Evaluation of Possible Neobavaisoflavone Chemosensitizing Properties towards Doxorubicin and Etoposide in SW1783 Anaplastic Astrocytoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cell Count Assay
2.5. Detection of Apoptotic Cells
2.6. Mitochondrial Membrane Potential Assay
2.7. Analysis of Cell Cycle
2.8. Statistical Analysis
3. Results
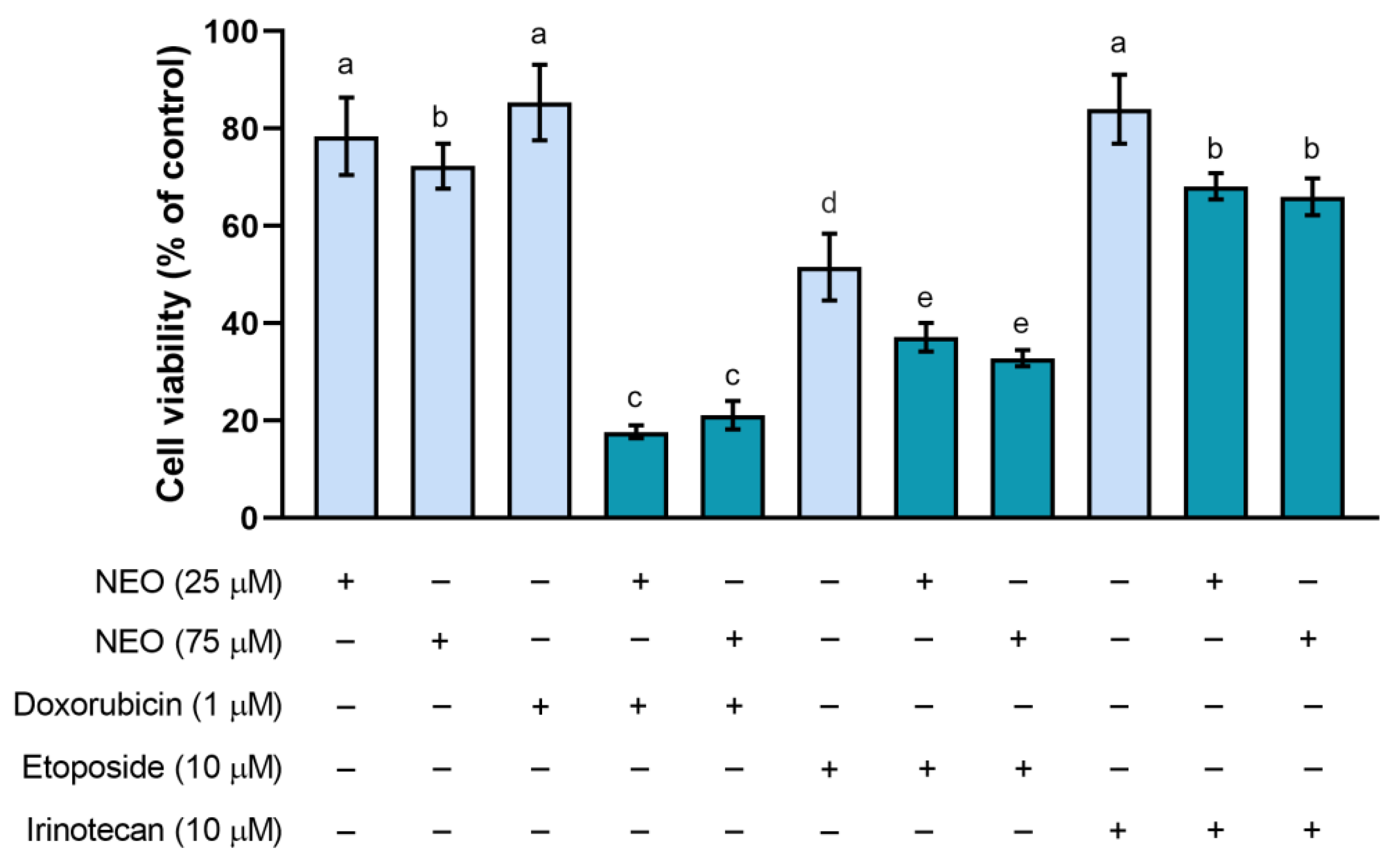
3.1. The Influence of NEO, Doxorubicin, Etoposide, and Irinotecan on the Cell Viability in Single and Combined Treatments
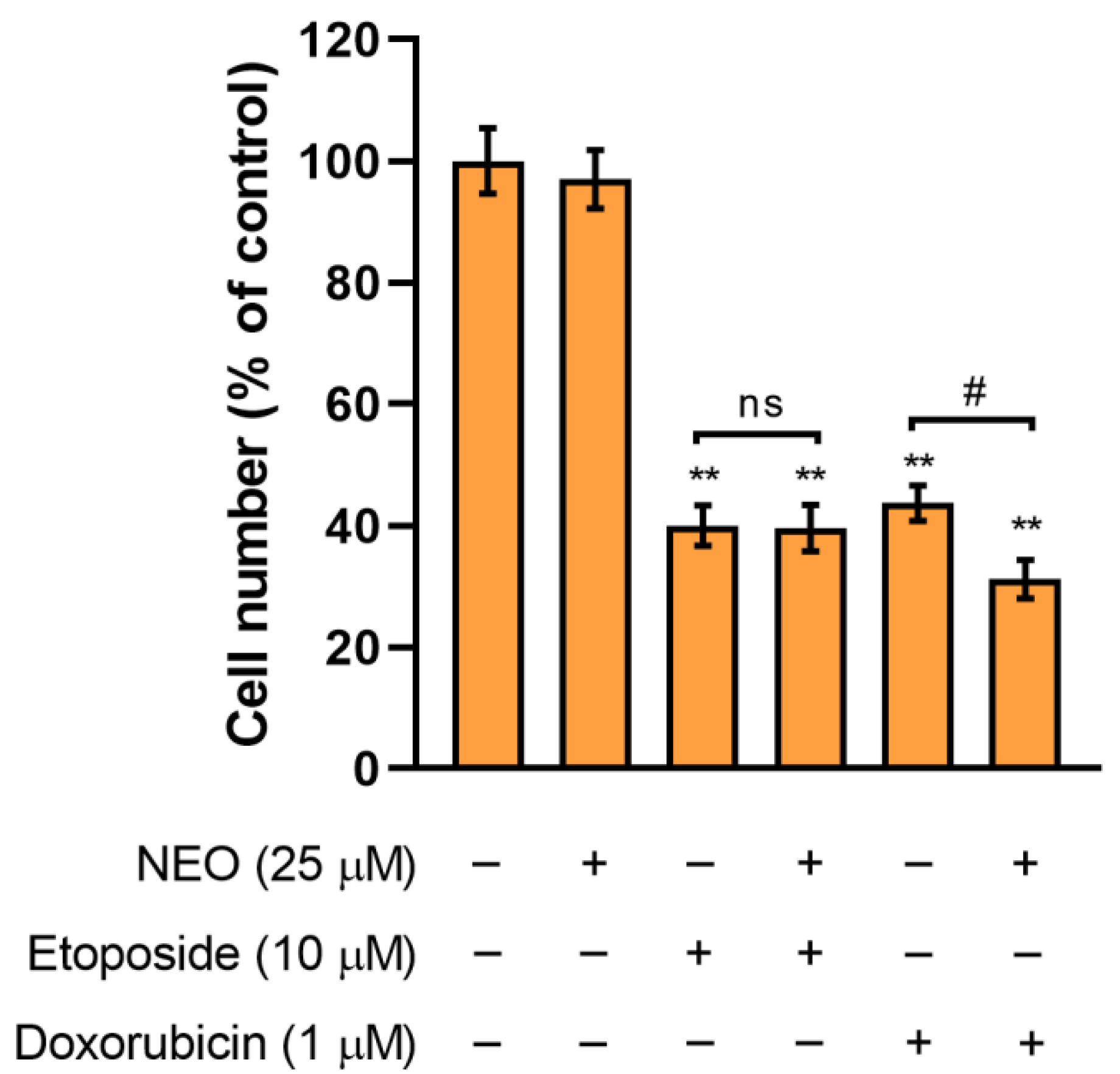
3.2. The Assessment of SW1783 Cell Growth Incubated with Doxorubicin or Etoposide Alone and in Combination with NEO
3.3. Combination of NEO and Etoposide Increases Apoptotic Subpopulation in SW1783 Cells
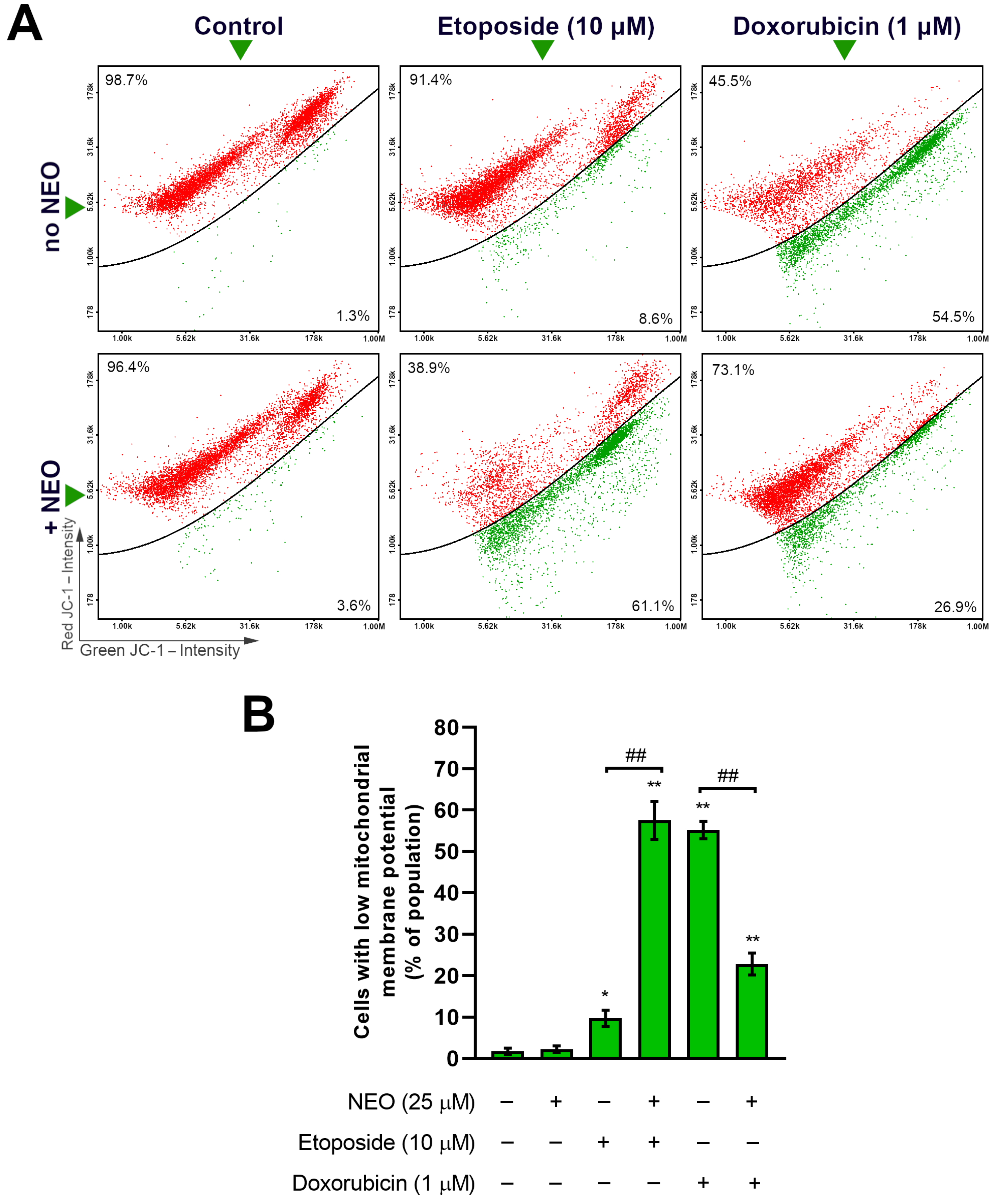
3.4. Co-Treatment of NEO and Doxorubicin or Etoposide Prompts Changes in the Mitochondrial Membrane Potential of SW1783 Cells
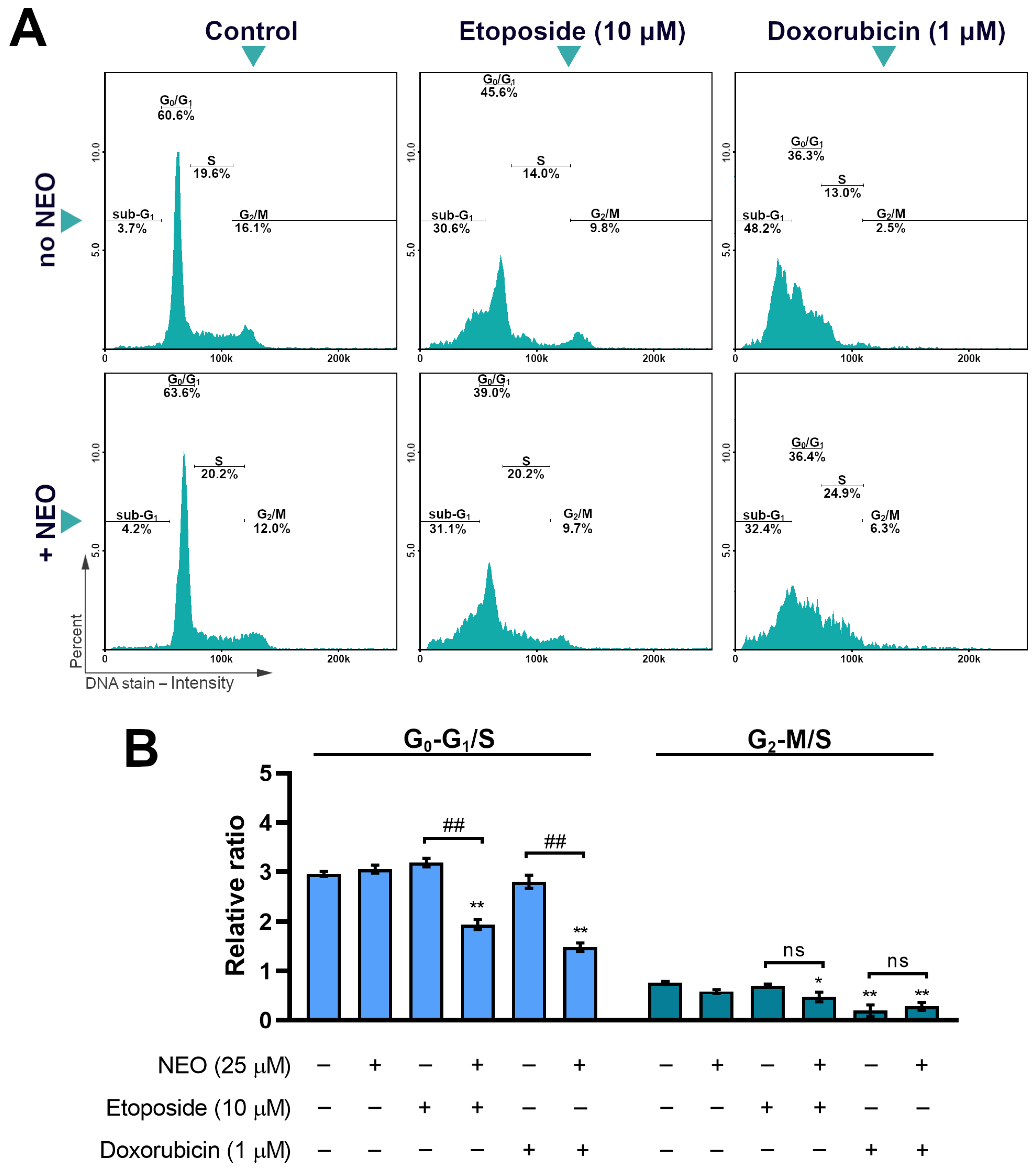
3.5. The Analysis of Cell Cycle in SW1783 Cells Treated with Doxorubicin or Etoposide Alone and in Combination with NEO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food. Sci. Nutr. 2018, 60, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Marunaka, Y. Metabolic functions of flavonoids: From human epidemiology to molecular mechanism. Neuropeptides 2021, 88, 102163. [Google Scholar] [CrossRef]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary Flavonoids and the risk of colorectal cancer: An updated meta-analysis of epidemiological studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, Flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef]
