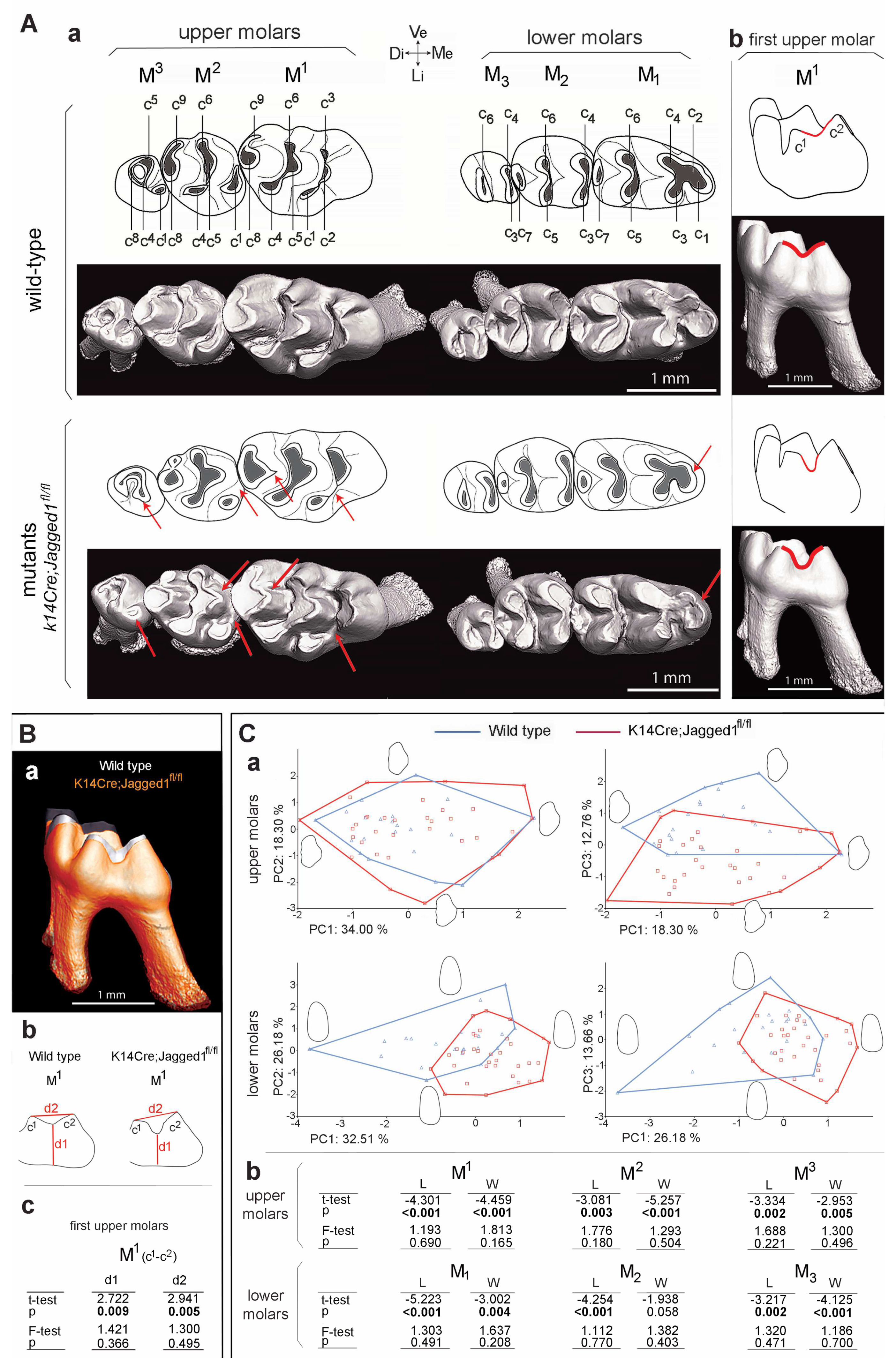
3.1. Morphological Modifications in the Crown of K14Cre;Jagged1fl/fl Molars
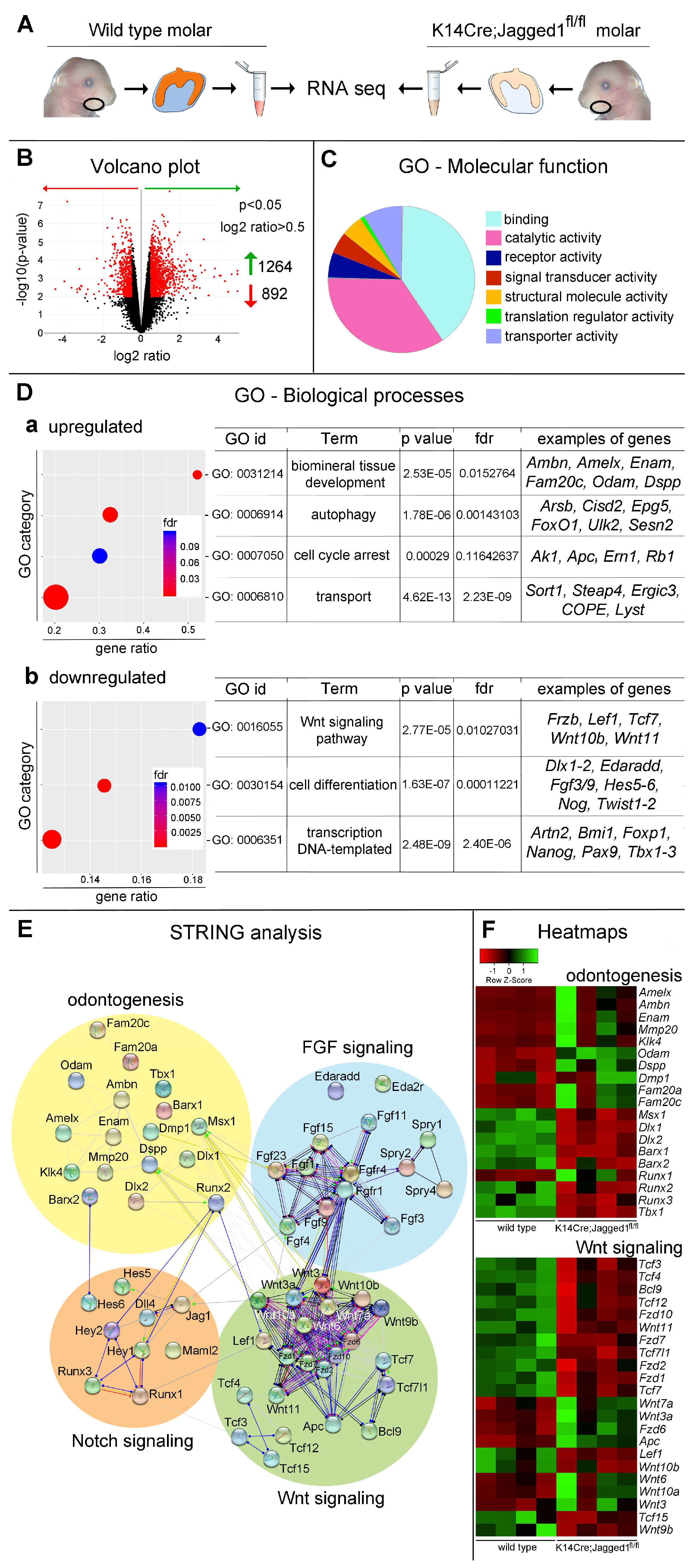
3.2. Transcriptome Modifications in K14Cre;Jagged1fl/fl Molars
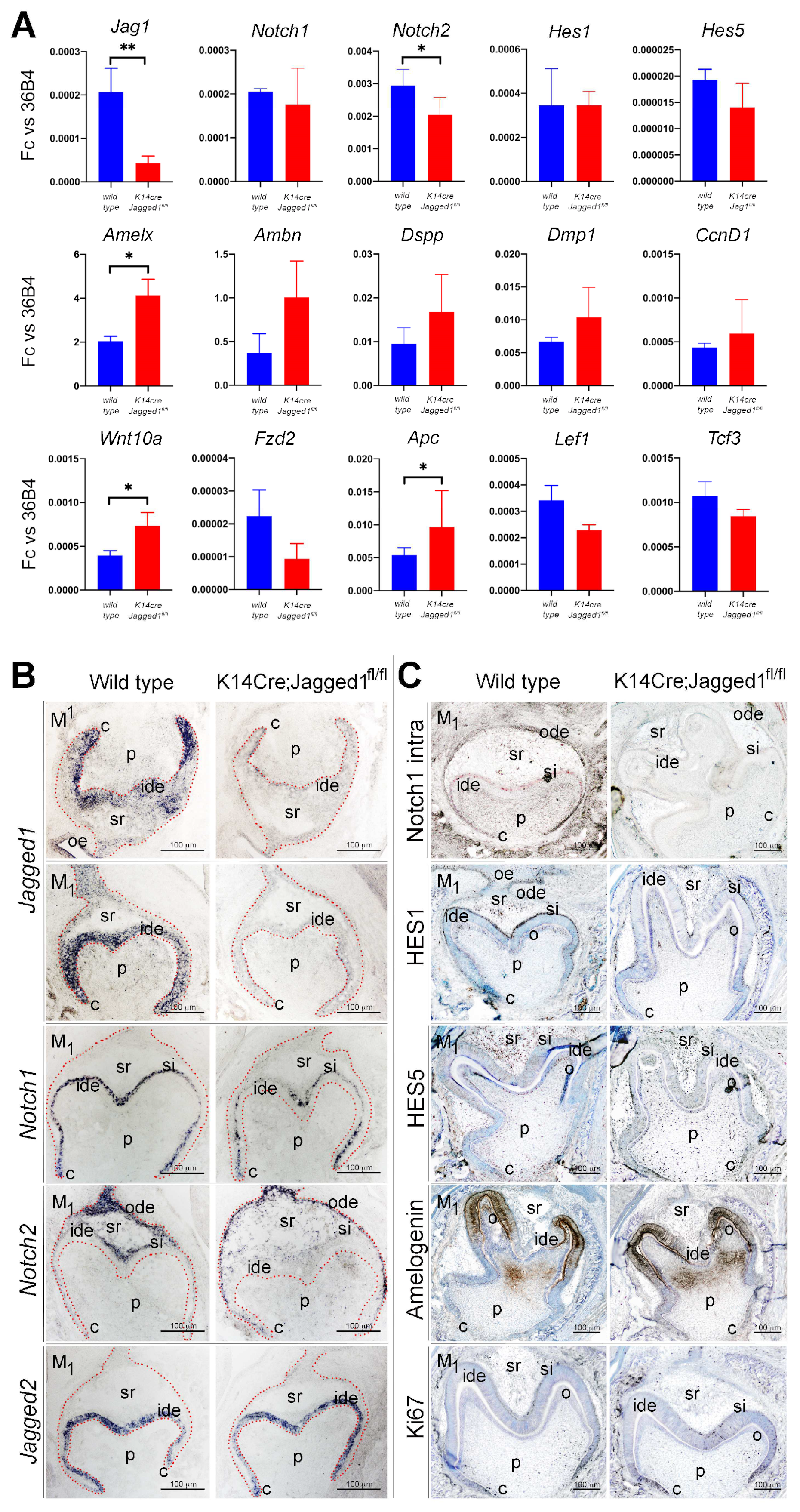
3.3. The Expression Patterns of Genes and Proteins Is Affected in K14Cre;Jagged1fl/fl Molars
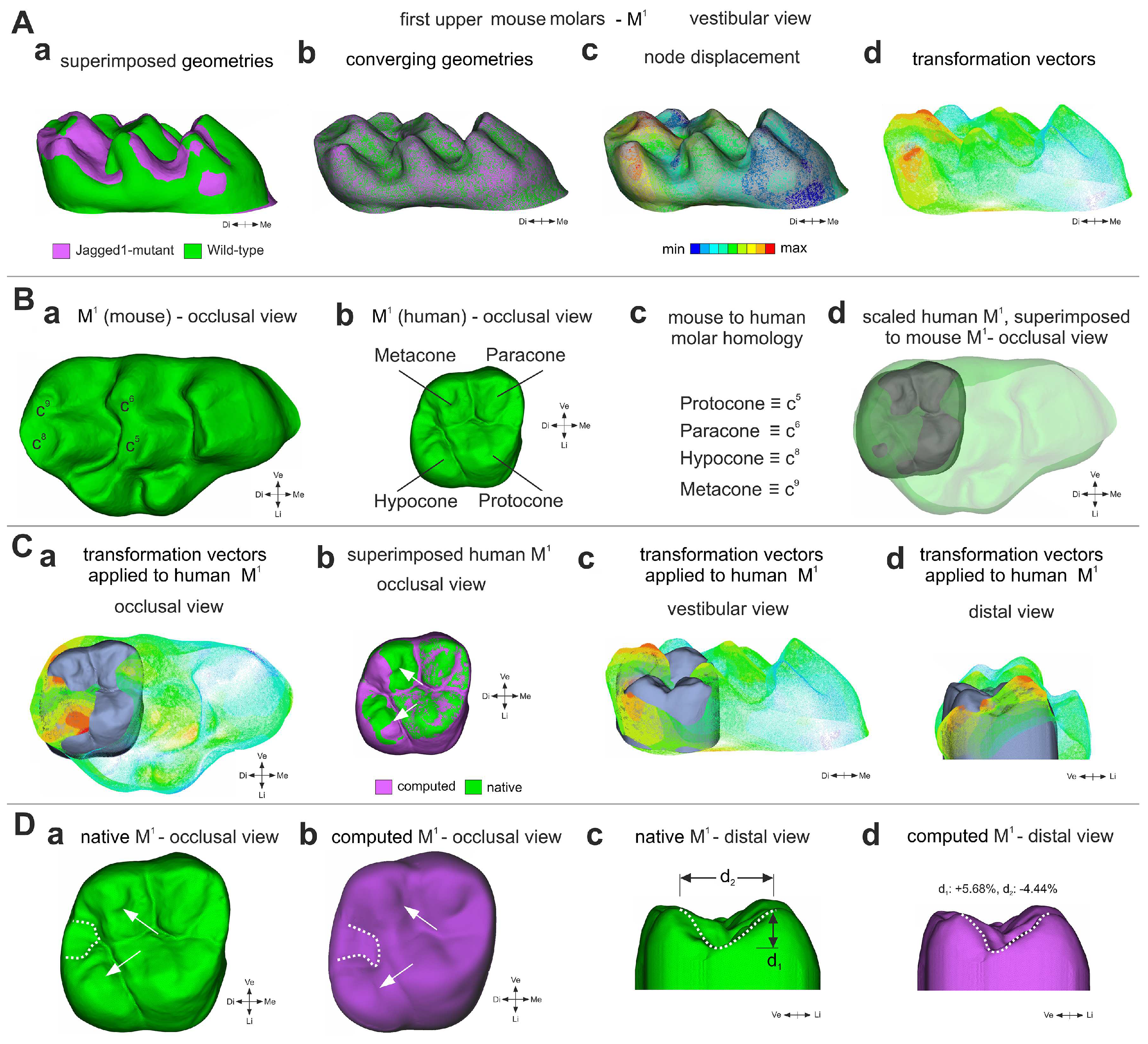
3.4. A Computation Mathematical Three-Dimensional (3D) Model Predicting the Shape of Human Molars upon Jagged1 Mutation
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berkovitz, B.; Shellis, P. The Teeth of Mammalian Vertebrates, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Martin, T.; Jäger, K.R.K.; Plogschties, T.; Schwermann, A.H.; Brinkkötter, J.J.; Schultz, J.A. Molar diversity and functional adaptations in Mesozoic mammals. In Mammalian Teeth—Form and Function; Verlag Dr. Friedrich Pfeil: München, Germany, 2021; pp. 187–214. [Google Scholar]
- Jernvall, J.; Hunter, J.P.; Fortelius, M. Molar tooth diversity, disparity, and ecology in Cenozoic ungulate radiations. Science 1996, 274, 1489–1492. [Google Scholar] [CrossRef]
- Luo, Z.X. Transformation and diversification in early mammal evolution. Nature 2007, 450, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.D.; Evans, A.R.; Jernvall, J. Predicting evolutionary patterns of mammalian teeth from development. Nature 2007, 449, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Lazzari, V.; Tafforeau, P.; Schimmang, T.; Tekin, M.; Klein, O.; Viriot, L. Modulation of Fgf3 dosage in mouse and men mirrors evolution of mammalian dentition. Proc. Natl. Acad. Sci. USA 2009, 106, 22364–22368. [Google Scholar] [CrossRef] [PubMed]
- Harjunmaa, E.; Kallonen, A.; Voutilainen, M.; Hamalainen, K.; Mikkola, M.L.; Jernvall, J. On the difficulty of increasing dental complexity. Nature 2012, 483, 324–327. [Google Scholar] [CrossRef]
- Gomes Rodrigues, H.; Renaud, S.; Charles, C.; Le Poul, Y.; Sole, F.; Aguilar, J.P.; Michaux, J.; Tafforeau, P.; Headon, D.; Jernvall, J.; et al. Roles of dental development and adaptation in rodent evolution. Nat. Commun. 2013, 4, 2504. [Google Scholar] [CrossRef]
- Morita, W.; Morimoto, N.; Jernvall, J. Mapping molar shapes on signaling pathways. PLoS Comput. Biol. 2020, 16, e1008436. [Google Scholar] [CrossRef] [PubMed]
- Savriama, Y.; Romestaing, C.; Clair, A.; Averty, L.; Ulmann, J.; Ledevin, R.; Renaud, S. Wild versus lab house mice: Effects of age, diet, and genetics on molar geometry and topography. J. Anat. 2022, 240, 66–83. [Google Scholar] [CrossRef]
- Jernvall, J.; Thesleff, I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000, 92, 19–29. [Google Scholar] [CrossRef]
- Hayden, L.; Lochovska, K.; Semon, M.; Renaud, S.; Delignette-Muller, M.L.; Vilcot, M.; Peterkova, R.; Hovorakova, M.; Pantalacci, S. Developmental variability channels mouse molar evolution. eLife 2020, 9, e50103. [Google Scholar] [CrossRef]
