The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Results of the Investigations Using the NucleoCounter (ChemoMetec)
3.2. Validity of the Lipoaspirate Specimens Investigated Using Flow Cytometry As Representative Sample of the Lipoaspirate Specimens Investigated with the NucleoCounter (ChemoMetec)
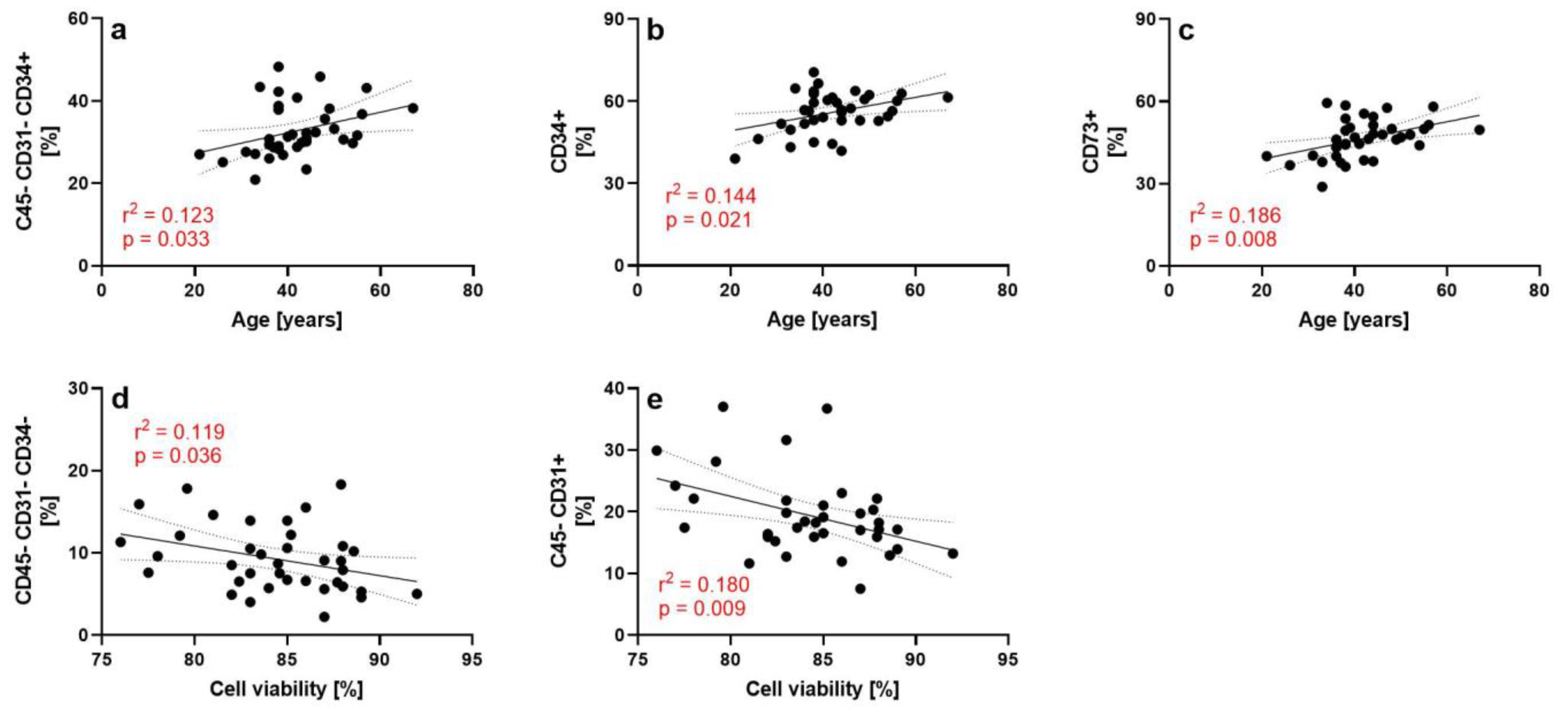
3.3. Results of the Flow Cytometry Investigations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurd, J.L.; Facile, T.R.; Weiss, J.; Hayes, M.; Hayes, M.; Furia, J.P.; Maffulli, N.; Winnier, G.E.; Alt, C.; Schmitz, C.; et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tear with fresh, uncultured, unmodified, autologous adipose derived regenerative cells (UA-ADRCs) isolated at the point of care: A prospective, randomized, controlled first-in-human pilot study. J. Orthop. Surg. Res. 2020, 15, 122. [Google Scholar] [PubMed]
- Alt, E.; Rothoerl, R.; Hoppert, M.; Frank, H.G.; Wuerfel, T.; Alt, C.; Schmitz, C. First immunohistochemical evidence of human tendon repair following stem cell injection: A case report and review of literature. World J. Stem Cells 2021, 13, 944–970. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Wuerfel, T.; Alt, C.; Alt, E.U. Behandlung von Sehnenrupturen mit Stammzellen: Eine aktuelle Übersicht [Treatment of tendon tears with stem cells: A current overview]. Obere Extrem. 2022, 17, 141–153. (In German) [Google Scholar] [CrossRef]
- Solakoglu, Ö.; Götz, W.; Kiessling, M.C.; Alt, C.; Schmitz, C.; Alt, E.U. Improved guided bone regeneration by combined application of unmodified, fresh autologous adipose derived regenerative cells and plasma rich in growth factors: A first-in-human case report and literature review. World J. Stem Cells 2019, 11, 124–146. [Google Scholar] [CrossRef]
- Alt, E.U.; Schmitz, C.; Bai, X. Perspective: Why and how ubiquitously distributed, vascular-associated, pluripotent stem cells in the adult body (vaPS Cells) are the next generation of medicine. Cells 2021, 10, 2303. [Google Scholar] [CrossRef]
- Schmitz, C.; Alt, C.; Pearce, D.A.; Furia, J.P.; Maffulli, N.; Alt, E.U. Methodological flaws in meta-analyses of clinical studies on the management of knee osteoarthritis with stem cells: A systematic review. Cells 2022, 11, 965. [Google Scholar] [CrossRef]
- Furia, J.P.; Lundeen, M.A.; Hurd, J.L.; Pearce, D.A.; Alt, C.; Alt, E.U.; Schmitz, C.; Maffulli, N. Why and how to use the body’s own stem cells for regeneration in musculoskeletal disorders: A primer. J. Orthop. Surg. Res. 2022, 17, 36. [Google Scholar] [CrossRef]