- Nde, C.; Zingue, S.; Winter, E.; Creczynski-Pasa, T.; Michel, T.; Fernandez, X.; Njamen, D.; Clyne, C. Flavonoids, breast cancer chemopreventive and/or chemotherapeutic agents. Curr. Med. Chem. 2015, 22, 3434–3446. [Google Scholar] [CrossRef]
- Geybels, M.S.; Verhage, B.A.; Arts, I.C.; Van Schooten, F.J.; Goldbohm, R.A.; Van den Brandt, P.A. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am. J. Epidemiol. 2013, 177, 1388–1398. [Google Scholar] [CrossRef]
- Sak, K. Current epidemiological knowledge about the role of flavonoids in prostate carcinogenesis. Exp. Oncol. 2017, 39, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Reale, G.; Russo, G.I.; Di Mauro, M.; Regis, F.; Campisi, D.; Giudice, A.L.; Marranzano, M.; Ragusa, R.; Castelli, T.; Cimino, S.; et al. Association between dietary flavonoids intake and prostate cancer risk: A case-control study in Sicily. Complement. Ther. Med. 2018, 39, 14–18. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer. 2017, 36, 50. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.; Pavan, I.C.; Mancini, M.C.; Da Silva, L.G.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.; Simabuco, F.M. The hallmarks of flavonoids in cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef]
- Mai, Z.; Blackburn, G.L.; Zhou, J.R. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog. 2007, 46, 534–542. [Google Scholar] [CrossRef]