- Thesleff, I.; Keranen, S.; Jernvall, J. Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv. Dent. Res. 2001, 15, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Henrique, D.; Thesleff, I.; Lendahl, U. Mouse Serrate-1 (Jagged-1): Expression in the developing tooth is regulated by epithelial-mesenchymal interactions and fibroblast growth factor-4. Development 1997, 124, 1473–1483. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Hirsinger, E.; Lendahl, U.; Goridis, C. Delta-notch signaling in odontogenesis: Correlation with cytodifferentiation and evidence for feedback regulation. Dev. Biol. 1998, 204, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Lardelli, M.; Lendahl, U.; Thesleff, I. Expression of Notch 1, 2 and 3 is regulated by epithelial-mesenchymal interactions and retinoic acid in the developing mouse tooth and associated with determination of ameloblast cell fate. J. Cell Biol. 1995, 130, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Mucchielli, M.L.; Mitsiadis, T.A. Correlation of asymmetric Notch2 expression and mouse incisor rotation. Mech. Dev. 2000, 91, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Matsuno, K.; Fortini, M.E. Notch signaling. Science 1995, 268, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cell 2017, 41, 228–241. [Google Scholar] [CrossRef]
- Ho, D.M.; Guruharsha, K.G.; Artavanis-Tsakonas, S. The Notch Interactome: Complexity in Signaling Circuitry. Adv. Exp. Med. Biol. 2018, 1066, 125–140. [Google Scholar] [CrossRef]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Joutel, A.; Tournier-Lasserve, E. Notch signalling pathway and human diseases. Semin. Cell Dev. Biol. 1998, 9, 619–625. [Google Scholar] [CrossRef]
- McDaniell, R.; Warthen, D.M.; Sanchez-Lara, P.A.; Pai, A.; Krantz, I.D.; Piccoli, D.A.; Spinner, N.B. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 2006, 79, 169–173. [Google Scholar] [CrossRef]
- Spinner, N.B.; Colliton, R.P.; Crosnier, C.; Krantz, I.D.; Hadchouel, M.; Meunier-Rotival, M. Jagged1 mutations in Alagille syndrome. Hum. Mutat. 2001, 17, 18–33. [Google Scholar] [CrossRef]
- Swiatek, P.J.; Lindsell, C.E.; del Amo, F.F.; Weinmaster, G.; Gridley, T. Notch1 is essential for postimplantation development in mice. Genes. Dev. 1994, 8, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Kadokawa, Y.; Okabe, M.; Ikawa, M.; Coleman, J.R.; Tsujimoto, Y. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development 1999, 126, 3415–3424. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, X.; Lindsell, C.E.; Norton, C.R.; Chang, B.; Hicks, C.; Gendron-Maguire, M.; Rand, E.B.; Weinmaster, G.; Gridley, T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 1999, 8, 723–730. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Graf, D.; Luder, H.; Gridley, T.; Bluteau, G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development 2010, 137, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Hrabe de Angelis, M.; McIntyre, J., 2nd; Gossler, A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 1997, 386, 717–721. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Jimenez-Rojo, L.; Balic, A.; Weber, S.; Saftig, P.; Pagella, P. Adam10-dependent Notch signaling establishes dental epithelial cell boundaries required for enamel formation. iScience 2022, 25, 105154. [Google Scholar] [CrossRef]
