Mitochondrial Dysfunction in Cardiac Arrhythmias
Abstract
1. Introduction
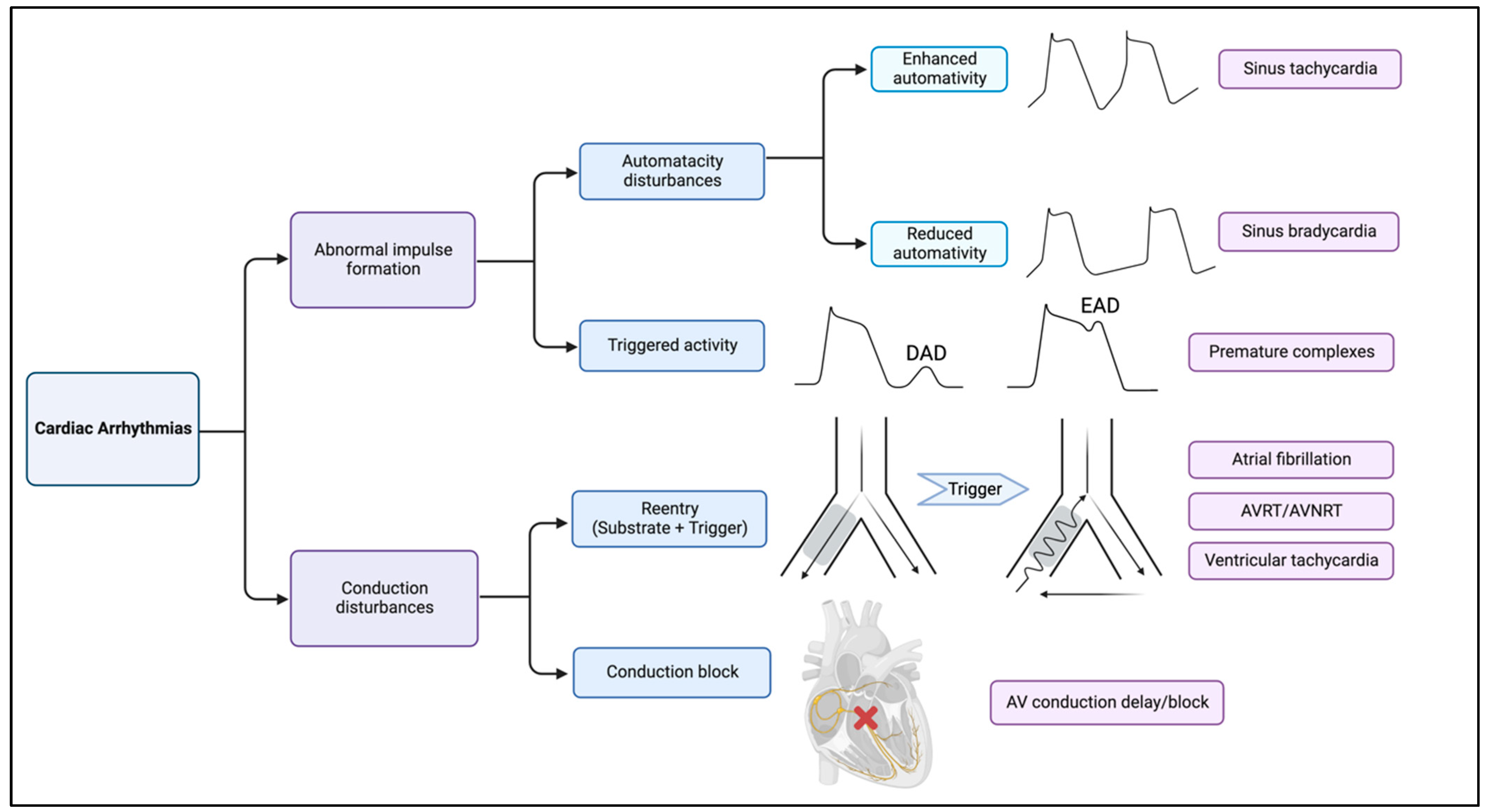
2. Basic Mechanisms of Arrhythmias
2.1. Phases of Action Potential (AP)
2.2. Abnormal Impulse Formation
2.2.1. Automaticity Disturbances
2.2.2. Triggered Activity
2.3. Conduction Disturbances
2.3.1. Reentry Tachycardia
2.3.2. Conduction Block
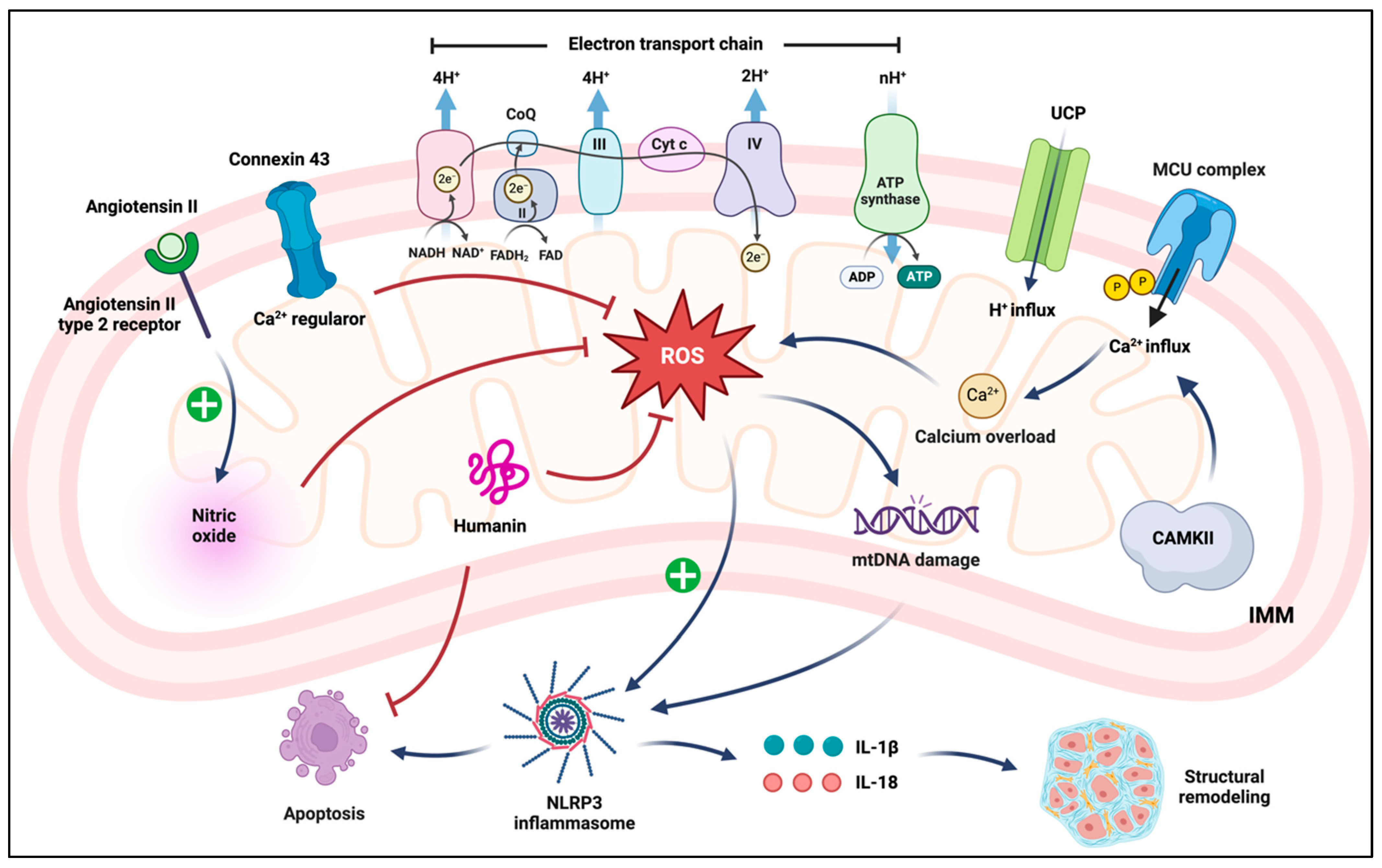
3. Mitochondrial Function and Dysfunction
4. Mitochondrial Dysfunction in Arrhythmogenic Pathogenesis
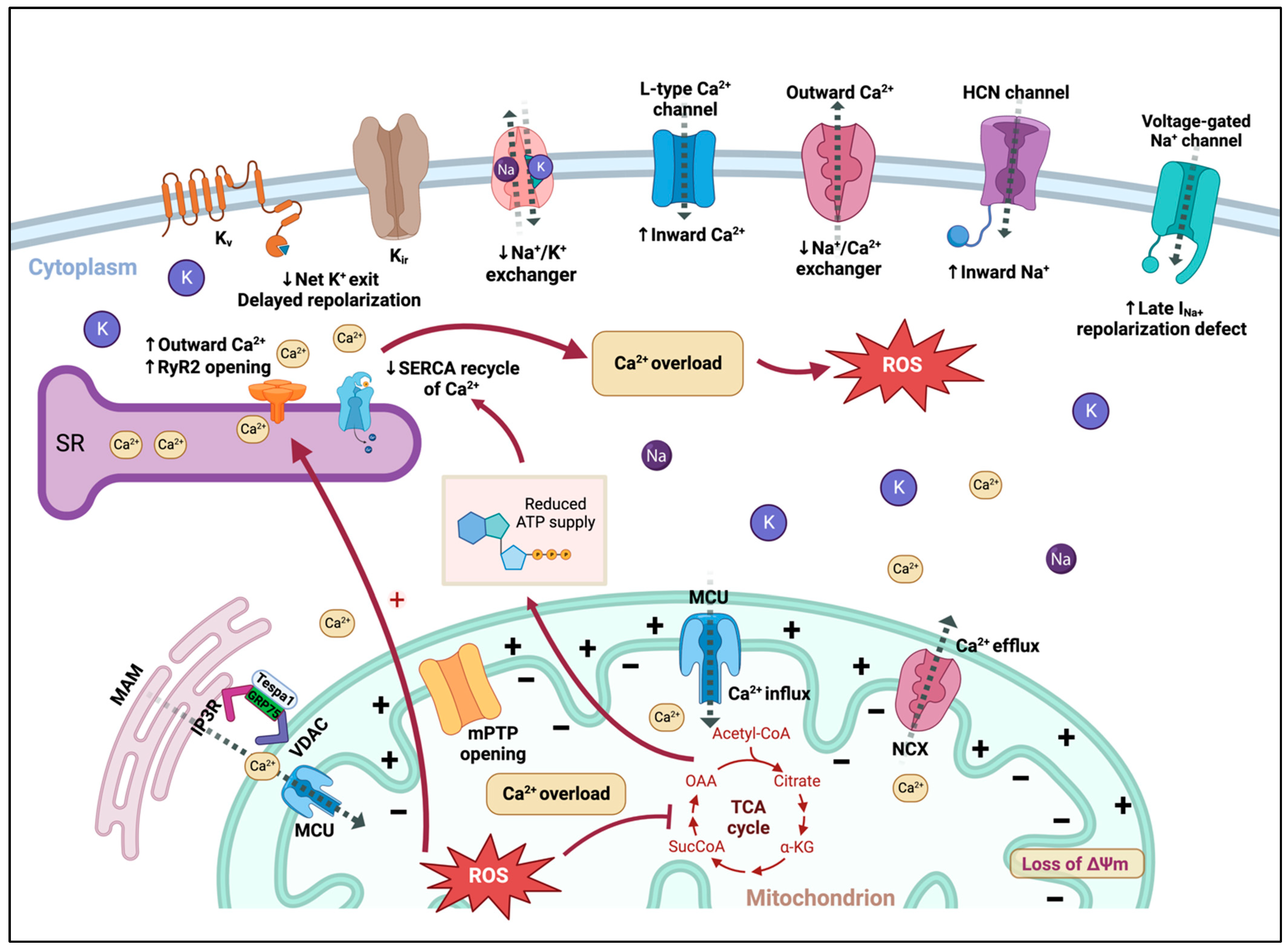
4.1. Sarcolemmal and Intracellular Ion Balance (Ca2+, Na+, K+)
4.1.1. Ca2+
4.1.2. Na+
4.1.3. K+
4.2. Mitochondria-Associated Proteins
4.2.1. Mitochondrial Ca2+ Transport Proteins
4.2.2. Connexin (Cx) Proteins
4.2.3. Mitochondrial RAS
4.2.4. MDPs
4.2.5. Mitochondrial GRKs and β-Arrestins
4.3. Inflammatory Signaling
4.4. Mitochondria-Associated ER Membranes (MAMs)
5. Mitochondrial Dysfunction in Different Arrhythmias
5.1. mtDNA and nDNA Mutation Associated Arrhythmias
5.2. SAN Dysfunction and AVN Dysfunction
5.3. Reentrant Tachyarrhythmias
5.3.1. AF
Electrical Stimulation-Induced AF
Aging-Associated Mitochondrial Dysfunction in AF
Gut Microbiota-Associated Mitochondrial Dysfunction in AF
Other Factors Induced AF
5.3.2. Ventricular Arrhythmias (VAs)
Cardiac Ischemia and I/R Injury-Induced VAs
Electrical Stimulation-Induced VAs
Other Factors Induced VA
5.3.3. Hereditary Muscular Dystrophy-Associated Arrhythmias
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; Al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of sudden cardiac death: Clinical and research implications. Prog. Cardiovasc. Dis. 2008, 51, 213–228. [Google Scholar] [CrossRef]
- Roberts-Thomson, K.C.; Lau, D.H.; Sanders, P. The diagnosis and management of ventricular arrhythmias. Nat. Rev. Cardiol. 2011, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A., 3rd; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013, 127, e283–e352. [Google Scholar] [CrossRef]
- Heijman, J.; Algalarrondo, V.; Voigt, N.; Melka, J.; Wehrens, X.H.; Dobrev, D.; Nattel, S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: A critical analysis. Cardiovasc. Res. 2016, 109, 467–479. [Google Scholar] [CrossRef]
- Harris, D.A.; Das, A.M. Control of mitochondrial ATP synthesis in the heart. Biochem. J. 1991, 280 Pt 3, 561–573. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Burashnikov, A. Overview of Basic Mechanisms of Cardiac Arrhythmia. Card Electrophysiol. Clin. 2011, 3, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Anumonwo, J.M.; Pandit, S.V. Ionic mechanisms of arrhythmogenesis. Trends Cardiovasc. Med. 2015, 25, 487–496. [Google Scholar] [CrossRef]
- Weiss, J.N.; Garfinkel, A.; Karagueuzian, H.S.; Nguyen, T.P.; Olcese, R.; Chen, P.S.; Qu, Z. Perspective: A dynamics-based classification of ventricular arrhythmias. J. Mol. Cell. Cardiol. 2015, 82, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Tse, G. Mechanisms of cardiac arrhythmias. J. Arrhythm. 2016, 32, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Temple, I.P.; Logantha, S.J.; Absi, M.; Zhang, Y.; Pervolaraki, E.; Yanni, J.; Atkinson, A.; Petkova, M.; Quigley, G.M.; Castro, S.; et al. Atrioventricular Node Dysfunction and Ion Channel Transcriptome in Pulmonary Hypertension. Circ. Arrhythm. Electrophysiol. 2016, 9, e003432. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Dai, L.; Zhan, H.; Dou, J.; Xia, L.; Zhang, H. Theoretical investigation of the mechanism of heart failure using a canine ventricular cell model: Especially the role of up-regulated CaMKII and SR Ca2+ leak. J. Mol. Cell. Cardiol. 2013, 56, 34–43. [Google Scholar] [CrossRef]
- Dai, L.; Zang, Y.; Zheng, D.; Xia, L.; Gong, Y. Role of CaMKII and PKA in Early Afterdepolarization of Human Ventricular Myocardium Cell: A Computational Model Study. Comput. Math. Methods Med. 2016, 2016, 4576313. [Google Scholar] [CrossRef]
- Liu, C.F.; Cheung, J.W.; Ip, J.E.; Thomas, G.; Yang, H.; Sharma, S.; Markowitz, S.M.; Lerman, B.B. Unifying Algorithm for Mechanistic Diagnosis of Atrial Tachycardia. Circ. Arrhythm. Electrophysiol. 2016, 9, e004028. [Google Scholar] [CrossRef]
- Kass, R.S.; Tsien, R.W.; Weingart, R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J. Physiol. 1978, 281, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, A.C.; Goodrow, R.J.; Weigel, C.M. INaCa and ICl(Ca) contribute to isoproterenol-induced delayed after depolarizations in midmyocardial cells. Am. J. Physiol. 1998, 275, H1979–H1992. [Google Scholar] [CrossRef]
- Goyal, A.; Basit, H.; Bhyan, P.; Zeltser, R. Reentry Arrhythmia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gaztañaga, L.; Marchlinski, F.E.; Betensky, B.P. Mechanisms of cardiac arrhythmias. Rev. Esp. Cardiol. 2012, 65, 174–185. [Google Scholar] [CrossRef]
- Baruscotti, M.; Bucchi, A.; Viscomi, C.; Mandelli, G.; Consalez, G.; Gnecchi-Rusconi, T.; Montano, N.; Casali, K.R.; Micheloni, S.; Barbuti, A.; et al. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc. Natl. Acad. Sci. USA 2011, 108, 1705–1710. [Google Scholar] [CrossRef]
- Verrier, R.L.; Sobrado, M.F.; Pagotto, V.P.; Kanas, A.F.; Machado, A.D.; Varone, B.B.; Sobrado, L.F.; Nearing, B.D.; Zeng, D.; Belardinelli, L. Inhibition of I(f) in the atrioventricular node as a mechanism for dronedarone’s reduction in ventricular rate during atrial fibrillation. Heart Rhythm. 2013, 10, 1692–1697. [Google Scholar] [CrossRef]
- Marger, L.; Mesirca, P.; Alig, J.; Torrente, A.; Dubel, S.; Engeland, B.; Kanani, S.; Fontanaud, P.; Striessnig, J.; Shin, H.S.; et al. Functional roles of Ca(v)1.3, Ca(v)3.1 and HCN channels in automaticity of mouse atrioventricular cells: Insights into the atrioventricular pacemaker mechanism. Channels 2011, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Matthes, J.; Yildirim, L.; Wietzorrek, G.; Reimer, D.; Striessnig, J.; Herzig, S. Disturbed atrio-ventricular conduction and normal contractile function in isolated hearts from Cav1.3-knockout mice. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 554–562. [Google Scholar] [CrossRef]
