Genotoxic Risks to Male Reproductive Health from Radiofrequency Radiation
Abstract
1. Introduction
2. Literature Search and Methodology
3. RFR-Induced Genotoxicity on Male Reproduction
3.1. In Vitro Studies
3.1.1. DNA Damage
3.1.2. Micronuclei and Genomic Instability
3.1.3. Sister Chromatid Exchange and Chromosomal Aberration
3.2. In Vivo Studies
3.2.1. DNA Damage
3.2.2. Micronuclei and Genomic Instability
3.2.3. Sister Chromatid Exchange and Chromosomal Aberrations
4. Genotoxicity and Oxidative Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wdowiak, A.; Skrzypek, M.; Stec, M.; Panasiuk, L. Effect of ionizing radiation on the male reproductive system. Ann. Agric. Environ. Med. 2019, 26, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kliukiene, J.; Tynes, T.; Andersen, A. Residential and occupational exposures to 50-Hz magnetic fields and breast cancer in women: A population-based study. Am. J. Epidemiol. 2004, 159, 852–861. [Google Scholar] [CrossRef]
- Kheifets, L.; Ahlbom, A.; Crespi, C.; Feychting, M.; Johansen, C.; Monroe, J.; Murphy, M.F.G.; Oksuzyan, S.; Preston-Martin, S.; Roman, E.; et al. A Pooled Analysis of Extremely Low-Frequency Magnetic Fields and Childhood Brain Tumors. Am. J. Epidemiol. 2010, 172, 752–761. [Google Scholar] [CrossRef]
- Górski, R.; Nowak-Terpiłowska, A.; Śledziński, P.; Baranowski, M.; Wosiński, S. Morphological and cytophysiological changes in selected lines of normal and cancer human cells under the influence of a radio-frequency electromagnetic field. Ann. Agric. Environ. Med. 2021, 28, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Durusoy, R.; Hassoy, H.; Özkurt, A.; Karababa, A.O. Mobile phone use, school electromagnetic field levels and related symptoms: A cross-sectional survey among 2150 high school students in Izmir. Environ. Health 2017, 16, 1–14. [Google Scholar] [CrossRef]
- Usman, J.D.; Isyaku, M.U.; Fasanmade, A.A. Evaluation of heart rate variability, blood pressure and lipid profile alterations from dual transceiver mobile phone radiation exposure. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 951–957. [Google Scholar] [CrossRef]
- Jangid, P.; Rai, U.; Sharma, R.S.; Singh, R. The role of non-ionizing electromagnetic radiation on female fertility: A review. Int. J. Environ. Health Res. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-ionizing radiation, Part 2: Radiofrequency electromagnetic fields. IARC Monogr. Eval. Carcinog. Risks Hum. 2013, 102 Pt 2, 1–460. [Google Scholar]
- Deepinder, F.; Makker, K.; Agarwal, A. Cell phones and male infertility: Dissecting the relationship. Reprod. Biomed. Online 2007, 15, 266–270. [Google Scholar] [CrossRef]
- World Health Organization. WHO Research Agenda for Radiofrequency Fields. 2010. Available online: http://www.who.int/peh-emf/research/rf_research_agenda_2006.pdf (accessed on 30 March 2022).
- Straume, A.; Oftedal, G.; Johnsson, A. Skin temperature increase caused by a mobile phone: A methodological infrared camera study. Bioelectromagnetics 2005, 26, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.G.; Agresti, M.; Bruce, T.; Yan, Y.H.; Granlund, A.; Matloub, H.S. Effects of cellular phone emissions on sperm motility in rats. Fertil. Steril. 2007, 88, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef]
- Leszczynski, D.; Joenväärä, S.; Reivinen, J.; Kuokka, R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: Molecular mechanism for cancer-and blood-brain barrier-related effects. Differentiation 2002, 70, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Adair, R.K. Biophysical limits on athermal effects of RF and microwave radiation. Bioelectromagnetics 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Prohofsky, E.W. RF absorption involving biological macromolecules. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2004, 25, 441–451. [Google Scholar] [CrossRef]
- Sheppard, A.R.; Swicord, M.L.; Balzano, Q. Quantitative evaluations of mechanisms of radiofrequency interactions with biological molecules and processes. Health Phys. 2008, 95, 365–396. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Wu, T.; Hadjem, A.; Wong, M.-F.; Gati, A.; Picon, O.; Wiart, J. Whole-body new-born and young rats’ exposure assessment in a reverberating chamber operating at 2.4 GHz. Phys. Med. Biol. 2010, 55, 1619–1630. [Google Scholar] [CrossRef]
- Agarwal, A.; Singh, A.; Hamada, A.; Kesari, K. Cell phones and male infertility: A review of recent innovations in technology and consequences. Int. Braz. J. Urol. 2011, 37, 432–454. [Google Scholar] [CrossRef]
- Dasdag, S.; Taş, M.; Akdag, M.Z.; Yegin, K. Effect of long-term exposure of 2.4 GHz radiofrequency radiation emitted from Wi-Fi equipment on testes functions. Electromagn. Biol. Med. 2015, 34, 37–42. [Google Scholar] [CrossRef]
- Othman, H.; Ammari, M.; Sakly, M.; Abdelmelek, H. Effects of prenatal exposure to WIFI signal (2.45 GHz) on postnatal development and behavior in rat: Influence of maternal restraint. Behav. Brain Res. 2017, 326, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Nath, R.; Mathur, A.K.; Sharma, R.S. Effect of radiofrequency radiation on reproductive health. Indian J. Med Res. 2018, 148, S92–S99. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Bennetts, L.E.; Sawyer, D.; Wiklendt, A.M.; King, B.V. Impact of radio frequency electro-magnetic radiation on DNA integrity in the male germline. Int. J. Androl. 2005, 28, 171–179. [Google Scholar] [CrossRef]