- Berset Convenor, F.; Caristo, M.E.; Ferrara, F.; Hardy, P.; Oropeza-Moe, M.; Waters, R. Federation of European Laboratory Animal Science Associations recommendations of best practices for the health management of ruminants and pigs used for scientific and educational purposes. Lab Anim. 2021, 55, 117–128. [Google Scholar] [CrossRef]
- Mancini, S.J.; Mantei, N.; Dumortier, A.; Suter, U.; MacDonald, H.R.; Radtke, F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 2005, 105, 2340–2342. [Google Scholar] [CrossRef] [PubMed]
- Gomes Rodrigues, H.; Sole, F.; Charles, C.; Tafforeau, P.; Vianey-Liaud, M.; Viriot, L. Evolutionary and biological implications of dental mesial drift in rodents: The case of the Ctenodactylidae (Rodentia, Mammalia). PLoS ONE 2012, 7, e50197. [Google Scholar] [CrossRef] [PubMed]
- Ferson, S.; Rohlf, F.; Koehn, R.K. Measuring shape variation of two-dimensional outlines. Syst. Zool. 1985, 34, 59–68. [Google Scholar] [CrossRef]
- Kuhl, F.; Giardina, C. Elliptic Fourier features of a closed contour. Comput. Graph. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Renaud, S.; Michaux, J.; Jaeger, J.J.; Auffray, J.C. Fourier analysis applied to Stephanomys (Rodentia, Muridae) Molars: Nonprogressive Evolutionary Pattern in a Gradual Lineage. Paleobiology 1996, 22, 255–265. [Google Scholar] [CrossRef]
- Crampton, J.S. Elliptic Fourier shape analysis of fossil bivalves: Some practical considerations. Lethaia 1995, 28, 179–186. [Google Scholar] [CrossRef]
- Conover, W.J.; Iman, R.L. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981, 35, 124–129. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Lawrence, M.; Huber, W.; Pages, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Blanz, V.; Mehl, A.; Vetter, T.; Seidel, H.P. A Statistical Method for Robust 3D Surface Reconstruction from Sparse Data. In Proceedings of the 3DPVT’04: 3D Data Processing, Visualization, and Transmission, 2nd International Symposium, Thessaloniki, Greece, 9 September 2004; pp. 293–300. [Google Scholar]
- Mehl, A.; Blanz, V.; Hickel, R. A new mathematical process for the calculation of average forms of teeth. J. Prosthet. Dent. 2005, 94, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Mehl, A.; Gloger, W.; Kunzelmann, K.H.; Hickel, R. A new optical 3-D device for the detection of wear. J. Dent. Res. 1997, 76, 1799–1807. [Google Scholar] [CrossRef]
- Blanz, V.; Vetter, T. A morphable model for the synthesis of 3D faces. In Proceedings of the SIGGRAPH’99: 26th Annual Conference on Computer Graphics and Interactive Techniques, New York, NY, USA, 8–13 August 1999; pp. 187–194. [Google Scholar]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef]