- Kim, D.; Shinohara, T.; Joung, B.; Maruyama, M.; Choi, E.K.; On, Y.K.; Han, S.; Fishbein, M.C.; Lin, S.F.; Chen, P.S. Calcium dynamics and the mechanisms of atrioventricular junctional rhythm. J. Am. Coll. Cardiol. 2010, 56, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Shoffner, J.M. Mitochondrial myopathy diagnosis. Neurol. Clin. 2000, 18, 105–123. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Brand, M.D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000, 35, 811–820. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolaki, N.E.; Apostolopoulos, E.J.; Melita, H.; Katsiki, N. Mitochondrial dysfunction in cardiovascular disease: Current status of translational research/clinical and therapeutic implications. Med. Res. Rev. 2021, 41, 275–313. [Google Scholar] [CrossRef] [PubMed]
- Armoundas, A.A.; Hobai, I.A.; Tomaselli, G.F.; Winslow, R.L.; O’Rourke, B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ. Res. 2003, 93, 46–53. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Ryosuke, T.; Noriko, O.; Tsuyoshi, S. Mitochondrial Cardiomyopathy. In Current Perspectives on Cardiomyopathies; Angelos, T., Ed.; IntechOpen: Rijeka, Croatia, 2018; p. 1. [Google Scholar]
- Hameed, S.; Tadi, P. Myoclonic Epilepsy and Ragged Red Fibers. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yu-Wai-Man, P.; Griffiths, P.G.; Hudson, G.; Chinnery, P.F. Inherited mitochondrial optic neuropathies. J. Med. Genet. 2009, 46, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Birtel, J.; von Landenberg, C.; Gliem, M.; Gliem, C.; Reimann, J.; Kunz, W.S.; Herrmann, P.; Betz, C.; Caswell, R.; Nesbitt, V.; et al. Mitochondrial Retinopathy. Ophthalmol. Retin. 2022, 6, 65–79. [Google Scholar] [CrossRef]
- Lemoine, S.; Panaye, M.; Rabeyrin, M.; Errazuriz-Cerda, E.; Mousson de Camaret, B.; Petiot, P.; Juillard, L.; Guebre-Egziabher, F. Renal Involvement in Neuropathy, Ataxia, Retinitis Pigmentosa (NARP) Syndrome: A Case Report. Am. J. Kidney Dis. 2018, 71, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Lake, N.J.; Compton, A.G.; Rahman, S.; Thorburn, D.R. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann. Neurol. 2016, 79, 190–203. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Adesina, A.M.; Jones, J.; Scaglia, F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015, 116, 4–12. [Google Scholar] [CrossRef]
- Yamashita, S.; Nishino, I.; Nonaka, I.; Goto, Y.I. Genotype and phenotype analyses in 136 patients with single large-scale mitochondrial DNA deletions. J. Hum. Genet. 2008, 53, 598. [Google Scholar] [CrossRef]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Mitochondrial Disorder: Kearns-Sayre Syndrome. Adv. Exp. Med. Biol. 2018, 1085, 161–162. [Google Scholar] [CrossRef]
- Clarke, S.L.; Bowron, A.; Gonzalez, I.L.; Groves, S.J.; Newbury-Ecob, R.; Clayton, N.; Martin, R.P.; Tsai-Goodman, B.; Garratt, V.; Ashworth, M.; et al. Barth syndrome. Orphanet. J. Rare Dis. 2013, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Giunti, P. Friedreich’s ataxia: Clinical features, pathogenesis and management. Br. Med. Bull. 2017, 124, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bassani, J.W.; Bassani, R.A.; Bers, D.M. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J. Physiol. 1994, 476, 279–293. [Google Scholar] [CrossRef]
- Salazar-Ramírez, F.; Ramos-Mondragón, R.; García-Rivas, G. Mitochondrial and Sarcoplasmic Reticulum Interconnection in Cardiac Arrhythmia. Front. Cell Dev. Biol. 2020, 8, 623381. [Google Scholar] [CrossRef]
- Liu, T.; O’Rourke, B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 2009, 41, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Beutner, G.; Kinnally, K.W.; Dirksen, R.T.; Sheu, S.S. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J. Biol. Chem. 2011, 286, 21324–21329. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, L.; Clapham, D.E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 2009, 326, 144–147. [Google Scholar] [CrossRef]
- Feng, S.; Li, H.; Tai, Y.; Huang, J.; Su, Y.; Abramowitz, J.; Zhu, M.X.; Birnbaumer, L.; Wang, Y. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 11011–11016. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, A.I.; Jean-Quartier, C.; Malli, R.; Graier, W.F. Characterization of distinct single-channel properties of Ca2+ inward currents in mitochondria. Pflugers Arch 2013, 465, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Palty, R.; Silverman, W.F.; Hershfinkel, M.; Caporale, T.; Sensi, S.L.; Parnis, J.; Nolte, C.; Fishman, D.; Shoshan-Barmatz, V.; Herrmann, S.; et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA 2010, 107, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Cortassa, S.; Aon, M.A.; Ganesan, A.N.; Liu, T.; O’Rourke, B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 2006, 99, 172–182. [Google Scholar] [CrossRef]
- Bernardi, P.; von Stockum, S. The permeability transition pore as a Ca2+ release channel: New answers to an old question. Cell Calcium 2012, 52, 22–27. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Goldhaber, J.I.; Ji, S.; Lamp, S.T.; Weiss, J.N. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J. Clin. Investig. 1989, 83, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Eigel, B.N.; Gursahani, H.; Hadley, R.W. ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H955–H963. [Google Scholar] [CrossRef]
- Hinata, M.; Matsuoka, I.; Iwamoto, T.; Watanabe, Y.; Kimura, J. Mechanism of Na+/Ca2+ exchanger activation by hydrogen peroxide in guinea-pig ventricular myocytes. J. Pharmacol. Sci. 2007, 103, 283–292. [Google Scholar] [CrossRef]
- Liu, T.; O’Rourke, B. Regulation of the Na+/Ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J. Biol. Chem. 2013, 288, 31984–31992. [Google Scholar] [CrossRef]
- Viola, H.M.; Arthur, P.G.; Hool, L.C. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ. Res. 2007, 100, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, W.A.; Opie, L.H. Effects of oxygen free radicals on isolated cardiac myocytes from guinea-pig ventricle: Electrophysiological studies. J. Mol. Cell. Cardiol. 1992, 24, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.L.; Li, W.; Lu, Y.; Centracchio, J.; Terentyeva, R.; Koren, G.; Terentyev, D. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J. Physiol. 2013, 591, 5895–5911. [Google Scholar] [CrossRef]