- Kesari, K.K.; Behari, J. Microwave Exposure Affecting Reproductive System in Male Rats. Appl. Biochem. Biotechnol. 2009, 162, 416–428. [Google Scholar] [CrossRef]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8 GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Yuksel, M.; Köse, S.A.; Özkaya, M.O. Recent Reports of Wi-Fi and Mobile Phone-Induced Radiation on Oxidative Stress and Reproductive Signaling Pathways in Females and Males. J. Membr. Biol. 2013, 246, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, J.; Cao, H.; Yi, C.; Li, J.X.; Nie, J.; Chen, L.L.; Wang, J.; Tong, J. Effects of 1800-MHz radiofrequency fields on circadian rhythm of plasma melatonin and testosterone in male rats. J. Toxicol. Environ. Health Part A 2012, 75, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile Phone Radiation Induces Reactive Oxygen Species Production and DNA Damage in Human Spermatozoa In Vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Singh, R. Association between reproductive health and nonionizing radiation exposure. Electromagn. Biol. Med. 2021, 40, 92–102. [Google Scholar] [CrossRef]
- Fang, H.H.; Zeng, G.Y.; Nie, Q.; Kang, J.B.; Ren, D.Q.; Zhou, J.X.; Li, Y.M. Effects on structure and secretion of pituitary gland in rats after electromagnetic pulse exposure. Zhonghua Yi Xue Za Zhi 2010, 90, 3231–3234. [Google Scholar]
- O’Shaughnessy, P.J.; Monteiro, A.; Fowler, P.A.; Morris, I.D. Identification of Leydig cell-specific mRNA transcripts in the adult rat testis. Reproduction 2014, 147, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.H. Study the electromagnetic radiation effects on testicular function of male rats by biochemical and histopathological. EurAsian J. BioSciences 2020, 14, 3869–3873. [Google Scholar]
- Romano-Spica, V.; Mucci, N.; Ursini, C.; Ianni, A.; Bhat, N. Ets1 oncogene induction by ELF-modulated 50 MHz radiofrequency electromagnetic field. Bioelectromagnetics 2000, 21, 8–18. [Google Scholar] [CrossRef]
- Meltz, M.L. Radiofrequency exposure and mammalian cell toxicity, genotoxicity, and transformation. Bioelectromagnetics 2003, 24, S196–S213. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, R.S.; Singh, R. Non-ionizing radiation as possible carcinogen. Int. J. Environ. Health Res. 2022, 32, 916–940. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, J.; Oftedal, G.; Feychting, M.; van Rongen, E.; Scarfì, M.R.; Mann, S.; Wong, R.; van Deventer, E. Prioritizing health outcomes when assessing the effects of exposure to radiofrequency electromagnetic fields: A survey among experts. Environ. Int. 2021, 146, 106300. [Google Scholar] [CrossRef]
- Romeo, S.; Zeni, O.; Sannino, A.; Lagorio, S.; Biffoni, M.; Scarfì, M. Genotoxicity of radiofrequency electromagnetic fields: Protocol for a systematic review of in vitro studies. Environ. Int. 2021, 148, 106386. [Google Scholar] [CrossRef]
- Merhi, Z.O. Challenging cell phone impact on reproduction: A Review. J. Assist. Reprod. Genet. 2012, 29, 293–297. [Google Scholar] [CrossRef]
- Meena, R.; Kumari, K.; Kumar, J.; Rajamani, P.; Verma, H.N.; Kesari, K.K. Therapeutic approaches of melatonin in microwave radiations-induced oxidative stress-mediated toxicity on male fertility pattern of Wistar rats. Electromagn. Biol. Med. 2014, 33, 81–91. [Google Scholar] [CrossRef]
- Kumar, S.; Nirala, J.P.; Behari, J.; Paulraj, R. Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian J. Exp. Biol. 2014, 52, 890–897. [Google Scholar]
- Diem, E.; Schwarz, C.; Adlkofer, F.; Jahn, O.; Rüdiger, H. Non-thermal DNA breakage by mobile-phone radiation (1800MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 583, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Behari, J.; Sisodia, R. Impact of Microwave at X-Band in the aetiology of male infertility. Electromagn. Biol. Med. 2012, 31, 223–232. [Google Scholar] [CrossRef]
- Lai, H. Single-and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int. J. Radiat. Biol. 1996, 69, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Spierings, D.; McStay, G.; Saleh, M.; Bender, C.; Chipuk, J.; Maurer, U.; Green, D.R. Connected to Death: The (Unexpurgated) Mitochondrial Pathway of Apoptosis. Science 2005, 310, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Mashevich, M.; Folkman, D.; Kesar, A.; Barbul, A.; Korenstein, R.; Jerby, E.; Avivi, L. Exposure of human peripheral blood lymphocytes to electromagnetic fields associated with cellular phones leads to chromosomal instability. Bioelectromagnetics 2003, 24, 82–90. [Google Scholar] [CrossRef]
- Tice, R.R.; Hook, G.G.; Donner, M.; McRee, D.I.; Guy, A.W. Genotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics 2002, 23, 113–126. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Xu, Z.P.; Chiang, H.; Lu, D.Q.; Zeng, Q.L. Effects of GSM 1800 MHz radiofrequency electromagnetic fields on DNA damage in Chinese hamster lung cells. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2006, 40, 149–152. [Google Scholar]
- Saliev, T.; Begimbetova, D.; Masoud, A.R.; Matkarimov, B. Biological effects of non-ionizing electro-magnetic fields: Two sides of a coin. Prog. Biophys. Mol. Biol. 2019, 141, 25–36. [Google Scholar] [CrossRef]
- Vornoli, A.; Falcioni, L.; Mandrioli, D.; Bua, L.; Belpoggi, F. The Contribution of In Vivo Mammalian Studies to the Knowledge of Adverse Effects of Radiofrequency Radiation on Human Health. Int. J. Environ. Res. Public Health 2019, 16, 3379. [Google Scholar] [CrossRef]