- Bronckers, A.L. Ion Transport by Ameloblasts during Amelogenesis. J. Dent. Res. 2017, 96, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Harjunmaa, E.; Seidel, K.; Hakkinen, T.; Renvoise, E.; Corfe, I.J.; Kallonen, A.; Zhang, Z.Q.; Evans, A.R.; Mikkola, M.L.; Salazar-Ciudad, I.; et al. Replaying evolutionary transitions from the dental fossil record. Nature 2014, 512, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, L. Dynamique Évolutive D’un Sous-Genre de Muridae, Apodemus (Sylveamus): Etude Biométrique des Caractères Dentaires de Populations Fossiles et Actuelles d’Europe Occidentale; Université des Sciences et Techniques du Languedoc: Languedoc-Roussillon, France, 1974. [Google Scholar]
- Maridet, O. Révision du Genre Democricetodon (Mammalia, Rodentia, Cricetinae) et Dynamique des Faunes de Rongeurs du Néogène d’Europe Occidentale: Évolution, Paléobiodiversité et Paléobiogéographie; University Claude Bernard: Lyon, France, 2003. [Google Scholar]
- Oliver, A.; Pelàez-Campomanes, P. Megacricetodon vandermeuleni, sp. nov. (Rodentia, Mammalia), from the Spanish Miocene: A new evolutionary framework for Megacricetodon. J. Vertebr. Paleontol. 2012, 33, 943–955. [Google Scholar] [CrossRef]
- Lopez-Antonanzas, R.; Renaud, S.; Pelaez-Campomanes, P.; Azar, D.; Kachacha, G.; Knoll, F. First levantine fossil murines shed new light on the earliest intercontinental dispersal of mice. Sci. Rep. 2019, 9, 11874. [Google Scholar] [CrossRef]
- Guruharsha, K.G.; Kankel, M.W.; Artavanis-Tsakonas, S. The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 2012, 13, 654–666. [Google Scholar] [CrossRef]
- Collu, G.M.; Hidalgo-Sastre, A.; Brennan, K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol. Life Sci. 2014, 71, 3553–3567. [Google Scholar] [CrossRef]
- Fre, S.; Pallavi, S.K.; Huyghe, M.; Lae, M.; Janssen, K.P.; Robine, S.; Artavanis-Tsakonas, S.; Louvard, D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc. Natl. Acad. Sci. USA 2009, 106, 6309–6314. [Google Scholar] [CrossRef]
- Kay, S.K.; Harrington, H.A.; Shepherd, S.; Brennan, K.; Dale, T.; Osborne, J.M.; Gavaghan, D.J.; Byrne, H.M. The role of the Hes1 crosstalk hub in Notch-Wnt interactions of the intestinal crypt. PLoS Comput. Biol. 2017, 13, e1005400. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, C.; Hausmann, G.; Basler, K. Beta-catenin hits chromatin: Regulation of Wnt target gene activation. Nat. Rev. Mol. Cell Biol. 2009, 10, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.T.; Wallingford, J.B. Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Bergendal, B.; Norderyd, J.; Zhou, X.; Klar, J.; Dahl, N. Abnormal primary and permanent dentitions with ectodermal symptoms predict WNT10A deficiency. BMC Med. Genet. 2016, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Kantaputra, P.N.; Kapoor, S.; Verma, P.; Kaewgahya, M.; Kawasaki, K.; Ohazama, A.; Ketudat Cairns, J.R. Al-Awadi-Raas-Rothschild syndrome with dental anomalies and a novel WNT7A mutation. Eur. J. Med. Genet. 2017, 60, 695–700. [Google Scholar] [CrossRef]