- Hamilton, S.; Terentyeva, R.; Martin, B.; Perger, F.; Li, J.; Stepanov, A.; Bonilla, I.M.; Knollmann, B.C.; Radwański, P.B.; Györke, S.; et al. Increased RyR2 activity is exacerbated by calcium leak-induced mitochondrial ROS. Basic. Res. Cardiol. 2020, 115, 38. [Google Scholar] [CrossRef]
- Xu, K.Y.; Zweier, J.L.; Becker, L.C. Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPase function by direct attack on the ATP binding site. Circ. Res. 1997, 80, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.T.; Makielski, J.C. Redox control of cardiac excitability. Antioxid. Redox Signal. 2013, 18, 432–468. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Morin, D.; Assaly, R.; Paradis, S.; Berdeaux, A. Inhibition of mitochondrial membrane permeability as a putative pharmacological target for cardioprotection. Curr. Med. Chem. 2009, 16, 4382–4398. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Mochizuki, S.; MacLeod, K.T. Effects of hypoxia and metabolic inhibition on increases in intracellular Ca2+ concentration induced by Na+/Ca2+ exchange in isolated guinea-pig cardiac myocytes. J. Mol. Cell. Cardiol. 1997, 29, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Killeen, M.J.; Grace, A.A.; Huang, C.L. Pharmacological separation of early afterdepolarizations from arrhythmogenic substrate in DeltaKPQ Scn5a murine hearts modelling human long QT 3 syndrome. Acta. Physiol. 2008, 192, 505–517. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Nesterenko, V.; Shryock, J.C.; Rajamani, S.; Song, Y.; Belardinelli, L. The role of late I Na in development of cardiac arrhythmias. Handb. Exp. Pharmacol. 2014, 221, 137–168. [Google Scholar] [CrossRef] [PubMed]
- Le Masson, G.; Przedborski, S.; Abbott, L.F. A computational model of motor neuron degeneration. Neuron 2014, 83, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Marder, E. Interactions among diameter, myelination, and the Na/K pump affect axonal resilience to high-frequency spiking. Proc. Natl. Acad. Sci. USA 2021, 118, e2105795118. [Google Scholar] [CrossRef]
- Glitsch, H.G. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol. Rev. 2001, 81, 1791–1826. [Google Scholar] [CrossRef]
- Bers, D.M.; Barry, W.H.; Despa, S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc. Res. 2003, 57, 897–912. [Google Scholar] [CrossRef]
- Greenstein, J.L.; Wu, R.; Po, S.; Tomaselli, G.F.; Winslow, R.L. Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circ. Res. 2000, 87, 1026–1033. [Google Scholar] [CrossRef]
- Nerbonne, J.M.; Kass, R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 2005, 85, 1205–1253. [Google Scholar] [CrossRef]
- Lopatin, A.N.; Nichols, C.G. Inward rectifiers in the heart: An update on I(K1). J. Mol. Cell. Cardiol. 2001, 33, 625–638. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lopatin, A.N. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997, 59, 171–191. [Google Scholar] [CrossRef]
- Zhuo, M.L.; Huang, Y.; Liu, D.P.; Liang, C.C. KATP channel: Relation with cell metabolism and role in the cardiovascular system. Int. J. Biochem. Cell Biol. 2005, 37, 751–764. [Google Scholar] [CrossRef]
- Chang, G.J.; Su, M.J.; Wu, T.S.; Chen, W.P.; Kuo, C.M. Electromechanical characterization of cinnamophilin, a natural thromboxane A2 receptor antagonist with anti-arrhythmic activity, in guinea-pig heart. Br. J. Pharmacol. 2008, 153, 110–123. [Google Scholar] [CrossRef]
- Billman, G.E. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacol. Ther. 2008, 120, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; O’Rourke, B. Mitochondria are sources of metabolic sink and arrhythmias. Pharmacol. Ther. 2011, 131, 287–294. [Google Scholar] [CrossRef]
- van Opbergen, C.J.M.; den Braven, L.; Delmar, M.; van Veen, T.A.B. Mitochondrial Dysfunction as Substrate for Arrhythmogenic Cardiomyopathy: A Search for New Disease Mechanisms. Front. Physiol. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Heinen, A.; Camara, A.K.; Aldakkak, M.; Rhodes, S.S.; Riess, M.L.; Stowe, D.F. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am. J. Physiol. Cell Physiol 2007, 292, C148–C156. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional Role of Mitochondria in Arrhythmogenesis. Adv. Exp. Med. Biol. 2017, 982, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, H. Role of ATP-sensitive K+ channels in cardiac arrhythmias. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 237–243. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Tarasova, N.V.; Vishnyakova, P.A.; Logashina, Y.A.; Elchaninov, A.V. Mitochondrial Calcium Uniporter Structure and Function in Different Types of Muscle Tissues in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4823. [Google Scholar] [CrossRef]
- Xie, A.; Song, Z.; Liu, H.; Zhou, A.; Shi, G.; Wang, Q.; Gu, L.; Liu, M.; Xie, L.H.; Qu, Z.; et al. Mitochondrial Ca2+ Influx Contributes to Arrhythmic Risk in Nonischemic Cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e007805. [Google Scholar] [CrossRef]
- Joseph, L.C.; Reyes, M.V.; Homan, E.A.; Gowen, B.; Avula, U.M.R.; Goulbourne, C.N.; Wan, E.Y.; Elrod, J.W.; Morrow, J.P. The mitochondrial calcium uniporter promotes arrhythmias caused by high-fat diet. Sci. Rep. 2021, 11, 17808. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shanmughapriya, S.; Tomar, D.; Siddiqui, N.; Lynch, S.; Nemani, N.; Breves, S.L.; Zhang, X.; Tripathi, A.; Palaniappan, P.; et al. Mitochondrial Ca2+ Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity. Mol. Cell 2017, 65, 1014–1028.e1017. [Google Scholar] [CrossRef]
- Bertero, E.; Nickel, A.; Kohlhaas, M.; Hohl, M.; Sequeira, V.; Brune, C.; Schwemmlein, J.; Abeßer, M.; Schuh, K.; Kutschka, I.; et al. Loss of Mitochondrial Ca2+ Uniporter Limits Inotropic Reserve and Provides Trigger and Substrate for Arrhythmias in Barth Syndrome Cardiomyopathy. Circulation 2021, 144, 1694–1713. [Google Scholar] [CrossRef]