- Zeni, O.; Romano, M.; Perrotta, A.; Lioi, M.; Barbieri, R.; D’Ambrosio, G.; Massa, R.; Scarfì, M. Evaluation of genotoxic effects in human peripheral blood leukocytes following an acute in vitro exposure to 900 MHz radiofrequency fields. Bioelectromagnetics 2005, 26, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, N.; Komatsubara, Y.; Takeda, H.; Hirose, H.; Sekijima, M.; Nojima, T.; Miyakoshi, J. DNA strand breaks are not induced in human cells exposed to 2.1425 GHz band CW and W-CDMA modulated radiofrequency fields allocated to mobile radio base stations. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2006, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chemeris, N.; Gapeyev, A.; Sirota, N.; Gudkova, O.; Tankanag, A.; Konovalov, I.; Buzoverya, M.; Suvorov, V.; Logunov, V. Lack of direct DNA damage in human blood leukocytes and lymphocytes after in vitro exposure to high power microwave pulses. Bioelectromagnetics 2006, 27, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Komatsubara, Y.; Hirose, H.; Sakurai, T.; Koyama, S.; Suzuki, Y.; Taki, M.; Miyakoshi, J. Effect of high-frequency electromagnetic fields with a wide range of SARs on chromosomal aberrations in murine m5S cells. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 587, 114–119. [Google Scholar] [CrossRef]
- Figueiredo, A.; Alves, R.N.; Ramalho, A.T. Cytogenetic analysis of the effects of 2.5 and 10.5 GHz microwaves on human lymphocytes. Genet. Mol. Biol. 2004, 27, 460–466. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.B.; Yang, J.Q.; Liu, Y.; Zhang, G.B. Influence of electromagnetic irradiation on P450scc mRNA expression in rat testis tissues and protective effect of the shield. Zhonghua Nan Ke Xue = Natl. J. Androl. 2005, 11, 269–271. [Google Scholar]
- Wang, S.M.; Wang, D.W.; Peng, R.Y.; Gao, Y.B.; Yang, Y.; Hu, W.H.; Chen, H.Y.; Zhang, Y.R.; Gao, Y. Effect of electromagnetic pulse irradiation on structure and function of Leydig cells in mice. Zhonghua Nan Ke Xue = Natl. J. Androl. 2003, 9, 327–330. [Google Scholar]
- Salama, N.; Kishimoto, T.; Kanayama, H.-O.; Kagawa, S. RETRACTED: The Mobile Phone Decreases Fructose But Not Citrate in Rabbit Semen: A Longitudinal Study. Syst. Biol. Reprod. Med. 2009, 55, 181–187. [Google Scholar] [CrossRef]
- Yadav, H.; Rai, U.; Singh, R. Radiofrequency radiation: A possible threat to male fertility. Reprod. Toxicol. 2021, 100, 90–100. [Google Scholar] [CrossRef]
- Kesari, K.K.; Behari, J. Effects of microwave at 2.45 GHz radiations on reproductive system of male rats. Toxicol. Environ. Chem. 2010, 92, 1135–1147. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Melatonin and N-tert-butyl-α-phenylnitrone block 60-Hz magnetic field-induced DNA single and double strand breaks in rat brain cells. J. Pineal Res. 1997, 22, 152–162. [Google Scholar] [CrossRef]
- Simkó, M. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 2007, 14, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Singh, N.P. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ. Health Perspect. 2004, 112, 687–694. [Google Scholar] [CrossRef]
- Schuermann, D.; Ziemann, C.; Barekati, Z.; Capstick, M.; Oertel, A.; Focke, F.; Murbach, M.; Kuster, N.; Dasenbrock, C.; Schär, P. Assessment of Genotoxicity in Human Cells Exposed to Modulated Electromagnetic Fields of Wireless Communication Devices. Genes 2020, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, N.; Grindon, C.; Combes, R.; Balls, M. Toxicity testing: Creating a revolution based on new technologies. Trends Biotechnol. 2005, 23, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Kamal, K.; Phang, D.; Willis, J.; Jarvi, K. Biologic variability of sperm DNA denaturation in infertile men. Urology 2001, 58, 258–261. [Google Scholar] [CrossRef]
- Aitken, R.J. The Amoroso Lecture The human spermatozoon—A cell in crisis? Reproduction 1999, 115, 1–7. [Google Scholar] [CrossRef]
- Schulte, R.T.; Ohl, D.A.; Sigman, M.; Smith, G.D. Sperm DNA damage in male infertility: Etiologies, assays, and outcomes. J. Assist. Reprod. Genet. 2009, 27, 3–12. [Google Scholar] [CrossRef]
- Zini, A.; Bielecki, R.; Phang, D.; Zenzes, M.T. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil. Steril. 2001, 75, 674–677. [Google Scholar] [CrossRef]
- Falzone, N.; Huyser, C.; Franken, D.R.; Leszczynski, D. Mobile Phone Radiation Does Not Induce Pro-apoptosis Effects in Human Spermatozoa. Radiat. Res. 2010, 174, 169–176. [Google Scholar] [CrossRef]
- Houston, B.; Nixon, B.; King, B.V.; Aitken, R.J.; De Iuliis, G.N. Probing the Origins of 1,800 MHz Radio Frequency Electromagnetic Radiation Induced Damage in Mouse Immortalized Germ Cells and Spermatozoa in vitro. Front. Public Health 2018, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, W.; Xu, S.; Chen, C.; He, M.; Zhang, L.; Yu, Z.; Zhou, Z. Exposure to 1800 MHz radiofrequency electromagnetic radiation induces oxidative DNA base damage in a mouse spermatocyte-derived cell line. Toxicol. Lett. 2013, 218, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, M.; Li, L.; Zhao, L.; Zhang, T.; Gao, X.; Zhang, D.; Zhu, Y.; Peng, Q.; Luo, X.; et al. The Protective Effect of Autophagy on DNA Damage in Mouse Spermatocyte-Derived Cells Exposed to 1800 MHz Radiofrequency Electromagnetic Fields. Cell. Physiol. Biochem. 2018, 48, 29–41. [Google Scholar] [CrossRef]
- Duan, W.; Liu, C.; Zhang, L.; He, M.; Xu, S.; Chen, C.; Pi, H.; Gao, P.; Zhang, Y.; Zhong, M.; et al. Comparison of the genotoxic effects induced by 50 Hz extremely low-frequency electromagnetic fields and 1800 MHz radiofrequency electromagnetic fields in GC-2 cells. Radiat. Res. 2015, 183, 305–314. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Wu, T.; Liu, J.Y.; Gao, P.; Li, K.C.; Guo, Q.Y.; Yuan, M.; Lang, H.; Zeng, L.; Guo, G.Z. 1950MHz radio frequency electromagnetic radiation inhibits testosterone secretion of mouse leydig cells. Int. J. Environ. Res. Public Health 2018, 15, 17. [Google Scholar] [CrossRef]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- McNamee, J.P.; Bellier, P.V.; Gajda, G.B.; Miller, S.M.; Lemay, E.P.; Lavallee, B.F.; Marro, L.; Thansandote, A. DNA damage and micronucleus induction in human leukocytes after acute in vitro exposure to a 1.9 GHz continuous-wave radiofrequency field. Radiat. Res. 2002, 158, 523–533. [Google Scholar] [CrossRef]