- Alt, E.U.; Winnier, G.; Haenel, A.; Rothoerl, R.; Solakoglu, O.; Alt, C.; Schmitz, C. Towards a comprehensive understanding of UA-ADRCs (uncultured, autologous, fresh, unmodified, adipose derived regenerative cells, isolated at point of care) in regenerative medicine. Cells 2020, 9, 1097. [Google Scholar] [CrossRef]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells 2011, 29, 749–754. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 5, 641–648. [Google Scholar]
- Andia, I.; Maffulli, N.; Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther. 2019, 19, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Winnier, G.E.; Valenzuela, N.; Peters-Hall, J.; Kellner, J.; Alt, C.; Alt, E.U. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS ONE 2019, 14, e0221457. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; Stevens, H.P.; Parvizi, M.; van der Lei, B.; Harmsen, M.C. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen. 2016, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Copcu, H.E.; Ozta, N.S. Not stromal vascular fraction (SVF) or nanofat, but total stromal-cells (TOST): A new definition. Systemic review of mechanical stromal-cell extraction techniques. Tissue Eng. Regen. Med. 2021, 18, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Tiryaki, T.; Womack, H.A.; Canikyan, S.; Schlaudraff, K.U.; Scheflan, M. Cellular optimization of nanofat: Comparison of two nanofat processing devices in terms of cell count and viability. Aesth. S. J. Open Forum 2019, 1, ojz028. [Google Scholar]
- Priglinger, E.; Schuh, C.M.A.P.; Steffenhagen, C.; Wurzer, C.; Maier, J.; Nuernberger, S.; Holnthoner, W.; Fuchs, C.; Suessner, S.; Rünzler, D.; et al. Improvement of adipose tissue-derived cells by low-energy extracorporeal shock wave therapy. Cytotherapy 2017, 19, 1079–1095. [Google Scholar] [CrossRef]
- Behfar, M.; Sarrafzadeh-Rezaei, F.; Hobbenaghi, R.; Delirezh, N.; Dalir-Naghadeh, B. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Curr. Stem Cell Res. Ther. 2012, 7, 173–178. [Google Scholar] [CrossRef]
- Behfar, M.; Javanmardi, S.; Sarrafzadeh-Rezaei, F. Comparative study on functional effects of allotransplantation of bone marrow stromal cells and adipose derived stromal vascular fraction on tendon repair: A biomechanical study in rabbits. Cell J. 2014, 16, 263–270. [Google Scholar]