- Liu, T.; Yang, N.; Sidor, A.; O’Rourke, B. MCU Overexpression Rescues Inotropy and Reverses Heart Failure by Reducing SR Ca2+ Leak. Circ. Res. 2021, 128, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Koshenov, Z.; Oflaz, F.E.; Hirtl, M.; Bachkoenig, O.A.; Rost, R.; Osibow, K.; Gottschalk, B.; Madreiter-Sokolowski, C.T.; Waldeck-Weiermair, M.; Malli, R.; et al. The contribution of uncoupling protein 2 to mitochondrial Ca2+ homeostasis in health and disease-A short revisit. Mitochondrion 2020, 55, 164–173. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef]
- Krauss, S.; Zhang, C.Y.; Lowell, B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Arsenijevic, D.; Onuma, H.; Pecqueur, C.; Raimbault, S.; Manning, B.S.; Miroux, B.; Couplan, E.; Alves-Guerra, M.C.; Goubern, M.; Surwit, R.; et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000, 26, 435–439. [Google Scholar] [CrossRef]
- Motloch, L.J.; Gebing, T.; Reda, S.; Schwaiger, A.; Wolny, M.; Hoppe, U.C. UCP3 Regulates Single-Channel Activity of the Cardiac mCa1. J. Membr. Biol. 2016, 249, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Larbig, R.; Reda, S.; Paar, V.; Trost, A.; Leitner, J.; Weichselbaumer, S.; Motloch, K.A.; Wernly, B.; Arrer, A.; Strauss, B.; et al. Through modulation of cardiac Ca2+ handling, UCP2 affects cardiac electrophysiology and influences the susceptibility for Ca2+ -mediated arrhythmias. Exp. Physiol. 2017, 102, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Gaspers, L.D.; Wang, G.; Thomas, A.P. Uncoupling protein-2 modulates myocardial excitation-contraction coupling. Circ. Res. 2010, 106, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W. Role of gap junctions in cardiac conduction and development: Insights from the connexin knockout mice. Circ. Res. 2000, 87, 346–348. [Google Scholar] [CrossRef]
- King, J.H.; Huang, C.L.; Fraser, J.A. Determinants of myocardial conduction velocity: Implications for arrhythmogenesis. Front. Physiol. 2013, 4, 154. [Google Scholar] [CrossRef]
- Sovari, A.A.; Rutledge, C.A.; Jeong, E.M.; Dolmatova, E.; Arasu, D.; Liu, H.; Vahdani, N.; Gu, L.; Zandieh, S.; Xiao, L.; et al. Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ. Arrhythm. Electrophysiol. 2013, 6, 623–631. [Google Scholar] [CrossRef]
- Lerner, D.L.; Yamada, K.A.; Schuessler, R.B.; Saffitz, J.E. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation 2000, 101, 547–552. [Google Scholar] [CrossRef]
- Boengler, K.; Leybaert, L.; Ruiz-Meana, M.; Schulz, R. Connexin 43 in Mitochondria: What Do We Really Know About Its Function? Front. Physiol. 2022, 13, 928934. [Google Scholar] [CrossRef]
- Papp, R.; Gönczi, M.; Kovács, M.; Seprényi, G.; Végh, A. Gap junctional uncoupling plays a trigger role in the antiarrhythmic effect of ischaemic preconditioning. Cardiovasc. Res. 2007, 74, 396–405. [Google Scholar] [CrossRef]
- Satoh, W.; Sato, H.; Kumasaka, K.; Shindoh, C.; Miura, M. B-PO02-019 Mitochondrial Connexin43 Is Involved in the Occurrence of Arrhythmias with Modulation of Mitochondrial KAtp Channels. Heart Rhythm 2021, 18, S103. [Google Scholar] [CrossRef]
- Oldenburg, O.; Cohen, M.V.; Yellon, D.M.; Downey, J.M. Mitochondrial K(ATP) channels: Role in cardioprotection. Cardiovasc. Res. 2002, 55, 429–437. [Google Scholar] [CrossRef]
- Abadir, P.M.; Foster, D.B.; Crow, M.; Cooke, C.A.; Rucker, J.J.; Jain, A.; Smith, B.J.; Burks, T.N.; Cohn, R.D.; Fedarko, N.S.; et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl. Acad. Sci. USA 2011, 108, 14849–14854. [Google Scholar] [CrossRef] [PubMed]
- Parodi-Rullan, R.; Barreto-Torres, G.; Ruiz, L.; Casasnovas, J.; Javadov, S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol. Biochem. 2012, 29, 841–850. [Google Scholar] [CrossRef]
- Escobales, N.; Nuñez, R.E.; Javadov, S. Mitochondrial angiotensin receptors and cardioprotective pathways. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1426–H1438. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Zhou, X.; Zhuo, J.L. Evidence for a Physiological Mitochondrial Angiotensin II System in the Kidney Proximal Tubules: Novel Roles of Mitochondrial Ang II/AT1a/O2− and Ang II/AT2/NO Signaling. Hypertension 2020, 76, 121–132. [Google Scholar] [CrossRef]
- Zhao, Z.; Fefelova, N.; Shanmugam, M.; Bishara, P.; Babu, G.J.; Xie, L.H. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J. Mol. Cell. Cardiol. 2011, 50, 128–136. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, L.; Zhuang, Z.; Hu, X.; Dong, D. Mitochondrial-Derived Peptides in Diabetes and Its Complications. Front. Endocrinol. 2021, 12, 808120. [Google Scholar] [CrossRef] [PubMed]
- Thummasorn, S.; Apaijai, N.; Kerdphoo, S.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc. Ther. 2016, 34, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Thummasorn, S.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. High-dose Humanin analogue applied during ischemia exerts cardioprotection against ischemia/reperfusion injury by reducing mitochondrial dysfunction. Cardiovasc. Ther. 2017, 35, e12289. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Gambardella, J.; Fiordelisi, A.; Iaccarino, G.; Illario, M. GRKs and β-Arrestins: “Gatekeepers” of Mitochondrial Function in the Failing Heart. Front. Pharmacol. 2019, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, K.; Pfleger, J.M.; Chuprun, K.; Barr, E.; Gao, E.; Koch, W.J. Abstract MP247: Mitochondrial Complex V Is Regulated By GRK2 in the Heart. Circ. Res. 2021, 129, AMP247. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, H.; Shen, J.; Wang, N.; Zhang, Y. Different roles of β-arrestin and the PKA pathway in mitochondrial ROS production induced by acute β-adrenergic receptor stimulation in neonatal mouse cardiomyocytes. Biochem. Biophys. Res. Commun. 2017, 489, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Singh, A.; Patil, S.; Dhiman, R.; et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int. J. Biochem. Cell Biol. 2021, 136, 106013. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Lee, M.S. Mitochondria and the NLRP3 inflammasome: Physiological and pathological relevance. Arch Pharm. Res. 2016, 39, 1503–1518. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Muna, A.P.; Veleva, T.; Molina, C.E.; Sutanto, H.; Tekook, M.; Wang, Q.; Abu-Taha, I.H.; Gorka, M.; Künzel, S.; et al. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circ. Res. 2020, 127, 1036–1055. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L., Jr.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.D.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Xie, A.; Kim, T.Y.; Terentyeva, R.; Liu, M.; Shi, G.; Feng, F.; Choi, B.R.; Terentyev, D.; et al. Interleukin-1β, Oxidative Stress, and Abnormal Calcium Handling Mediate Diabetic Arrhythmic Risk. JACC Basic. Transl. Sci. 2021, 6, 42–52. [Google Scholar] [CrossRef]