- Franzellitti, S.; Valbonesi, P.; Ciancaglini, N.; Biondi, C.; Contin, A.; Bersani, F.; Fabbri, E. Transient DNA damage induced by high-frequency electromagnetic fields (GSM 1.8 GHz) in the human trophoblast HTR-8/SVneo cell line evaluated with the alkaline comet assay. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2010, 683, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bisht, K.S.; LaGroye, I.; Zhang, P.; Straube, W.L.; Moros, E.G.; Roti Roti, J.L. Measurement of DNA damage in mammalian cells exposed in vitro to radiofrequency fields at SARs of 3–5 W/kg. Radiat. Res. 2001, 156, 328–332. [Google Scholar] [CrossRef]
- Speit, G.; Schütz, P.; Hoffmann, H. Genotoxic effects of exposure to radiofrequency electromagnetic fields (RF-EMF) in cultured mammalian cells are not independently reproducible. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 626, 42–47. [Google Scholar] [CrossRef]
- Zeni, O.; Schiavoni, A.; Perrotta, A.; Forigo, D.; Deplano, M.; Scarfi, M. Evaluation of genotoxic effects in human leukocytes after in vitro exposure to 1950 MHz UMTS radiofrequency field. Bioelectromagnetics 2007, 29, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hook, G.J.; Zhang, P.; Lagroye, I.; Li, L.; Higashikubo, R.; Moros, E.G.; Straube, W.L.; Pickard, W.F.; Baty, J.D.; Roti Roti, J.L. Measurement of DNA damage and apoptosis in Molt-4 cells after in vitro exposure to radiofrequency radiation. Radiat. Res. 2004, 161, 193–200. [Google Scholar] [CrossRef]
- Leal, B.Z.; Szilagyi, M.; Prihoda, T.J.; Meltz, M.L. Primary DNA damage in human blood lymphocytes exposed in vitro to 2450 MHz radiofrequency radiation. Radiat. Res. 2000, 153, 479–486. [Google Scholar] [CrossRef]
- Gorpinchenko, I.; Nikitin, O.; Banyra, O.; Shulyak, A. The influence of direct mobile phone radiation on sperm quality. Cent. Eur. J. Urol. 2014, 67, 65–71. [Google Scholar] [CrossRef]
- Vasan, S.; Veerachari, S.B. Mobile Phone Electromagnetic Waves and Its Effect on Human Ejaculated Semen: An in vitro Study. Int. J. Infertil. Fetal Med. 2012, 3, 15–21. [Google Scholar] [CrossRef]
- Rago, R.; Salacone, P.; Caponecchia, L.; Sebastianelli, A.; Marcucci, I.; Calogero, A.E.; Condorelli, R.A.; Vicari, E.; Morgia, G.; Favilla, V.; et al. The semen quality of the mobile phone users. J. Endocrinol. Investig. 2013, 36, 970–974. [Google Scholar] [CrossRef]
- Baah, E. In-Vitro Effect of Non-Ionising Radiation from Cellular Phone on Human Sperm Quality in Men. Ph.D. Dissertation, Kwame Nkrumah University of Science & Technology, Kumasi, Ghana, 2017. [Google Scholar]
- Hagras, A.M.; Toraih, E.A.; Fawzy, M.S. Mobile phones electromagnetic radiation and NAD+-dependent isocitrate dehydrogenase as a mitochondrial marker in asthenozoospermia. Biochim. Open 2016, 3, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Baah, E.; Obirikorang, C.; Asmah, R.H.; Acheampong, E.; Anto, E.O.; Yakass, M.B.; Mawusi, D. Seminal antioxidant capacity to oxidative stress induced by electromagnetic waves emitting from cellular phones on sperm quality: An in vitro simulation model. Adv. Reprod. Sci. 2019, 7, 94–105. [Google Scholar] [CrossRef]
- Zalata, A.; El-Samanoudy, A.Z.; Shaalan, D.; El-Baiomy, Y.; Mostafa, T. In Vitro Effect of Cell Phone Radiation on Motility, DNA Fragmentation and Clusterin Gene Expression in Human Sperm. Int. J. Fertil. Steril. 2015, 9, 129–136. [Google Scholar] [CrossRef]
- Avendaño, C.; Mata, A.; Sarmiento, C.A.S.; Doncel, G.F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012, 97, 39–45.e2. [Google Scholar] [CrossRef]
- Ding, S.S.; Ping, S.; Hong, T. Association between daily exposure to electromagnetic radiation from 4G smartphone and 2.45-GHz wi-fi and oxidative damage to semen of males attending a genetics clinic: A primary study. Int. J. Clin. Exp. Med. 2018, 11, 2821–2830. [Google Scholar]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef]
- Knudsen, L.E.; Kirsch-Volders, M. Micronuclei, reproduction and child health. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108345. [Google Scholar] [CrossRef]
- Kesari, K.K.; Kumar, S.; Behari, J. Effects of radiofrequency electromagnetic wave exposure from cellular phones on the reproductive pattern in male Wistar rats. Appl. Biochem. Biotechnol. 2011, 164, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Garaj-Vrhovac, V.; Horvat, D.; Koren, Z. The relationship between colony-forming ability, chromosome aberrations and incidence of micronuclei in V79 Chinese hamster cells exposed to microwave radiation. Mutat. Res. Lett. 1991, 263, 143–149. [Google Scholar] [CrossRef]
- Sannino, A.; Zeni, O.; Romeo, S.; Massa, R.; Scarfi, M.R. Adverse and beneficial effects in Chinese hamster lung fibroblast cells following radiofrequency exposure. Bioelectromagnetics 2017, 38, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.S.; Moros, E.G.; Straube, W.L.; Baty, J.D.; Roti, J.L.R. The effect of 835.62 MHz FDMA or 847.74 MHz CDMA modulated radiofrequency radiation on the induction of micronuclei in C3H 10T½ cells. Radiat. Res. 2017, 157, 506–515. [Google Scholar] [CrossRef]