- Millar, S.E.; Koyama, E.; Reddy, S.T.; Andl, T.; Gaddapara, T.; Piddington, R.; Gibson, C.W. Over- and ectopic expression of Wnt3 causes progressive loss of ameloblasts in postnatal mouse incisor teeth. Connect. Tissue Res. 2003, 44 (Suppl. S1), 124–129. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, C.; Jung, S.; Niederreither, K.; Prasad, M.; Hadj-Rabia, S.; Philip, N.; Mallet, A.; Consolino, E.; Sfeir, E.; Noueiri, B.; et al. Dental and extra-oral clinical features in 41 patients with WNT10A gene mutations: A multicentric genotype-phenotype study. Clin. Genet. 2017, 92, 477–486. [Google Scholar] [CrossRef]
- Xu, M.; Horrell, J.; Snitow, M.; Cui, J.; Gochnauer, H.; Syrett, C.M.; Kallish, S.; Seykora, J.T.; Liu, F.; Gaillard, D.; et al. WNT10A mutation causes ectodermal dysplasia by impairing progenitor cell proliferation and KLF4-mediated differentiation. Nat. Commun. 2017, 8, 15397. [Google Scholar] [CrossRef]
- Yu, P.; Yang, W.; Han, D.; Wang, X.; Guo, S.; Li, J.; Li, F.; Zhang, X.; Wong, S.W.; Bai, B.; et al. Mutations in WNT10B Are Identified in Individuals with Oligodontia. Am. J. Hum. Genet. 2016, 99, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.D.; Minowada, G.; Peterkova, R.; Kangas, A.; Yu, B.D.; Lesot, H.; Peterka, M.; Jernvall, J.; Martin, G.R. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell 2006, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Boran, T.; Peterkova, R.; Lesot, H.; Lyons, D.B.; Peterka, M.; Klein, O.D. Temporal analysis of ectopic enamel production in incisors from sprouty mutant mice. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.M.; Artavanis-Tsakonas, S. The Notch-Mediated Proliferation Circuitry. Curr. Top. Dev. Biol. 2016, 116, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Michaux, J.; Pasquier, L. Dynamique des populations de mulots (Rodentia, Apodemus) en Europe durant le Quaternaire. Bull. Soc. Géolog. Fr. 1974, 7, 431–439. [Google Scholar] [CrossRef]
- Vianey-Liaud, M.; Gomes-Rodrigues, H.; Michaux, J. L’espèce en paléontologie: De l’utilisation du binôme linnéen chez les rongeurs fossiles. Comptes Rendus. Palevol. 2011, 10, 117–131. [Google Scholar] [CrossRef]
- Hofmann, J.J.; Zovein, A.C.; Koh, H.; Radtke, F.; Weinmaster, G.; Iruela-Arispe, M.L. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: Insights into Alagille syndrome. Development 2010, 137, 4061–4072. [Google Scholar] [CrossRef]
- Lorent, K.; Yeo, S.Y.; Oda, T.; Chandrasekharappa, S.; Chitnis, A.; Matthews, R.P.; Pack, M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development 2004, 131, 5753–5766. [Google Scholar] [CrossRef]
- Jheon, A.H.; Seidel, K.; Biehs, B.; Klein, O.D. From molecules to mastication: The development and evolution of teeth. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 165–182. [Google Scholar] [CrossRef]