- Kosaka, M.; Nakase, J.; Hayashi, K.; Tsuchiya, H. Adipose-derived regenerative cells promote tendon-bone healing in a rabbit model. Arthroscopy 2016, 32, 851–859. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Ellenhorn, J.D. Adipose stromal vascular fraction isolation: A head-to-head comparison of four commercial cell separation systems. Plast. Reconstr. Surg. 2013, 132, 932e–939e. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef] [PubMed]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell. Regen. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S.; Birnbaum, Z.E. Adipose stromal vascular fraction isolation: A head-to-head comparison of 4 cell separation systems #2. Ann. Plast. Surg. 2016, 77, 354–362. [Google Scholar]
- Condé-Green, A.; Kotamarti, V.S.; Sherman, L.S.; Keith, J.D.; Lee, E.S.; Granick, M.S.; Rameshwar, P. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: Review of upcoming techniques. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1017. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Shang, H.; Li, Y.; Yang, N.; Patel, N.; Katz, A.J. Isolation of adipose-derived stromal vascular fraction cells using a novel point-of-care device: Cell characterization and review of the literature. Tissue Eng. Part C Methods 2017, 23, 125–135. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: A systematic review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274. [Google Scholar] [CrossRef] [PubMed]
- Vilaboa, S.D.-A.; Navarro-Palou, M.; Llull, R. Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy 2014, 16, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Dykstra, J.A.; Blue, E.D.; Negrão de Assis, P.L.; Weimer, J.M.; Kota, D.J. Human adipose-derived stromal vascular fraction: Characterization, safety and therapeutic potential in an experimental mouse model of articular injury. J. Stem Cells Regen. Med. 2020, 16, 16–25. [Google Scholar]
- Chia, C.T.; Neinstein, R.M.; Theodorou, S.J. Evidence-based medicine: Liposuction. Plast. Reconstr. Surg. 2017, 139, 267e–274e. [Google Scholar] [CrossRef]
- Johnson, S.M.; Saint John, B.E.; Dine, A.P. Local anesthetics as antimicrobial agents: A review. Surg. Infect. 2008, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Fazly Bazzaz, B.S. A review and new insights to antimicrobial action of local anesthetics. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315.e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. CD4+ CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002, 2, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.G.; Hubbard, N.W.; Messmer, M.N.; Kofman, S.B.; Hagan, C.E.; Orozco, S.L.; Chiang, K.; Daniels, B.P.; Baker, D.; Oberst, A. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci. Immunol. 2019, 4, eaaw2004. [Google Scholar] [CrossRef]
- Labarre, K.W.; Zimmermann, G. Infiltration of the Hoffa’s fat pad with stromal vascular fraction in patients with osteoarthritis of the knee -Results after one year of follow-up-. Bone Rep. 2022, 16, 101168. [Google Scholar] [CrossRef]