- Chen, Y.; Csordás, G.; Jowdy, C.; Schneider, T.G.; Csordás, N.; Wang, W.; Liu, Y.; Kohlhaas, M.; Meiser, M.; Bergem, S.; et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ. Res. 2012, 111, 863–875. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wen, Y.; Li, S.; Lu, X.; Xu, R.; Li, C. Endoplasmic Reticulum-Mitochondria Contacts: A Potential Therapy Target for Cardiovascular Remodeling-Associated Diseases. Front. Cell Dev. Biol. 2021, 9, 774989. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Fujimoto, T.; Tanaka, M.; Shirasawa, S. Tespa1 is a novel component of mitochondria-associated endoplasmic reticulum membranes and affects mitochondrial calcium flux. Biochem. Biophys. Res. Commun. 2013, 433, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Li, J.; Qi, X.; Ramos, K.S.; Lanters, E.; Keijer, J.; de Groot, N.; Brundel, B.; Zhang, D. Disruption of Sarcoplasmic Reticulum-Mitochondrial Contacts Underlies Contractile Dysfunction in Experimental and Human Atrial Fibrillation: A Key Role of Mitofusin 2. J. Am. Heart Assoc. 2022, 11, e024478. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003, 348, 2656–2668. [Google Scholar] [CrossRef]
- Bates, M.G.; Bourke, J.P.; Giordano, C.; d’Amati, G.; Turnbull, D.M.; Taylor, R.W. Cardiac involvement in mitochondrial DNA disease: Clinical spectrum, diagnosis, and management. Eur. Heart J. 2012, 33, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, C.; Lach, B.; Shoubridge, E.A. Variable distribution of mutant mitochondrial DNAs (tRNA(Leu[3243])) in tissues of symptomatic relatives with MELAS: The role of mitotic segregation. Neurology 1993, 43, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Majamaa-Voltti, K.; Turkka, J.; Kortelainen, M.L.; Huikuri, H.; Majamaa, K. Causes of death in pedigrees with the 3243A>G mutation in mitochondrial DNA. J. Neurol. Neurosurg. Psychiatry 2008, 79, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, G.; Tome-Esteban, M.; Dejthevaporn, C.; Rahman, S.; Hanna, M.G.; Elliott, P.M. Prevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain disease. Eur. J. Heart Fail. 2010, 12, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kabunga, P.; Lau, A.K.; Phan, K.; Puranik, R.; Liang, C.; Davis, R.L.; Sue, C.M.; Sy, R.W. Systematic review of cardiac electrical disease in Kearns-Sayre syndrome and mitochondrial cytopathy. Int. J. Cardiol. 2015, 181, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Behjati, M.; Sabri, M.R.; Etemadi Far, M.; Nejati, M. Cardiac complications in inherited mitochondrial diseases. Heart Fail. Rev. 2021, 26, 391–403. [Google Scholar] [CrossRef]
- Finsterer, J.; Kothari, S. Cardiac manifestations of primary mitochondrial disorders. Int. J. Cardiol. 2014, 177, 754–763. [Google Scholar] [CrossRef]
- Oginosawa, Y.; Abe, H.; Nagatomo, T.; Mizuki, T.; Nakashima, Y. Sustained polymorphic ventricular tachycardia unassociated with QT prolongation or bradycardia in the Kearns-Sayre syndrome. Pacing Clin. Electrophysiol. 2003, 26, 1911–1912. [Google Scholar] [CrossRef]
- Wilmin, S.; De Bels, D.; Knecht, S.; Gottignies, P.; Gazagnes, M.D.; Devriendt, J. Torsade de pointes in Kearns-Sayre syndrome. Pract. Neurol. 2012, 12, 199–201. [Google Scholar] [CrossRef]
- Nakano, T.; Imanaka, K.; Uchida, H.; Isaka, N.; Takezawa, H. Myocardial ultrastructure in Kearns-Sayre syndrome. Angiology 1987, 38, 28–35. [Google Scholar] [CrossRef]
- Katsanos, K.H.; Pappas, C.J.; Patsouras, D.; Michalis, L.K.; Kitsios, G.; Elisaf, M.; Tsianos, E.V. Alarming atrioventricular block and mitral valve prolapse in the Kearns-Sayre syndrome. Int. J. Cardiol. 2002, 83, 179–181. [Google Scholar] [CrossRef]
- Wahbi, K.; Larue, S.; Jardel, C.; Meune, C.; Stojkovic, T.; Ziegler, F.; Lombès, A.; Eymard, B.; Duboc, D.; Laforêt, P. Cardiac involvement is frequent in patients with the m.8344A>G mutation of mitochondrial DNA. Neurology 2010, 74, 674–677. [Google Scholar] [CrossRef]
- Majamaa-Voltti, K.; Peuhkurinen, K.; Kortelainen, M.L.; Hassinen, I.E.; Majamaa, K. Cardiac abnormalities in patients with mitochondrial DNA mutation 3243A>G. BMC Cardiovasc. Disord 2002, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Nikoskelainen, E.K.; Savontaus, M.L.; Huoponen, K.; Antila, K.; Hartiala, J. Pre-excitation syndrome in Leber’s hereditary optic neuropathy. Lancet 1994, 344, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Ichida, F. Left ventricular noncompaction. Circ J 2009, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Baris, O.R.; Ederer, S.; Neuhaus, J.F.; von Kleist-Retzow, J.C.; Wunderlich, C.M.; Pal, M.; Wunderlich, F.T.; Peeva, V.; Zsurka, G.; Kunz, W.S.; et al. Mosaic Deficiency in Mitochondrial Oxidative Metabolism Promotes Cardiac Arrhythmia during Aging. Cell Metab. 2015, 21, 667–677. [Google Scholar] [CrossRef]
- Wang, J.; Wilhelmsson, H.; Graff, C.; Li, H.; Oldfors, A.; Rustin, P.; Brüning, J.C.; Kahn, C.R.; Clayton, D.A.; Barsh, G.S.; et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 1999, 21, 133–137. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Daneshgar, N.; Chu, Y.; Chen, B.; Hefti, M.; Vikram, A.; Irani, K.; Song, L.S.; Brenner, C.; Abel, E.D.; et al. Metabolic rescue ameliorates mitochondrial encephalo-cardiomyopathy in murine and human iPSC models of Leigh syndrome. Clin. Transl. Med. 2022, 12, e954. [Google Scholar] [CrossRef]
- Anan, R.; Nakagawa, M.; Miyata, M.; Higuchi, I.; Nakao, S.; Suehara, M.; Osame, M.; Tanaka, H. Cardiac involvement in mitochondrial diseases. A study on 17 patients with documented mitochondrial DNA defects. Circulation 1995, 91, 955–961. [Google Scholar] [CrossRef]