- Line, S.R. Variation of tooth number in mammalian dentition: Connecting genetics, development, and evolution. Evol. Dev. 2003, 5, 295–304. [Google Scholar] [CrossRef]
- Ludwig, M.; Berrier, S.; Tetlaff, M.; Meyer, G. 3D shape and texture morphing using 2D projection and reconstruction. Comput. Graph. 2015, 51, 146–156. [Google Scholar] [CrossRef]

| Adapter | Sequence |
|---|---|
| TruSeq Universal Adapter | 5′ AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT |
| TruSeq Adapter, Index 1 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACATCACGATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 2 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACCGATGTATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 3 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACTTAGGCATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 4 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACTGACCAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 5 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACACAGTGATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 6 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGCCAATATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 7 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACCAGATCATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 8 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACACTTGAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 9 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGATCAGATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 10 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACTAGCTTATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 11 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGGCTACATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 12 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACCTTGTAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 13 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACAGTCAACAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 14 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACAGTTCCGTATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 15 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACATGTCAGAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 16 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACCCGTCCCGATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 18 4 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGTCCGCACATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 19 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGTGAAACGATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 20 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGTGGCCTTATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 21 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGTTTCGGAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 22 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACCGTACGTAATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 23 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACGAGTGGATATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 25 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACACTGATATATCTCGTATGCCGTCTTCTGCTTG |