- Solomon, L.A.; Ali, S.; Banerjee, S.; Munkarah, A.R.; Morris, R.T.; Sarkar, F.H. Sensitization of ovarian cancer cells to cisplatin bygenistein: The role of NF-kappaB. J. Ovarian Res. 2008, 1, 9. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Basak, N.; Caglayan, B.; Kilic, U.; Sahin, F.; Kucuk, O. Sensitization of cervical cancer cells to cisplatin by genistein: The role of NFκB and Akt/mTOR signaling pathways. J. Oncol. 2012, 2012, 461562. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef]
- Jia, H.; Yang, Q.; Wang, T.; Cao, Y.; Jiang, Q.Y.; Ma, H.D.; Sun, H.W.; Hou, M.X.; Yang, Y.P.; Feng, F. Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents. Biochim. Biophys. Acta 2016, 1860, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Nozhat, Z.; Heydarzadeh, S.; Memariani, Z.; Ahmadi, A. Chemoprotective and chemosensitizing effects of apigenin on cancer therapy. Cancer Cell Int. 2021, 21, 574. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol can reverse the 5-Fu resistance of colorectal cancer cells by inhibiting PKM2-mediated glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.-W.; Morgan, E.R.; Mason, W.P. Contemporary management of high-grade gliomas. CNS Oncol. 2018, 7, 51–65. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, F.; Qi Xie, X. Advanced treatment in high-grade gliomas. J. BUON 2019, 24, 424–430. [Google Scholar] [PubMed]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Noiphithak, R.; Veerasarn, K. Clinical Predictors for survival and treatment outcome of high-grade glioma in Prasat Neurological Institute. Asian J. Neurosurg. 2017, 12, 28–33. [Google Scholar] [CrossRef]