- Yang, B.; Huang, Y.; Zhang, H.; Huang, Y.; Zhou, H.J.; Young, L.; Xiao, H.; Min, W. Mitochondrial thioredoxin-2 maintains HCN4 expression and prevents oxidative stress-mediated sick sinus syndrome. J. Mol. Cell. Cardiol. 2020, 138, 291–303. [Google Scholar] [CrossRef]
- Li, Q.; Su, D.; O’Rourke, B.; Pogwizd, S.M.; Zhou, L. Mitochondria-derived ROS bursts disturb Ca2+ cycling and induce abnormal automaticity in guinea pig cardiomyocytes: A theoretical study. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H623–H636. [Google Scholar] [CrossRef]
- Yaniv, Y.; Juhaszova, M.; Lyashkov, A.E.; Spurgeon, H.A.; Sollott, S.J.; Lakatta, E.G. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. J. Mol. Cell. Cardiol. 2011, 51, 740–748. [Google Scholar] [CrossRef]
- Ren, L.; Gopireddy, R.R.; Perkins, G.; Zhang, H.; Timofeyev, V.; Lyu, Y.; Diloretto, D.A.; Trinh, P.; Sirish, P.; Overton, J.L.; et al. Disruption of mitochondria-sarcoplasmic reticulum microdomain connectomics contributes to sinus node dysfunction in heart failure. Proc. Natl. Acad. Sci. USA 2022, 119, e2206708119. [Google Scholar] [CrossRef]
- Donoghue, M.; Wakimoto, H.; Maguire, C.T.; Acton, S.; Hales, P.; Stagliano, N.; Fairchild-Huntress, V.; Xu, J.; Lorenz, J.N.; Kadambi, V.; et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J. Mol. Cell. Cardiol. 2003, 35, 1043–1053. [Google Scholar] [CrossRef]
- Xiao, H.D.; Fuchs, S.; Campbell, D.J.; Lewis, W.; Dudley, S.C., Jr.; Kasi, V.S.; Hoit, B.D.; Keshelava, G.; Zhao, H.; Capecchi, M.R.; et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am. J. Pathol. 2004, 165, 1019–1032. [Google Scholar] [CrossRef]
- Yang, K.C.; Bonini, M.G.; Dudley, S.C., Jr. Mitochondria and arrhythmias. Free Radic. Biol. Med. 2014, 71, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Uguccioni, G.; Hood, D.A. The importance of PGC-1alpha in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E361–E371. [Google Scholar] [CrossRef] [PubMed]
- Holgersen, E.M.; Gandhi, S.; Zhou, Y.; Kim, J.; Vaz, B.; Bogojeski, J.; Bugno, M.; Shalev, Z.; Cheung-Ong, K.; Goncalves, J.; et al. Transcriptome-Wide Off-Target Effects of Steric-Blocking Oligonucleotides. Nucleic. Acid. Ther. 2021, 31, 392–403. [Google Scholar] [CrossRef]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Khan, R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc. Res. 2004, 64, 387–394. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Wakili, R.; Voigt, N.; Kääb, S.; Dobrev, D.; Nattel, S. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Investig. 2011, 121, 2955–2968. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Holm, H.; Gretarsdottir, S.; Thorleifsson, G.; Walters, G.B.; Thorgeirsson, G.; Gulcher, J.; Mathiesen, E.B.; Njølstad, I.; Nyrnes, A.; et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat. Genet. 2009, 41, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Ellinor, P.T.; Lunetta, K.L.; Albert, C.M.; Glazer, N.L.; Ritchie, M.D.; Smith, A.V.; Arking, D.E.; Müller-Nurasyid, M.; Krijthe, B.P.; Lubitz, S.A.; et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat. Genet. 2012, 44, 670–675. [Google Scholar] [CrossRef]
- Dridi, H.; Kushnir, A.; Zalk, R.; Yuan, Q.; Melville, Z.; Marks, A.R. Intracellular calcium leak in heart failure and atrial fibrillation: A unifying mechanism and therapeutic target. Nat. Rev. Cardiol. 2020, 17, 732–747. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, M.; van Marion, D.M.S.; Wüst, R.C.I.; Houtkooper, R.H.; Zhang, D.; Groot, N.M.S.; Henning, R.H.; Brundel, B. Mitochondrial Dysfunction Underlies Cardiomyocyte Remodeling in Experimental and Clinical Atrial Fibrillation. Cells 2019, 8, 1202. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Bao, L.; Kefaloyianni, E.; Taskin, E.; Okorie, U.; Hong, M.; Dhar-Chowdhury, P.; Kaneko, M.; Coetzee, W.A. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J. Mol. Cell. Cardiol. 2012, 52, 410–418. [Google Scholar] [CrossRef]
- Sasaki, N.; Sato, T.; Marbán, E.; O’Rourke, B. ATP consumption by uncoupled mitochondria activates sarcolemmal K(ATP) channels in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol 2001, 280, H1882–H1888. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Dudley, S.C., Jr. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ. Res. 2010, 107, 967–974. [Google Scholar] [CrossRef]
- Yoo, S.; Aistrup, G.; Shiferaw, Y.; Ng, J.; Mohler, P.J.; Hund, T.J.; Waugh, T.; Browne, S.; Gussak, G.; Gilani, M.; et al. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight 2018, 3, e120728. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Xie, W.; Betzenhauser, M.; Reiken, S.; Chen, B.X.; Wronska, A.; Marks, A.R. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 2012, 111, 708–717. [Google Scholar] [CrossRef]
- Schrickel, J.W.; Bielik, H.; Yang, A.; Schimpf, R.; Shlevkov, N.; Burkhardt, D.; Meyer, R.; Grohé, C.; Fink, K.; Tiemann, K.; et al. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic. Res. Cardiol. 2002, 97, 452–460. [Google Scholar] [CrossRef]
- Lyu, J.J.; Mehta, J.L.; Li, Y.; Ye, L.; Sun, S.N.; Sun, H.S.; Li, J.C.; Zhang, D.M.; Wei, J. Mitochondrial Autophagy and NLRP3 Inflammasome in Pulmonary Tissues from Severe Combined Immunodeficient Mice after Cardiac Arrest and Cardiopulmonary Resuscitation. Chin. Med. J. 2018, 131, 1174–1184. [Google Scholar] [CrossRef]
- Bukowska, A.; Schild, L.; Keilhoff, G.; Hirte, D.; Neumann, M.; Gardemann, A.; Neumann, K.H.; Röhl, F.W.; Huth, C.; Goette, A.; et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp. Biol. Med. 2008, 233, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, J.; Zhang, M.; Liu, G.; Wang, X.; Liu, Y.; Yang, N.; Liu, Y.; Zhao, G.; Sun, J.; et al. β3-Adrenoceptor Impairs Mitochondrial Biogenesis and Energy Metabolism during Rapid Atrial Pacing-Induced Atrial Fibrillation. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Meng, L.; Lee, S.; Tse, G.; Gong, M.; Zhang, Z.; Zhao, J.; Zhao, Y.; Li, G.; Liu, T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2019, 18, 165. [Google Scholar] [CrossRef]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Muller-Hocker, J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart—An age-related phenomenon. A histochemical ultracytochemical study. Am. J. Pathol. 1989, 134, 1167–1173. [Google Scholar]