| TruSeq Adapter, Index 27 | 5′ GATCGGAAGAGCACACGTCTGAACTCCAGTCACATTCCTTTATCTCGTATGCCGTCTTCTGCTTG |
| Specimen | Cohort | M1 | M2 | M3 | Upper Molars | ||||
|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | ||
| c1-c2 | c1-c2 | c1 | c1 | c1 | c1 | Anormal cusp | Anormal Cusp | ||
| D1399 | Jag1fl/fl | V-shape | V-shape | - | - | reduced | - | - | - |
| D1400 | Jag1fl/fl | U-shape | U-shape | no spur | - | - | - | - | c8M2 |
| D1401 | Jag1fl/fl | V-shape | V-shape | - | - | reduced | - | c5M2 | c5M1, c8M2 |
| D1402 | Jag1fl/fl | U-shape | U-shape | - | - | - | - | c8M2 | c8M2 |
| D1404 | Jag1fl/fl | U-shape | U-shape | no spur | no spur | - | reduced | c5M2 | c8M2 |
| D1405 | Jag1fl/fl | U-shape | V-shape | no spur | no spur | - | - | c8M2 | - |
| D1406 | Jag1fl/fl | V-shape | V-shape | no spur | no spur | - | - | c8M2 | c8M2 |
| D1407 | Jag1fl/fl | V-shape | V-shape | no spur | - | - | - | - | - |
| D1408 | Jag1fl/fl | U-shape | U-shape | - | - | - | - | c8M2 | - |
| D1409 | Jag1fl/fl | U-shape | V-shape | - | no spur | - | - | c8M1-2 | c8M2 |
| D1413 | Jag1fl/fl | U-shape | U-shape | no spur | no spur | - | - | - | - |
| D1414 | Jag1fl/fl | U-shape | V-shape | no spur | no spur | - | - | - | c8M1-2 |
| D1415 | Jag1fl/fl | U-shape | U-shape | no spur | no spur | - | - | c8M1-2 | c8M1-2 |
| D1433 | Jag1fl/fl | U-shape | V-shape | - | no spur | - | reduced | c8M2 | c8M1-2 |
| D1434 | Jag1fl/fl | U-shape | U-shape | no spur | no spur | - | - | - | - |
| D1435 | Jag1fl/fl | V-shape | V-shape | no spur | no spur | - | - | c8M2 | c8M1-2 |
| D1436 | Jag1fl/fl | U-shape | U-shape | no spur | no spur | - | - | - | c8M2 |
| D1439 | Jag1fl/fl | U-shape | V-shape | - | no spur | - | - | c8M2 | - |
| D1440 | Jag1fl/fl | V-shape | U-shape | no spur | no spur | - | - | c8M2 | c5-8M2 |
| D1443 | Jag1fl/fl | V-shape | U-shape | no spur | no spur | reduced | reduced | - | c8M1 |
| D1444 | Jag1fl/fl | V-shape | V-shape | no spur | no spur | reduced | reduced | c5M2 | - |
| M8 | Jag1fl/fl | U-shape | U-shape | - | no spur | big | - | c8M2 | c8M3 |
| M9 | Jag1fl/fl | V-shape | U-shape | no spur | no spur | reduced | reduced | - | c8M2 |
| M10 | Jag1fl/fl | V-shape | V-shape | no spur | - | reduced | reduced | c8M2 | c8M1-2 |
| M11 | Jag1fl/fl | U-shape | U-shape | - | no spur | reduced | reduced | - | c8M1 |
| M12 | Jag1fl/fl | U-shape | U-shape | - | - | reduced | reduced | - | - |
| M15 | Jag1fl/fl | V-shape | V-shape | - | - | - | - | - | - |
| M16 | Jag1fl/fl | V-shape | U-shape | no spur | no spur | reduced | reduced | c8M2 | c5M1, c8M2 |
| M18 | Jag1fl/fl | U-shape | U-shape | no spur | - | - | reduced | c8M2 | c5-8M2 |