- Maes, A.; Verschaeve, L.; Arroyo, A.; De Wagter, C.; Vercruyssen, L. In vitro cytogenetic effects of 2450 MHz waves on human peripheral blood lymphocytes. Bioelectromagnetics 1993, 14, 495–501. [Google Scholar] [CrossRef]
- Maes, A.; Collier, M.; Slaets, D.; Verschaeve, L. Cytogenetic effects of microwaves from mobile communication frequencies (954 MHz). Electro-Magn. 1995, 14, 91. [Google Scholar] [CrossRef]
- Garaj-Vrhovac, V.; Fučić, A.; Horvat, D. The correlation between the frequency of micronuclei and specific chromosome aberrations in human lymphocytes exposed to microwave radiation in vitro. Mutat. Res. Lett. 1992, 281, 181–186. [Google Scholar] [CrossRef]
- Khalil, A.M.; Qassem, W.F.; Suleiman, M.M. A preliminary study on the radiofrequency field-induced cytogenetic effects in cultured human lymphocytes. Dirasat 1993, 20, 121–130. [Google Scholar]
- Panagopoulos, D.J. Chromosome damage in human cells induced by UMTS mobile telephony radiation. Gen. Physiol. Biophys. 2019, 38, 54. [Google Scholar] [CrossRef]
- Panagopoulos, D.J. Comparing DNA damage induced by mobile telephony and other types of man-made electromagnetic fields. Mutat. Res. Mutat. Res. 2019, 781, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Uslu, N.; Demirhan, O.; Emre, M.; Seydaoğlu, G. The chromosomal effects of GSM-like electromagnetic radiation exposure on human fetal cells. Biomed. Res. Clin. Pr. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Wolff, S.; James, T.L.; Young, G.B.; Margulis, A.R.; Bodycote, J.; Afzal, V. Magnetic resonance imaging: Absence of in vitro cytogenetic damage. Radiology 1985, 155, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Verschaeve, L.; Juutilainen, J.; Lagroye, I.; Miyakoshi, J.; Saunders, R.; de Seze, R.; Tenforde, T.; van Rongen, E.; Veyret, B.; Xu, Z. In vitro and in vivo genotoxicity of radiofrequency fields. Mutat. Res. Mutat. Res. 2010, 705, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Mohan, N.; Meltz, M.L.; Wittler, M.A. Proliferation and cytogenetic studies in human blood lymphocytes exposed in vitro to 2450 MHz radiofrequency radiation. Int. J. Radiat. Biol. 1997, 72, 751–757. [Google Scholar] [CrossRef]
- Vijayalaxmi, K.S.; Bisht, W.F.; Pickard, M.L.; Meltz, J.L. Roti Roti and EG Moros, Chromosome damage and micronucleus formation in human blood lymphocytes exposed in vitro to radiofrequency radiation at a cellular telephone frequency (847.74 MHz, CDMA). Radiat. Res. 2001, 156, 430–432. [Google Scholar] [PubMed]
- Straubec, W.L.; Morosc, E.G. Cytogenetic studies in human blood lymphocytes exposed in vitro to radiofrequency radiation at a cellular telephone frequency (835.62 MHz, FDMA). Radiat. Res. 2001, 155, 113–121. [Google Scholar]
- Vijayalaxmi. Cytogenetic studies in human blood lymphocytes exposed in vitro to 2.45 GHz or 8.2 GHz radiofrequency radiation. Radiat. Res. 2006, 166, 532–538. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.K.; Kim, H.G.; Kim, K.B.; Kim, H.R. Possible Effects of Radiofrequency Electromagnetic Field Exposure on Central Nerve System. Biomol. Ther. (Seoul) 2019, 27, 265–275. [Google Scholar] [CrossRef]
- Gajski, G.; Ravlić, S.; Godschalk, R.; Collins, A.; Dusinska, M.; Brunborg, G. Application of the comet assay for the evaluation of DNA damage in mature sperm. Mutat. Res. Mutat. Res. 2021, 788, 108398. [Google Scholar] [CrossRef]
- Singh, N.P. The comet assay: Reflections on its development, evolution and applications. Mutat. Res. Mutat. Res. 2016, 767, 23–30. [Google Scholar] [CrossRef]
- Akdag, M.Z.; Dasdag, S.; Canturk, F.; Karabulut, D.; Caner, Y.; Adalier, N. Does prolonged radiofrequency radiation emitted from Wi-Fi devices induce DNA damage in various tissues of rats? J. Chem. Neuroanat. 2016, 75, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Behari, J.; Sisodia, R. Influence of electromagnetic fields on reproductive system of male rats. Int. J. Radiat. Biol. 2012, 89, 147–154. [Google Scholar] [CrossRef]
- Baverstock, K. Radiation-induced genomic instability: A paradigm-breaking phenomenon and its relevance to environmentally induced cancer. Mutat. Res. /Fundam. Mol. Mech. Mutagenes. 2000, 454, 89–109. [Google Scholar] [CrossRef]
- Nikolova, T.; Czyz, J.; Rolletschek, A.; Blyszczuk, P.; Fuchs, J.; Jovtchev, G.; Schulderer, J.; Kuster, N.; Wobus, A.M. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. 2005, 19, 1686–1688. [Google Scholar] [CrossRef]
- Houston, B.J.; Nixon, B.; McEwan, K.E.; Martin, J.H.; King, B.V.; Aitken, R.J.; De Iuliis, G.N. Whole-body exposures to radiofrequency-electromagnetic energy can cause DNA damage in mouse spermatozoa via an oxidative mechanism. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Smith-Roe, S.L.; Wyde, M.E.; Stout, M.D.; Winters, J.W.; Hobbs, C.A.; Shepard, K.G.; Green, A.S.; Kissling, G.E.; Shockley, K.R.; Tice, R.R.; et al. Evaluation of the genotoxicity of cell phone radiofrequency radiation in male and female rats and mice following subchronic exposure. Environ. Mol. Mutagenes. 2020, 61, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Giri, S. Melatonin attenuates radiofrequency radiation (900 MHz)-induced oxidative stress, DNA damage and cell cycle arrest in germ cells of male Swiss albino mice. Toxicol. Ind. Health 2018, 34, 315–327. [Google Scholar] [CrossRef]
- Pandey, N.; Giri, S.; Das, S.; Upadhaya, P. Radiofrequency radiation (900 MHz)-induced DNA damage and cell cycle arrest in testicular germ cells in swiss albino mice. Toxicol. Ind. Health 2017, 33, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Si, T.; Xu, X.; Liang, F.; Wang, L.; Pan, S. Electromagnetic radiation at 900 MHz induces sperm apoptosis through bcl-2, bax and caspase-3 signaling pathways in rats. Reprod. Health 2015, 12, 65. [Google Scholar] [CrossRef]