- Markarian, C.F.; Frey, G.Z.; Silveira, M.D.; Chem, E.M.; Milani, A.R.; Ely, P.B.; Horn, A.P.; Nardi, N.B.; Camassola, M. Isolation of adipose-derived stem cells: A comparison among different methods. Biotechnol. Lett. 2014, 36, 693–702. [Google Scholar] [CrossRef]
- Kokai, L.E.; Traktuev, D.O.; Zhang, L.; Merfeld-Clauss, S.; DiBernardo, G.; Lu, H.; Marra, K.G.; Donnenberg, A.; Donnenberg, V.; Meyer, E.M.; et al. Adipose stem cell function maintained with age: An intra-subject study of long-term cryopreserved cells. Aesthet. Surg. J. 2017, 37, 454–463. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.W.; Lee, C.Y.; Lee, J.; Shin, S.; Lim, S.; Lee, S.; Kim, I.K.; Lee, H.B.; Hwang, K.C. Effects of donor age on human adipose-derived adherent stromal cells under oxidative stress conditions. J. Int. Med. Res. 2018, 46, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.E.S.; Iminitoff, M.; Williams, E.; Damani, T.; Jackson-Patel, V.; Fan, V.; James, J.; Dunbar, P.R.; Feisst, V.; Sheppard, H.M. Ex vivo human adipose tissue derived mesenchymal stromal cells (ASC) are a heterogeneous population that demonstrate rapid culture-induced changes. Front. Pharmacol. 2020, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Ruoss, S.; Walker, J.T.; Nasamran, C.A.; Fisch, K.M.; Paez, C.J.; Parekh, J.N.; Ball, S.T.; Chen, J.L.; Ahmed, S.S.; Ward, S.R. Strategies to identify mesenchymal stromal cells in minimally manipulated human bone marrow aspirate concentrate lack consensus. Am. J. Sports Med. 2021, 49, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiang, Q.; Xu, F.; Huang, J.; Yu, N.; Zhang, Q.; Long, X.; Zhou, Z. Single-cell RNA-seq of cultured human adipose-derived mesenchymal stem cells. Sci. Data 2019, 6, 190031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes isolated from adipose-derived stem cells: A new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, Q.; Song, W.; Yu, W.; He, Y. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am. J. Sports Med. 2020, 48, 1456–1464. [Google Scholar] [CrossRef]
- Fu, G.; Lu, L.; Pan, Z.; Fan, A.; Yin, F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen. Med. 2021, 16, 359–372. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Shi, M.; Zhang, T.; Lu, W.; Yang, S.; Cui, Q.; Li, Z. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res. Ther. 2021, 12, 338. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, Z.Y.; Lin, Y.H.; Chen, S.H.; Chou, P.Y.; Kao, H.K.; Lin, F.H. Extracellular vesicles of adipose-derived stem cells promote the healing of traumatized Achilles tendons. Int. J. Mol. Sci. 2021, 22, 12373. [Google Scholar] [CrossRef]