- Taylor, J.W.; Schiff, D. Treatment considerations for MGMT-unmethylated glioblastoma. Curr. Neurol. Neurosci. Rep. 2014, 15, 507. [Google Scholar] [CrossRef] [PubMed]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lobardi, G. Recurrent glioblastoma: From molecular landscape to new treatment perspectives. Cancers 2020, 13, 47. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Dayani, F.; Morshed, R.A.; Okada, H.; Aghi, M.K. Immunotherapy for high-grade gliomas: A clinical update and practical considerations for neurosurgeons. World Neurosurg. 2019, 124, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Núñez, F.J.; Haase, S.; McClellan, B.L.; Faisal, S.M.; Carney, S.V.; Yu, J.; Alghamri, M.S.; Asad, A.S.; Candia, A.J.N.; et al. Current Approaches for Glioma Gene Therapy and Virotherapy. Front. Mol. Neurosci. 2021, 14, 621831. [Google Scholar] [CrossRef]
- Fulton, D.; Urtasun, R.; Forsyth, P. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma. J. Neurooncol. 1996, 27, 149–155. [Google Scholar] [CrossRef]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C.; et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer 2004, 100, 1199–1207. [Google Scholar] [CrossRef]
- Leonard, A.; Wolff, J.E. Etoposide improves survival in high-grade glioma: A meta-analysis. Anticancer Res. 2013, 33, 3307–3315. [Google Scholar] [PubMed]
- Carrillo, J.A.; Hsu, F.P.K.; Delashaw, J.; Bota, D.A. Efficacy and safety of bevacizumab and etoposide combination in patients with recurrent malignant gliomas who have failed bevacizumab. RHC 2014, 5, 23–32. [Google Scholar] [CrossRef]