- Shahin, N.N.; El-Nabarawy, N.A.; Gouda, A.S.; Mégarbane, B. The protective role of spermine against male reproductive aberrations induced by exposure to electromagnetic field—An experimental investigation in the rat. Toxicol. Appl. Pharmacol. 2019, 370, 117–130. [Google Scholar] [CrossRef]
- Mahmoud, N.M.; Gomaa, R.S.; Salem, A.E. Activation of liver X receptors ameliorates alterations in testicular function in rats exposed to electromagnetic radiation. Alex. J. Med. 2021, 57, 82–91. [Google Scholar] [CrossRef]
- Guo, L.; Lin, J.-J.; Xue, Y.-Z.; An, G.-Z.; Zhang, J.-P.; Zhang, K.-Y.; He, W.; Wang, H.; Li, W.; Ding, G.-R. Effects of 220 MHz Pulsed Modulated Radiofrequency Field on the Sperm Quality in Rats. Int. J. Environ. Res. Public Health 2019, 16, 1286. [Google Scholar] [CrossRef] [PubMed]
- Dasdag, S.; Zulkuf Akdag, M.; Aksen, F.; Yılmaz, F.; Bashan, M.; Mutlu Dasdag, M.; Salih Celik, M. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics 2003, 24, 182–188. [Google Scholar] [CrossRef]
- Dong, G.; Zhou, H.; Gao, Y.; Zhao, X.; Liu, Q.; Li, Z.; Zhao, X.; Yin, J.; Wang, C. Effects of 1.5-GHz high-power microwave exposure on the reproductive systems of male mice. Electromagn. Biol. Med. 2021, 40, 311–320. [Google Scholar] [CrossRef]
- Okechukwu, C.E. Effects of mobile phone radiation and exercise on testicular function in male Wistar rats. Niger. J. Exp. Clin. Biosci. 2018, 6, 51. [Google Scholar] [CrossRef]
- Hasan, I.; Amin, T.; Alam, R.; Islam, M.R. Hematobiochemical and histopathological alterations of kidney and testis due to exposure of 4G cell phone radiation in mice. Saudi J. Biol. Sci. 2021, 28, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Shahin, S.; Singh, S.P.; Chaturvedi, C.M. 1800 MHz mobile phone irradiation induced oxidative and nitrosative stress leads to p53 dependent Bax mediated testicular apoptosis in mice, Mus musculus. J. Cell. Physiol. 2018, 233, 7253–7267. [Google Scholar] [CrossRef]
- Alkiş, M.E.; Akdag, M.Z.; Dasdag, S.; Yegin, K.; Akpolat, V. Single-strand DNA breaks and oxidative changes in rat testes exposed to radiofrequency radiation emitted from cellular phones. Biotechnol. Biotechnol. Equip. 2019, 33, 1733–1740. [Google Scholar] [CrossRef]
- Yu, G.; Tang, Z.; Chen, H.; Chen, Z.; Wang, L.; Cao, H.; Wang, G.; Xing, J.; Shen, H.; Cheng, Q.; et al. Long-term exposure to 4G smartphone radiofrequency electromagnetic radiation diminished male reproductive potential by directly disrupting Spock3–MMP2-BTB axis in the testes of adult rats. Sci. Total Environ. 2020, 698, 133860. [Google Scholar] [CrossRef]
- Desai, N.R.; Kesari, K.K.; Agarwal, A. Pathophysiology of cell phone radiation: Oxidative stress and carcinogenesis with focus on male reproductive system. Reprod. Biol. Endocrinol. 2009, 7, 114. [Google Scholar] [CrossRef]
- Kesari, K.K.; Luukkonen, J.; Juutilainen, J.; Naarala, J. Genomic instability induced by 50Hz magnetic fields is a dynamically evolving process not blocked by antioxidant treatment. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 794, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Meena, R.; Nirala, J.; Kumar, J.; Verma, H.N. Effect of 3G cell phone exposure with computer controlled 2-D stepper motor on non-thermal activation of the hsp27/p38MAPK stress pathway in rat brain. Cell Biochem. Biophys. 2014, 68, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Manikowska-Czerska, E.; Czerskl, P.; Leach, W.M. Effects of 2.45 GHz microwaves on meiotic chromosomes of male CBA/CAY mice. J. Hered. 1985, 76, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Beechey, C.V.; Brooker, D.; Kowalczuk, C.I.; Saunders, R.D.; Searle, A.G. Cytogenetic effects of microwave irradiation on male germ cells of the mouse. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 50, 909–918. [Google Scholar] [CrossRef]
- Saunders, R.; Darby, S.; Kowalczuk, C. Dominant lethal studies in male mice after exposure to 2.45 GHz microwave radiation. Mutat. Res. Toxicol. 1983, 117, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. REVIEW ARTICLE: Clinical Relevance of Oxidative Stress in Male Factor Infertility: An Update. Am. J. Reprod. Immunol. 2007, 59, 2–11. [Google Scholar] [CrossRef]
- Desai, N.; Sharma, R.; Makker, K.; Sabanegh, E.; Agarwal, A. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil. Steril. 2009, 92, 1626–1631. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Athayde, K.S.; Cocuzza, M.; Agarwal, A.; Krajcir, N.; Lucon, A.M.; Srougi, M.; Hallak, J. Development of Normal Reference Values for Seminal Reactive Oxygen Species and Their Correlation With Leukocytes and Semen Parameters in a Fertile Population. J. Androl. 2007, 28, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.R.; Dehghani, V.; Tabibnezhad, N.; Vahidi, S. Reactive Oxygen Species (ROS) level in seminal plasma of infertile men and healthy donors. Iran J. Reprod. Med. 2007, 5, 51–55. [Google Scholar] [CrossRef]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef]
- Kullisaar, T.; Türk, S.; Kilk, K.; Ausmees, K.; Punab, M.; Mändar, R. Increased levels of hydrogen peroxide and nitric oxide in male partners of infertile couples. Andrology 2013, 1, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Behari, J. Evidence for mobile phone radiation exposure effects on reproductive pattern of male rats: Role of ROS. Electromagn. Biol. Med. 2012, 31, 213–222. [Google Scholar] [CrossRef]