- Uysal, A.C.; Mizuno, H. Differentiation of adipose-derived stem cells for tendon repair. Methods Mol. Biol. 2011, 702, 443–451. [Google Scholar] [CrossRef]
- Geburek, F.; Mundle, K.; Conrad, S.; Hellige, M.; Walliser, U.; van Schie, H.T.; van Weeren, R.; Skutella, T.; Stadler, P.M. Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions—A pilot study. Stem Cell Res. Ther. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Vindigni, V.; Tonello, C.; Lancerotto, L.; Abatangelo, G.; Cortivo, R.; Zavan, B.; Bassetto, F. Preliminary report of in vitro reconstruction of a vascularized tendonlike structure: A novel application for adipose-derived stem cells. Ann. Plast. Surg. 2013, 71, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Blaine, T.A.; Kim, Y.S.; Voloshin, I.; Chen, D.; Murakami, K.; Chang, S.S.; Winchester, R.; Lee, F.Y.; O’keefe, R.J.; Bigliani, L.U. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J. Shoulder Elb. Surg. 2005, 14, 84S–89S. [Google Scholar] [CrossRef] [PubMed]
- Shindle, M.K.; Chen, C.C.; Robertson, C.; DiTullio, A.E.; Paulus, M.C.; Clinton, C.M.; Cordasco, F.A.; Rodeo, S.A.; Warren, R.F. Full-thickness supraspinatus tears are associated with more synovial inflammation and tissue degeneration than partial-thickness tears. J. Shoulder Elb. Surg. 2011, 20, 917–927. [Google Scholar] [CrossRef]
- Brandt, L.; Schubert, S.; Scheibe, P.; Brehm, W.; Franzen, J.; Gross, C.; Burk, J. Tenogenic properties of mesenchymal progenitor cells are compromised in an inflammatory environment. Int. J. Mol. Sci. 2018, 19, 2549. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Schonk, M.M.; Kharaz, Y.A.; Comerford, E.; Mendias, C.L. Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight 2020, 5, e138295. [Google Scholar] [CrossRef]
- Li, J.; Stoppato, M.; Schiele, N.R.; Graybeal, K.L.; Nguyen, P.K.; Kuo, C.K. Embryonic and postnatal tendon cells respond differently to interleukin-1β. Ann. N. Y. Acad. Sci. 2019, 1442, 118–127. [Google Scholar] [CrossRef]
- Krane, S.M.; Byrne, M.H.; Lemaître, V.; Henriet, P.; Jeffrey, J.J.; Witter, J.P.; Liu, X.; Wu, H.; Jaenisch, R.; Eeckhout, Y. Different collagenase gene products have different roles in degradation of type I collagen. J. Biol. Chem. 1996, 271, 28509–28515. [Google Scholar] [CrossRef]