- Krishnan, K.J.; Greaves, L.C.; Reeve, A.K.; Turnbull, D. The ageing mitochondrial genome. Nucleic. Acids Res. 2007, 35, 7399–7405. [Google Scholar] [CrossRef]
- Emelyanova, L.; Preston, C.; Gupta, A.; Viqar, M.; Negmadjanov, U.; Edwards, S.; Kraft, K.; Devana, K.; Holmuhamedov, E.; O’Hair, D.; et al. Effect of Aging on Mitochondrial Energetics in the Human Atria. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 608–616. [Google Scholar] [CrossRef]
- Moslehi, J.; DePinho, R.A.; Sahin, E. Telomeres and mitochondria in the aging heart. Circ. Res. 2012, 110, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Anjaneyulu, M.; Inoue, T.; Choi, J.; Sagi, A.R.; Chen, C.; Ide, T.; Russell, J.W. Mitochondrial transcription factor A regulation of mitochondrial degeneration in experimental diabetic neuropathy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E132–E141. [Google Scholar] [CrossRef]
- Jeganathan, J.; Saraf, R.; Mahmood, F.; Pal, A.; Bhasin, M.K.; Huang, T.; Mittel, A.; Knio, Z.; Simons, R.; Khabbaz, K.; et al. Mitochondrial Dysfunction in Atrial Tissue of Patients Developing Postoperative Atrial Fibrillation. Ann. Thorac. Surg. 2017, 104, 1547–1555. [Google Scholar] [CrossRef]
- Liu, C.; Bai, J.; Dan, Q.; Yang, X.; Lin, K.; Fu, Z.; Lu, X.; Xie, X.; Liu, J.; Fan, L.; et al. Mitochondrial Dysfunction Contributes to Aging-Related Atrial Fibrillation. Oxid. Med. Cell Longev. 2021, 2021, 5530293. [Google Scholar] [CrossRef] [PubMed]
- Edling, C.E.; Fazmin, I.T.; Saadeh, K.; Chadda, K.R.; Ahmad, S.; Valli, H.; Huang, C.L.; Jeevaratnam, K. Molecular basis of arrhythmic substrate in ageing murine peroxisome proliferator-activated receptor gamma co-activator deficient hearts modelling mitochondrial dysfunction. Biosci. Rep. 2019, 39, BSR20190403. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.N.; Theiss, A.L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 2020, 11, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Prochnicki, T.; Latz, E. Inflammasomes on the Crossroads of Innate Immune Recognition and Metabolic Control. Cell Metab. 2017, 26, 71–93. [Google Scholar] [CrossRef]
- Ferko, M.; Andelová, N.; Szeiffová Bačová, B.; Jašová, M. Myocardial Adaptation in Pseudohypoxia: Signaling and Regulation of mPTP via Mitochondrial Connexin 43 and Cardiolipin. Cells 2019, 8, 1449. [Google Scholar] [CrossRef]
- Gawałko, M.; Agbaedeng, T.A.; Saljic, A.; Müller, D.N.; Wilck, N.; Schnabel, R.; Penders, J.; Rienstra, M.; van Gelder, I.; Jespersen, T.; et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc. Res. 2022, 118, 2415–2427. [Google Scholar] [CrossRef]
- Wan, E.; Abrams, J.; Weinberg, R.L.; Katchman, A.N.; Bayne, J.; Zakharov, S.I.; Yang, L.; Morrow, J.P.; Garan, H.; Marx, S.O. Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J. Clin. Investig. 2016, 126, 112–122. [Google Scholar] [CrossRef]
- Avula, U.M.R.; Dridi, H.; Chen, B.X.; Yuan, Q.; Katchman, A.N.; Reiken, S.R.; Desai, A.D.; Parsons, S.; Baksh, H.; Ma, E.; et al. Attenuating persistent sodium current-induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress. JCI Insight 2021, 6, e147371. [Google Scholar] [CrossRef] [PubMed]
- Krokhaleva, Y.; Vaseghi, M. Update on prevention and treatment of sudden cardiac arrest. Trends Cardiovasc. Med. 2019, 29, 394–400. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Gong, Y.; Zhang, J.; Zang, Y.; Xia, L. Exploring Impaired SERCA Pump-Caused Alternation Occurrence in Ischemia. Comput. Math. Methods Med. 2019, 2019, 8237071. [Google Scholar] [CrossRef] [PubMed]
- Gazmuri, R.J.; Radhakrishnan, J.; Ayoub, I.M. Sodium-Hydrogen Exchanger Isoform-1 Inhibition: A Promising Pharmacological Intervention for Resuscitation from Cardiac Arrest. Molecules 2019, 24, 1765. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.P.; Teshima, Y.; Akao, M.; Marbán, E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2003, 93, 697–699. [Google Scholar] [CrossRef]
- Acosta, D.; Ramos, K.; Li-Goldman, C.P. Cell injury of cultured rat myocardial cells after reoxygenation of hypoxic cultures in the presence and absence of calcium. In Vitro 1984, 20, 642–646. [Google Scholar] [CrossRef]
- Shibayama, J.; Taylor, T.G.; Venable, P.W.; Rhodes, N.L.; Gil, R.B.; Warren, M.; Wende, A.R.; Abel, E.D.; Cox, J.; Spitzer, K.W.; et al. Metabolic determinants of electrical failure in ex-vivo canine model of cardiac arrest: Evidence for the protective role of inorganic pyrophosphate. PLoS ONE 2013, 8, e57821. [Google Scholar] [CrossRef]
- Szendrei, L.; Turoczi, T.; Kovacs, P.; Vecsernyes, M.; Das, D.K.; Tosaki, A. Mitochondrial gene expression and ventricular fibrillation in ischemic/reperfused nondiabetic and diabetic myocardium. Biochem. Pharmacol. 2002, 63, 543–552. [Google Scholar] [CrossRef]
- García-Rivas Gde, J.; Carvajal, K.; Correa, F.; Zazueta, C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br. J. Pharmacol. 2006, 149, 829–837. [Google Scholar] [CrossRef]
- Fischbach, P.S.; White, A.; Barrett, T.D.; Lucchesi, B.R. Risk of ventricular proarrhythmia with selective opening of the myocardial sarcolemmal versus mitochondrial ATP-gated potassium channel. J. Pharmacol. Exp. Ther. 2004, 309, 554–559. [Google Scholar] [CrossRef]
- Stöckigt, F.; Beiert, T.; Knappe, V.; Baris, O.R.; Wiesner, R.J.; Clemen, C.S.; Nickenig, G.; Andrié, R.P.; Schrickel, J.W. Aging-related mitochondrial dysfunction facilitates the occurrence of serious arrhythmia after myocardial infarction. Biochem. Biophys. Res. Commun. 2017, 493, 604–610. [Google Scholar] [CrossRef]
- Fang, X.; Huang, Z.; Zhu, J.; Jiang, L.; Li, H.; Fu, Y.; Sun, S.; Tang, W. Ultrastructural evidence of mitochondrial abnormalities in postresuscitation myocardial dysfunction. Resuscitation 2012, 83, 386–394. [Google Scholar] [CrossRef]
- Wang, S.; Radhakrishnan, J.; Ayoub, I.M.; Kolarova, J.D.; Taglieri, D.M.; Gazmuri, R.J. Limiting sarcolemmal Na+ entry during resuscitation from ventricular fibrillation prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J. Appl. Physiol. 2007, 103, 55–65. [Google Scholar] [CrossRef]