- Horescu, C.; Cioc, C.E.; Tuta, C.; Sevastre, A.S.; Tache, D.E.; Alexandru, O.; Artene, S.A.; Danoiu, S.; Dricu, A.; Oana, P.S. The effect of temozolomide in combination with doxorubicin in glioblastoma cells in vitro. J. Immunoass. Immunochem. 2020, 31, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.Q.; Li, Y.; Hou, Y.J.; Yang, M.X.; Fu, X.Q.; Zhao, B.S.; Jiang, H.M.; Fu, X.Y. Enhanced anticancer efficiency of doxorubicin against human glioma by natural borneol through triggering ROS-mediated signal. Biomed. Pharm. 2019, 118, 109261. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chang, Y.H.; Rajesh, R. Targeted delivery of etoposide, carmustine and doxorubicin to human glioblastoma cells using methoxy poly(ethylene glycol)-poly(ε-caprolactone) nanoparticles conjugated with wheat germ agglutinin and folic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 114–128. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of drug resistant cancer cells: A matter of combination therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef]
- Matsuno, Y.; Hyodo, M.; Fujimori, H.; Shimizu, A.; Yoshioka, K.I. Sensitization of cancer cells to radiation and topoisomerase I inhibitor camptothecin using inhibitors of PARP and other signaling molecules. Cancers 2018, 10, 364. [Google Scholar] [CrossRef]
- George, A.; Sahin, I.; Carneiro, B.A.; Dizon, D.S.; Safran, H.P.; El-Deiry, W.S. Strategies to sensitize cancer cells to immunotherapy. Hum. Vaccin. Immunother. 2021, 17, 2595–2601. [Google Scholar] [CrossRef]
- Maszczyk, M.; Banach, K.; Karkoszka, M.; Rzepka, Z.; Rok, J.; Beberok, A.; Wrześniok, D. Chemosensitization of U-87 MG glioblastoma cells by neobavaisoflavone towards doxorubicin and etoposide. Int. J. Mol. Sci. 2022, 23, 5621. [Google Scholar] [CrossRef] [PubMed]