- Yaoita, H.; Foidart, J.M.; Katz, S.I. Localization of the collagenous component in skin basement membrane. J. Investig. Dermatol. 1978, 70, 191–193. [Google Scholar] [CrossRef]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Robey, P.G.; Abe, S. Partial purification and characterization of a neutral protease which cleaves type IV collagen. Biochemistry 1981, 20, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Chang, B.; Traktuev, D.O.; Sun, J.; March, K.; Chan, L.; Sage, E.H.; Pasqualini, R.; Arap, W.; Kolonin, M.G. IFATS collection: Combinatorial peptides identify alpha5beta1 integrin as a receptor for the matricellular protein SPARC on adipose stromal cells. Stem Cells 2008, 26, 2735–2745. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.; Kolonin, M.G. Proteolytic isoforms of SPARC induce adipose stromal cell mobilization in obesity. Stem Cells 2016, 34, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Hurd, J. Autologous Adult Adipose-Derived Regenerative Cell Injection into Chronic Partial-Thickness Rotator Cuff Tears. ClinicalTrials.gov Identifier: NCT03752827. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03752827 (accessed on 1 November 2022).
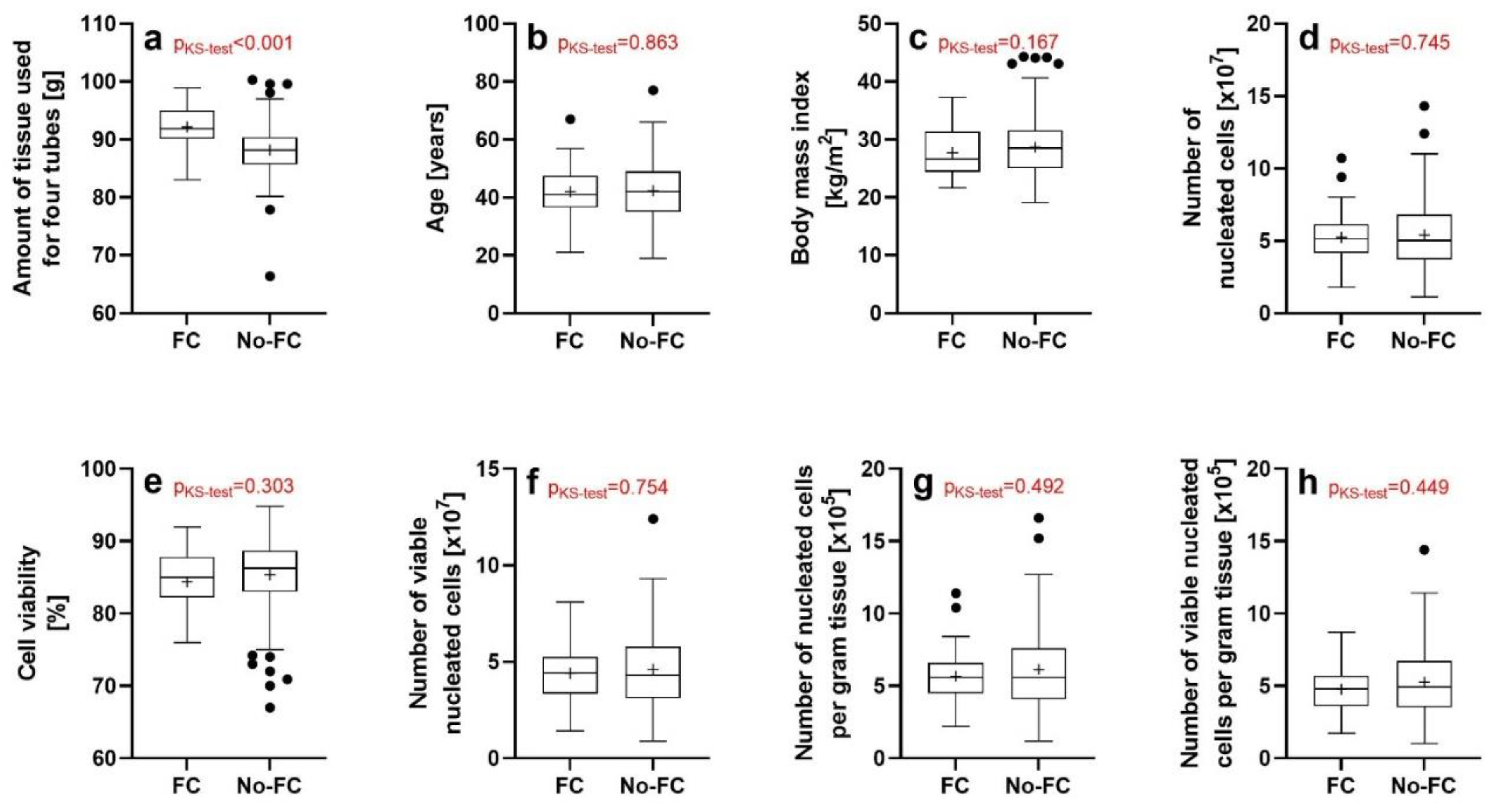
| Variable | Mean | SD | SEM | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| Age | 42.3 | 9.9 | 0.7 | 19 | 42 | 77 |
| BMI | 28.5 | 4.8 | 0.3 | 19.1 | 28.2 | 44.3 |
| Tissue [g] | 88.8 | 4.5 | 0.3 | 66.4 | 89.0 | 100.3 |
| Sex | Female, n = 207; Male, n = 25 | |||||
| Ethnicity | Caucasian, n = 153; Hispanic, n = 43; Black, n = 22; Asian, n = 7; African American, n = 3; Arabic, n = 2; Unknown, n = 2 | |||||
| Kit used | Transpose RT original, n = 60; Transpose RT ultra, n = 172 | |||||
| CD | Clone | Isotype | Conjugate | Provider | Catalog # |
|---|---|---|---|---|---|
| CD3 | OKT3 | IgG2a, kappa | PE | eBioscience/Thermo Fisher | 12-0037-42 |
| CD4 | RPA-T4 | IgG1, kappa | FITC | eBioscience/Thermo Fisher | 11-0049-42 |
| CD14 | 61D3 | IgG1, kappa | PE | eBioscience/Thermo Fisher | 12-0149-42 |
| CD16 | CB16 | IgG1, kappa | FITC | eBioscience/Thermo Fisher | 11-0168-42 |
| CD19 | HIB19 | IgG1, kappa | APC | eBioscience/Thermo Fisher | 17-0199-42 |
| CD25 | BC96 | IgG1, kappa | PE | eBioscience/Thermo Fisher | 12-0259-42 |
| CD31 | WM59 | IgG1, kappa | PE | eBioscience/Thermo Fisher | 12-0319-42 |
| CD33 | WM53 | IgG1, kappa | FITC | eBioscience/Thermo Fisher | 11-0338-42 |
| CD34 | 581 | IgG1, kappa | PE-Cy | BD Pharmingen/BD Biosciences | 555823 |
| CD45 | HI30 | IgG1 | PerCP | Thermo Fisher | MHCD4531 |
| CD45 | HI30 | IgG1, kappa | PerCP-eFluor71m | eBioscience/fisher scientific | 50-245-943 |
| CD73 | AD2 | IgG1, kappa | APC | eBioscience/Thermo Fisher | 17-0739-42 |
| CD90 | 5E10 | IgG1, kappa | PE | eBioscience/Thermo Fisher | 12-0909-42 |
| CD105 | MEM-226 | IgG2a | FITC | Thermo Fisher | MA1-19594 |