- Geng, L.; Wang, Z.; Cui, C.; Zhu, Y.; Shi, J.; Wang, J.; Chen, M. Rapid Electrical Stimulation Increased Cardiac Apoptosis Through Disturbance of Calcium Homeostasis and Mitochondrial Dysfunction in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Physiol. Biochem. 2018, 47, 1167–1180. [Google Scholar] [CrossRef]
- O’Rourke, B.; Kass, D.A.; Tomaselli, G.F.; Kääb, S.; Tunin, R.; Marbán, E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: Experimental studies. Circ. Res. 1999, 84, 562–570. [Google Scholar] [CrossRef]
- Zang, Y.L.; Xia, L. Cellular mechanism of cardiac alternans: An unresolved chicken or egg problem. J. Zhejiang Univ. Sci. B 2014, 15, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pogwizd, S.M.; Qi, M.; Yuan, W.; Samarel, A.M.; Bers, D.M. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 1999, 85, 1009–1019. [Google Scholar] [CrossRef]
- Hegyi, B.; Bossuyt, J.; Griffiths, L.G.; Shimkunas, R.; Coulibaly, Z.; Jian, Z.; Grimsrud, K.N.; Sondergaard, C.S.; Ginsburg, K.S.; Chiamvimonvat, N.; et al. Complex electrophysiological remodeling in postinfarction ischemic heart failure. Proc. Natl. Acad. Sci. USA 2018, 115, E3036–E3044. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Talanov, E.Y.; Tenkov, K.S.; Starinets, V.S.; Mikheeva, I.B.; Belosludtsev, K.N. Transport of Ca2+ and Ca2+-dependent permeability transition in heart mitochondria in the early stages of Duchenne muscular dystrophy. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148250. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.C.; Ramos, S.V.; Turnbull, P.C.; Edgett, B.A.; Huber, J.S.; Polidovitch, N.; Schlattner, U.; Backx, P.H.; Simpson, J.A.; Perry, C.G.R. Impairments in left ventricular mitochondrial bioenergetics precede overt cardiac dysfunction and remodelling in Duchenne muscular dystrophy. J. Physiol. 2020, 598, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Rubi, L.; Obermair, G.J.; Cervenka, R.; Dang, X.B.; Lukacs, P.; Kummer, S.; Bittner, R.E.; Kubista, H.; Todt, H.; et al. Enhanced currents through L-type calcium channels in cardiomyocytes disturb the electrophysiology of the dystrophic heart. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H564–H573. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, J.; Thireau, J.; Reiken, S.; Cassan, C.; Richard, S.; Matecki, S.; Marks, A.R.; Lacampagne, A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
| Inheritance Pattern | Clinical Syndrome | Mutations | Affected Genes/Proteins in Mitochondria | Arrhythmias Involved | Cardiac Manifestations | Other Systems Involved |
|---|---|---|---|---|---|---|
| Maternally Transmitted | Myoclonic epilepsy with ragged red fibers (MERRF) [35,36] | m.8344 A to G, m.8356 T to C, m.8363 G to A, and m.8361 G to A | MT-TK | Pre-excitation | Dilated and histiocytoid cardiomyopathy | Myoclonus, spasticity, myopathy |
| Maternally Transmitted | Leber hereditary optic neuropathy [37] | m.3460 G to A, m.11778 G to A, and m.14484 T to C | MT-ND1, MT-ND4, MT-ND4L, or MT-ND6 | Sudden death | Dilated cardiac myopathy | Loss of vision |
| Maternally Transmitted/Sporadic | Neuropathy, ataxia, and retinitis pigmentosa (NARP) [38,39] | m.8993 T to G | MT-ATP6 | Conduction block | Cardiomyopathy | Psychomotor retardation, epilepsy, ataxia, neuropathy, and myopathy |
| Maternally Transmitted | Leigh syndrome (Mt DNA associated subtype) [40] | More than 75 monogenic causes | MT-TL-1, MT-TK, MT-TI | Conduction block | Hypertrophic cardiomyopathy | Psychomotor regression, respiratory failure, muscular and movement disorder (death at young age) |
| Maternally Transmitted/Sporadic | Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) [41] | m.3243 A to G mutation in MT-TL-1 | MT-TL1, MT-TK, and MT-TE genes provide instructions for making tRNAs | Pre-excitation, bundle branch block | Dilated/hypertrophic cardiomyopathy | Severe encephalopathy, lactic acidosis, myoclonus |
| Maternally Transmitted/Sporadic | Kearns–Sayre syndrome [42,43] | 4.9 kb Mt DNA deletion (12 genes)/point mutation | Not determined, involved in mitochondrial protein expression and oxidative phosphorylation | Atrioventricular conduction defects | Cardiomyopathy, syncope, Adams–Stokes syndrome, sudden cardiac death | Anemia, myopathy, lactic acidosis, CNS abnormality, endocrine abnormality, renal disease, sensorineural deafness, and retinal involvement |
| X-Linked Recessive | Barth syndrome [44] | Mutations or deletions of the highly conserved Xq28 tafazzin (TAZ) gene | Tafazzin protein is essential for remodeling of cardiolipin, a principal phospholipid of the inner mitochondrial membrane | Ventricular arrhythmia, sudden cardiac death, prolonged QTc interval | Dilated/hypertrophic cardiomyopathy, endocardial fibroelastosis (EFE), left ventricular non-compaction (LVNC) | Skeletal myopathy, growth delay, neutropenia and increased urinary excretion of 3-methylglutaconic acid (3-MGCA) |
| Autosomal Recessive | Friedreich’s Ataxia[45] | GAA triplet repeat expansion in the first intron Frataxin (FXN) gene, silencing the gene | Frataxin: expressed in the mitochondria of tissue with high metabolic rates, involved in assembly of iron-sulfur clusters | Conduction block, atrial fibrillation, atrial/ventricular tachycardias, ECG repolarization abnormalities | Hypertrophic cardiomyopathy, heart failure | Gait and limb ataxia, dysarthria, loss of lower limb reflexes, optic neuropathy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Jiang, Y.; Chen, Z.B.; Rhee, J.-W.; Deng, Y.; Wang, Z.V. Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells 2023, 12, 679. https://doi.org/10.3390/cells12050679
Deng J, Jiang Y, Chen ZB, Rhee J-W, Deng Y, Wang ZV. Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells. 2023; 12(5):679. https://doi.org/10.3390/cells12050679
Chicago/Turabian StyleDeng, Jielin, Yunqiu Jiang, Zhen Bouman Chen, June-Wha Rhee, Yingfeng Deng, and Zhao V. Wang. 2023. "Mitochondrial Dysfunction in Cardiac Arrhythmias" Cells 12, no. 5: 679. https://doi.org/10.3390/cells12050679
APA StyleDeng, J., Jiang, Y., Chen, Z. B., Rhee, J.-W., Deng, Y., & Wang, Z. V. (2023). Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells, 12(5), 679. https://doi.org/10.3390/cells12050679