- Oyewopo, A.O.; Olaniyi, S.K.; Oyewopo, C.I.; Jimoh, A.T. Radiofrequency electromagnetic radiation from cell phone causes defective testicular function in male Wistar rats. Andrologia 2017, 49, e12772. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, K.V.; Nirala, J.; Murmu, N.N.; Meena, R.; Rajamani, P. Oxidative stress-mediated alterations on sperm parameters in male Wistar rats exposed to 3G mobile phone radiation. Andrologia 2018, 51, e13201. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Priyadarshini, E.; Nirala, J.P.; Meena, R.; Rajamani, P. Modulatory effects of Punica granatum L juice against 2115 MHz (3G) radiation-induced reproductive toxicity in male Wistar rat. Environ. Sci. Pollut. Res. 2021, 28, 54756–54765. [Google Scholar] [CrossRef]
- Fahmi, A.; Hussein, A.S.; Ibrahim, K.S.; Madboly, A.; Rahman, M. Effect of Cell Phone-Emitted Electromagnetic Waves on Levels of Male Sex Hormones and Oxidative Stress Biomarkers in Humans. J. Biol. Sci. 2021, 21, 221–227. [Google Scholar] [CrossRef]
- Schuermann, D.; Mevissen, M. Manmade Electromagnetic Fields and Oxidative Stress—Biological Effects and Consequences for Health. Int. J. Mol. Sci. 2021, 22, 3772. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Verma, H.N.; Sisodia, R.; Kesari, K.K. Microwave radiation (2.45 GHz)-induced oxidative stress: Whole-body exposure effect on histopathology of Wistar rats. Electromagn. Biol. Med. 2017, 36, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, S.; Darbandi, M. Lifestyle modifications on further reproductive problems. Cresco. J. Reprod. Sci. 2016, 1, 1–2. [Google Scholar]
- Zirkin, B.R.; Chen, H. Regulation of Leydig Cell Steroidogenic Function During Aging1. Biol. Reprod. 2000, 63, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Bang, H.J.; Lysiak, J.J. Experimental testicular torsion: Reperfusion blood flow and sub-sequent testicular venous plasma testosterone concentrations. Urology 2005, 65, 390–394. [Google Scholar] [CrossRef]
- Qin, F.; Shen, T.; Cao, H.; Qian, J.; Zou, D.; Ye, M.; Pei, H. CeO2NPs relieve radiofrequency radiation, improve testosterone synthesis, and clock gene expression in Leydig cells by enhancing antioxidation. Int. J. Nanomed. 2019, 14, 4601. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef]
- Maluin, S.M.; Osman, K.; Jaffar, F.H.F.; Ibrahim, S.F. Effect of Radiation Emitted by Wireless Devices on Male Reproductive Hormones: A Systematic Review. Front. Physiol. 2021, 12, 732420. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Altunkaynak, B.Z.; Kaplan, S. Biochemical, immunohistochemical and morpho-metrical investigation of the effect of thymoquinone on the rat testis following exposure to a 900-MHz electro-magnetic field. Acta Histochem. 2020, 122, 151467. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.N.; Lukose, S.T.; Arun, G.; Mohapatra, N.; Pamala, J.; Concessao, P.L.; Jetti, R.; Kedage, V.; Nalini, K.; Bhat, P.G. Modulatory effect of 900 MHz radiation on biochemical and reproductive parameters in rats. Bratisl. Med. J. 2018, 119, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Wang, M.; Wu, S.; Ma, X.; An, G.; Liu, H.; Xie, F. Oxidative changes and apoptosis induced by 1800-MHz electromagnetic radiation in NIH/3T3 cells. Electromagn. Biol. Med. 2013, 34, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, E.; Yurt, K.; Kaplan, A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Tsunoda, S. Redox regulation of fertilisation and the spermatogenic process. Asian J. Androl. 2011, 13, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Kravchenko, V.G. Resonance Effect of Low-Intensity Millimeter Waves on the Chromatin Conformational State of Rat Thymocytes. Z. Für Nat. C 1994, 49, 352–358. [Google Scholar] [CrossRef]
- Sharma, S.; Bahel, S.; Katnoria, J.K. Evaluation of oxidative stress and genotoxicity of 900 MHz electromagnetic radiations using Trigonella foenum-graecum test system. Protoplasma 2022, 260, 1–16. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
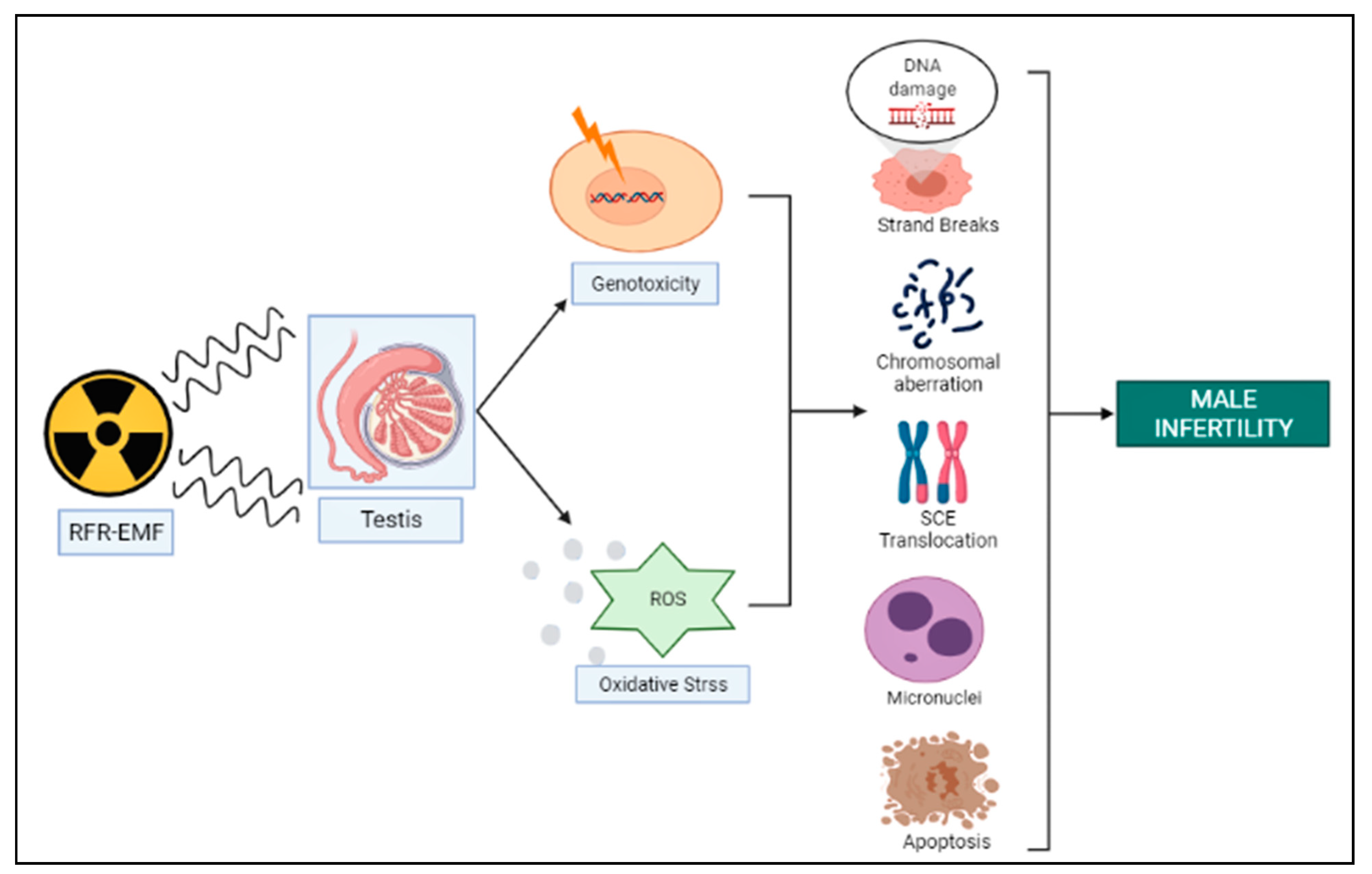
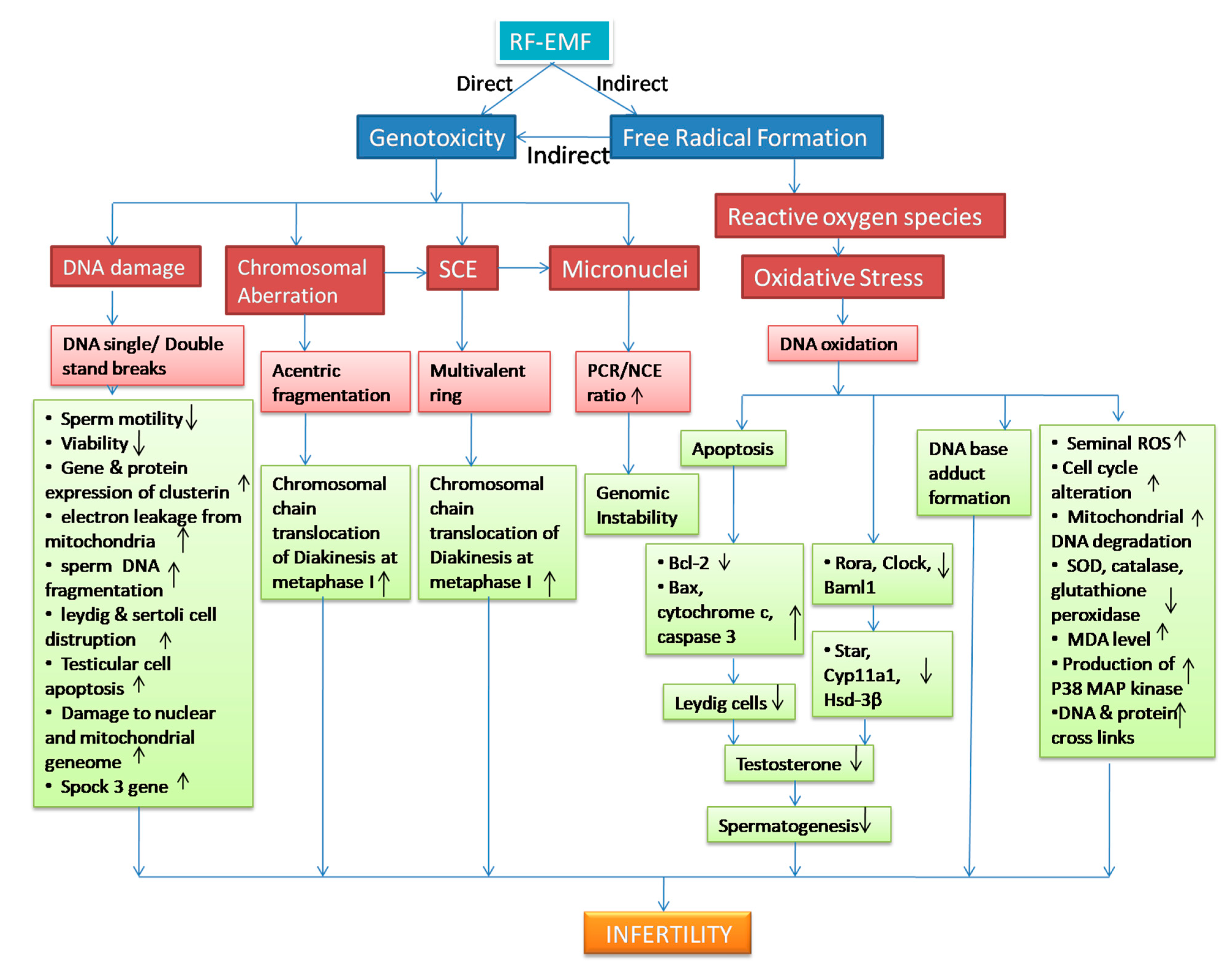
| Genotoxic Endpoints | Subject | Frequency (MHz) | SAR | Dose Duration | Findings | References |
|---|---|---|---|---|---|---|
| DNA Damage | Human semen | 850 | 1.46 W/kg | 1 h | Significant increase in sperm DNA damage with a rise in gene and protein expression of clustering | [91] |
| DNA damage | Human semen | 850 | 1.46 W/kg | 1 h | No significant destruction in DNA integrity while an increase in ROS level reported | [77] |
| DNA Damage | Human semen | 900 | 1.46 W/kg | 1 h | Significant decrease in sperm motility and viability with the increase in DNA damage | [86] |
| DNA Damage | Human semen | 900 | 2.0 and 5.7 W/kg | 1 h | No significant induction of apoptosis in spermatozoa and no DNA fragmentation or any ROS generation | [71] |
| DNA Damage | Human semen | 900/1800 | 5 h | Increase sperm DNA fragmentation with the decrease in sperm motility in exposed sperm | [85] | |
| DNA Damage | Human semen | 947.6 | 3.29 W/kg, 2.89 W/kg | 3 h | Decreased SOD activity with a rise in DNA fragmentation and decline in sperm motility and viability with increase in oxidative stress | [88] |
| DNA Damage | Human Semen | 947.6 | 3.29 and 2.89 W/kg | 180 min | Significant increase in DNA fragmentation | [90] |
| DNA Damage | Human spermatozoa | 1800 | 1.0 W/kg | 16 h | Damage in DNA and sperm function due to electron leakage from the mitochondria and increased ROS generation, reduced motility and viability | [30] |
| DNA Damage | Cultured Mouse spermatocyte derieved GC-2-cell | 1800 | 0.13 W/kg | 1/20 min, 24 h | Accumulation of single stranded DNA break | [73] |