| M19 | Jag1fl/fl | U-shape | U-shape | - | no spur | - | - | c8M2 | c8M1 |
| M21 | Jag1fl/fl | U-shape | V-shape | no spur | - | - | - | c8M1-2 | c8M1-2 |
| M22 | Jag1fl/fl | U-shape | U-shape | - | - | - | - | - | - |
| M23 | Jag1fl/fl | U-shape | V-shape | - | no spur | - | reduced | - | c8M1-2 |
| D1398 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1403 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1410 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1411 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1412 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1426 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1427 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1428 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1429 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1430 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1431 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1432 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1437 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1438 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1441 | WT | V-shape | V-shape | - | - | - | - | - | - |
| D1442 | WT | V-shape | V-shape | - | - | - | - | - | - |
| M13 | WT | V-shape | V-shape | - | - | - | - | - | - |
| M14 | WT | V-shape | V-shape | - | - | - | - | - | - |
| M17 | WT | V-shape | V-shape | - | - | - | - | - | - |
| M20 | WT | V-shape | V-shape | - | - | - | - | - | - |
| M24 | WT | V-shape | V-shape | - | - | - | - | - | - |
| M1 (c1-c2) | |||
|---|---|---|---|
| d3 | d4 | d5 | |
| t-test | −3.121 | −4.307 | −3.387 |
| p | 0.003 | <0.001 | 0.001 |
| F-test | 1.564 | 1.101 | 1.534 |
| p | 0.296 | 0.837 | 0.317 |
| MANOVA | Test | Value | F | d.f. | d.f. (Error) | p |
|---|---|---|---|---|---|---|
| M1 | Wilk | 0.340 | 7.427 | 11 | 42 | <0.001 |
| M1 | Wilk | 0.557 | 4.470 | 8 | 45 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsiadis, T.A.; Pagella, P.; Gomes Rodrigues, H.; Tsouknidas, A.; Ramenzoni, L.L.; Radtke, F.; Mehl, A.; Viriot, L. Notch Signaling Pathway in Tooth Shape Variations throughout Evolution. Cells 2023, 12, 761. https://doi.org/10.3390/cells12050761
Mitsiadis TA, Pagella P, Gomes Rodrigues H, Tsouknidas A, Ramenzoni LL, Radtke F, Mehl A, Viriot L. Notch Signaling Pathway in Tooth Shape Variations throughout Evolution. Cells. 2023; 12(5):761. https://doi.org/10.3390/cells12050761
Chicago/Turabian StyleMitsiadis, Thimios A., Pierfrancesco Pagella, Helder Gomes Rodrigues, Alexander Tsouknidas, Liza L. Ramenzoni, Freddy Radtke, Albert Mehl, and Laurent Viriot. 2023. "Notch Signaling Pathway in Tooth Shape Variations throughout Evolution" Cells 12, no. 5: 761. https://doi.org/10.3390/cells12050761
APA StyleMitsiadis, T. A., Pagella, P., Gomes Rodrigues, H., Tsouknidas, A., Ramenzoni, L. L., Radtke, F., Mehl, A., & Viriot, L. (2023). Notch Signaling Pathway in Tooth Shape Variations throughout Evolution. Cells, 12(5), 761. https://doi.org/10.3390/cells12050761