- Maszczyk, M.; Rzepka, Z.; Rok, J.; Beberok, A.; Wrzesniok, D. Neobavaisoflavone may modulate the activity of topoisomerase inhibitors towards U-87 MG Cells: An in vitro study. Molecules 2021, 26, 4516. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Meng, X.W.; Flatten, K.S.; Loegering, D.A.; Kaufmann, S.H. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2012, 20, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef]
- Don, M.J.; Lin, L.C.; Chiou, W.F. Neobavaisoflavone stimulates osteogenesis via p38-mediated up-regulation of transcription factors and osteoid genes expression in MC3T3-E1 cells. Phytomedicine 2012, 19, 551–561. [Google Scholar] [CrossRef]
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharm. 2020, 129, 110369. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, W.I.; Ko, H.; So, Y.; Kang, K.S.; Kim, I.; Kim, K.; Yoon, H.G.; Kim, T.J.; Choi, K.C. Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells. Life Sci. 2014, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Sedek, Ł.; Paradysz, A.; Król, W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011, 63, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhou, F.; Xie, X.; Zheng, D.; Yao, Y.; Zhao, C.; Huang, X.; Hu, K. Neobavaisoflavone demonstrates valid anti-tumor effects in non-small-cell lung cancer by inhibiting STAT3. Comb. Chem. High Throughput Screen. 2022, 25, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Drugging topoisomerases: Lessons and challenges. ACS Chem. Biol. 2013, 8, 82–95. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; De Bruin, R.A. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2021, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Jingwen, B.; Yaochen, L.; Guojun, Z. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, B.; Cosmai, L.; Porta, C. Conventional chemotherapy. In Onco-Nephrology, 1st ed.; Finkel, K., Perazella, M., Cohen, E., Eds.; Elsevier: Philadelphia, PA, USA, 2019; Volume 4, pp. 128–151. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maszczyk, M.; Banach, K.; Rok, J.; Rzepka, Z.; Beberok, A.; Wrześniok, D. Evaluation of Possible Neobavaisoflavone Chemosensitizing Properties towards Doxorubicin and Etoposide in SW1783 Anaplastic Astrocytoma Cells. Cells 2023, 12, 593. https://doi.org/10.3390/cells12040593
Maszczyk M, Banach K, Rok J, Rzepka Z, Beberok A, Wrześniok D. Evaluation of Possible Neobavaisoflavone Chemosensitizing Properties towards Doxorubicin and Etoposide in SW1783 Anaplastic Astrocytoma Cells. Cells. 2023; 12(4):593. https://doi.org/10.3390/cells12040593
Chicago/Turabian StyleMaszczyk, Mateusz, Klaudia Banach, Jakub Rok, Zuzanna Rzepka, Artur Beberok, and Dorota Wrześniok. 2023. "Evaluation of Possible Neobavaisoflavone Chemosensitizing Properties towards Doxorubicin and Etoposide in SW1783 Anaplastic Astrocytoma Cells" Cells 12, no. 4: 593. https://doi.org/10.3390/cells12040593
APA StyleMaszczyk, M., Banach, K., Rok, J., Rzepka, Z., Beberok, A., & Wrześniok, D. (2023). Evaluation of Possible Neobavaisoflavone Chemosensitizing Properties towards Doxorubicin and Etoposide in SW1783 Anaplastic Astrocytoma Cells. Cells, 12(4), 593. https://doi.org/10.3390/cells12040593