| DNA Damage | Mouse spermatocyte derieved GC-2-cell | 1800 | 4 W/kg | 24 h | Significant DNA damage via ROS generation | [74] |
| DNA Damage | Mouse spermatozoa | 1800 | 0.15 W/kg & 1.5 W/kg | 3 h | DNA fragmentation due to ROS generation under oxidative stress of RF exposure | [72] |
| DNA Damage | Human semen | Active mobile phone usage | More than 4 h/day | Sperm DNA fragmentation | [87] | |
| DNA Damage | Mouse leydig cells | 1950 | 3 W/kg | 24 h | Cell proliferation inhibition, cell cycle alteration, dysfunction of testosterone secretion with no effect on ROS levels and cell apoptosis | [76] |
| DNA Damage | Human semen | 2400 | 4 h | Sperm motility reduced progressively and sperm DNA damage increased. No significant difference observed in levels of dead sperm | [92] | |
| DNA Damage | Human Semen | 1800/2450 | >30 min <121 min | Increased 8-OHdG expression and sperm nuclear DNA fragmentation. Sperm count, vitality, and motility decreased significantly with increase in oxidative stress | [93] |
| Genotoxic Endpoints | Subject | Frequency (MHz) | SAR | Dose Duration | Findings | References |
|---|---|---|---|---|---|---|
| DNA Damage | Male Sprague-Dawley rat | 220 | 0.030 W/kg-whole body, 0.014 W/kg-testis | 1 h/day, 30 days | Leydig and sertoli cell disruption along with cell apoptosis in testes | [127] |
| DNA Damage | Sprague-Dawley rat | 250 | 0.52 W/kg | 20 min/day, 1 month | No significant alteration in testicular functions (MDA concentration, sperm count, p53 immune reactivity) | [128] |
| DNA Damage | Male Wistar rat | 890–915 | 0.69 W/kg | 3 h/day, 2 weeks | Significant increase in apoptotic gene expression (caspase 3) and decrease in Bcl2, and significant decrease in sperm count, motility, viability, FSH, LH and testosterone with increase in MDA concentration | [126] |
| DNA Damage | Male Swiss mice | 900 | 0.09 W/kg | 12 h/day, 7 days | Significant damage to the mitochondrial and nuclear genome | [25] |
| DNA Damage | Male Swiss Albino mice | 900 | 0.0054–0.0516 W/kg | 6 h/day, 35 days | Increased DNA fragmentation and spermatogenesis arrest at the premeiotic stage due to increase in ROS generation | [122] |
| DNA Damage | Rat | 900 | 0.66 ± 0.01 W/kg | 2 h/day, 50 days | Significant increase in apoptosis due to elevated ROS levels and decreased TAC in sperm | [124] |
| DNA Damage | Male Wistar Rat | 900 | 1.075 W/kg | 2 h/day, 8 weeks | Elevated oxidative, inflammatory, apoptotic and testicular DNA damage | [125] |
| DNA Damage | Male Swiss Albino | 902.4 | 0.0516 W/kg | 4 or 8 h/day, 35 days | Significant increase in DNA damage | [123] |
| DNA Damage | Male C57BL/6 mice | 905 | 2.2 W/kg | 12 h/day, 1, 3 or 5 weeks | Elevated DNA oxidation and fragmentation (single strand break) and increased mitochondrial ROS generation after 1 week of exposure | [120] |
| DNA Damage | Male Swiss Albino | 1800 | 0.05 W/kg | 3 h/day, 120 days | Significant increase in testicular apoptosis due to elevated ROS levels with decrease in serum testosterone levels, sperm count and viability | [132] |
| DNA Damage | Male Sprague Dawley rat | 1800/2100 | 0.166 W/kg, 0.174 W/kg | 2 h/day, 6 months | Significant DNA single -strand fragmentation due to oxidative stress | [133] |
| DNA Damage | Male Wistar rat | 1910.5 | 1.34 W/kg | 2 h/day, 60 days | Increased MDA level and DNA strand break in sperm cells | [42] |
| DNA Damage | Male Wistar rat | 2400 | 0.1 W/kg | 24 h/day, 12 months | Significant increase in DNA damage in testes tissues | [116] |
| DNA Damage | Male Wistar rat | 2450 | 0.14 W/kg | 2 h/day, 45 days | Significant increase in sperm DNA damage, ROS, MDA, apoptosis, protein carbonyl content with decrease in testosterone level in testes | [41] |
| DNA Damage | Male Wistar rat | 2450 | 0.11 W/kg | 2 h/day, 35 days | Rise in DNA damage and cellular apoptosis | [61] |
| DNA Damage | Male Wistar rat | 10,000 | 0.014 W/kg | 2 h/day, 45 days | DNA strand break observed in sperm DNA in comet assay | [117] |
| DNA Damage | Rat testicular cells | 4G | - | 6 h/day, 150 days | Long term exposure impaired rat testis and unregulated testicular Spock-3 gene | [134] |
| Micronuclei | Male Wistar rat | 900 | 0.9 W/kg | 2 h/day, 35 days | Increase in micronuclei formation along with calatalse activity, MDA and ROS generation along with alteration in sperm cell cycle | [96] |
| Chromosomal Aberration | CBA/CEY male mice | 2450 | 0.05–20 W/kg | 30 min/day, 6 days/week, 2 weeks | Significant increase in sperm cell chromosomal chain translocation observed at diakinesis at metaphase I | [138] |
| Chromosomal Aberration | Male mice | 2450 | - | 30 min/day, 6 days/week, 2 weeks | No increase in sperm cell chromosomal aberrations | [139] |
| SCE | CBA/CEY male mice | 2450 | 0.05–20 W/kg | 30 min/day, 6 days/week, 2 weeks | Significant increase in sperm cell chromosomal chain translocation observed at diakinesis at metaphase I | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, P.; Rai, U.; Singh, R. Genotoxic Risks to Male Reproductive Health from Radiofrequency Radiation. Cells 2023, 12, 594. https://doi.org/10.3390/cells12040594
Kaur P, Rai U, Singh R. Genotoxic Risks to Male Reproductive Health from Radiofrequency Radiation. Cells. 2023; 12(4):594. https://doi.org/10.3390/cells12040594
Chicago/Turabian StyleKaur, Puneet, Umesh Rai, and Rajeev Singh. 2023. "Genotoxic Risks to Male Reproductive Health from Radiofrequency Radiation" Cells 12, no. 4: 594. https://doi.org/10.3390/cells12040594
APA StyleKaur, P., Rai, U., & Singh, R. (2023). Genotoxic Risks to Male Reproductive Health from Radiofrequency Radiation. Cells, 12(4), 594. https://doi.org/10.3390/cells12040594