| CD117 | YB5.B8 | IgG1, kappa | PE | eBioscience/Thermo Fisher | 12-1179-42 |
| CD127 | eBioRDR5 | IgG1, kappa | APC | eBioscience/Thermo Fisher | 12-1179-42 |
| CD144 | 16B1 | IgG1 | Alexa Fluor 488 | eBioscience/Thermo Fisher | 53-1449-42 |
| CD146 | P1H12 | IgG1, kappa | FITC | eBioscience/Thermo Fisher | 11-1469-42 |
| CD206 | 19.2 | IgG1, kappa | APC | eBioscience/Thermo Fisher | 17-2069-42 |
| Flow Cytometry Tube 1 | Flow Cytometry Tube 2 | Flow Cytometry Tube 3 | Flow Cytometry Tube 4 | ||||
|---|---|---|---|---|---|---|---|
| M | C | M | C | M | C | M | C |
| CD45 | PerCP | CD45 | PerCP | CD45 | PerCP | CD45 | PerCP |
| CD73 | APC | CD4 | FITC | CD34 | PE-Cy | CD14 | PE |
| CD90 | PE | CD25 | PE | CD105 | FITC | CD16 | FITC |
| CD105 | FITC | CD127 | APC | CD117 | PE | CD206 | APC |
| Flow Cytometry Tube 5 | Flow Cytometry Tube 6 | Flow Cytometry Tube 7 | |||||
| M | C | M | C | M | C | ||
| CD45 | PerCP | CD45 | PerCP | CD45 | PerCP | ||
| CD3 | PE | CD31 | PE | CD31 | PE | ||
| CD19 | APC | CD34 | PE-Cy | CD34 | PE-Cy | ||
| CD33 | FITC | CD146 | FITC | CD144 | Alexa Fluor 488 | ||
| Variable | Mean | SD | SEM | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| V1 [×107] | 5.38 | 2.33 | 0.15 | 1.1 | 5.0 | 14.3 |
| V2 [%] | 85.2 | 4.78 | 0.31 | 67.0 | 86.0 | 94.8 |
| V3 [×107] | 4.59 | 1.98 | 0.13 | 0.9 | 4.4 | 12.4 |
| V4 [×105/g] | 6.06 | 2.67 | 0.18 | 1.2 | 5.6 | 16.6 |
| V5 [×105/g] | 5.18 | 2.28 | 0.15 | 1.0 | 4.9 | 14.4 |
| A | S | B | E | T | K | K × S | K × E | S × E | K × S × E | |
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 0.077 | 0.141 | 0.075 | 0.139 | 0.155 | 0.707 | 0.582 | 0.857 | 0.979 | 0.154 |
| V2 | 0.669 | 0.978 | 0.294 | 0.416 | 0.312 | 0.840 | 0.154 | 0.432 | 0.454 | 0.662 |
| V3 | 0.085 | 0.125 | 0.047 | 0.085 | 0.216 | 0.719 | 0.655 | 0.807 | 0.963 | 0.146 |
| V4 | 0.073 | 0.110 | 0.071 | 0.112 | 0.854 | 0.769 | 0.565 | 0.833 | 0.979 | 0.124 |
| V5 | 0.081 | 0.097 | 0.044 | 0.066 | 0.738 | 0.773 | 0.638 | 0.773 | 0.961 | 0.117 |
| Surface Markers | Cell Type | Reference | n | Mean | SD | SEM | Min | Med | Max |
|---|---|---|---|---|---|---|---|---|---|
| CD45- | CD45- cell group | 37 | 58.0 | 7.7 | 1.3 | 42.1 | 57.6 | 72.1 | |
| CD45- CD73+ CD90+ | (ADSCs) | [33] | 37 | 32.5 | 8.7 | 1.4 | 20.4 | 30.0 | 61.4 |
| CD45- CD73+ CD90+ CD105+ | ADSCs | [33] | 37 | 2.4 | 1.4 | 0.2 | 0.2 | 2.0 | 6.7 |
| CD45- CD31+ | Endothelial cells | [25] | 37 | 19.3 | 6.5 | 1.1 | 7.5 | 17.4 | 37.0 |
| CD45- CD31+ CD34+ | Endothelial progenitors | [25] | 37 | 15.3 | 4.5 | 0.7 | 7.3 | 14.4 | 28.1 |
| CD45- CD31+ CD34+ CD146+ | Pericytes | [25] | 37 | 13.2 | 4.0 | 0.7 | 3.8 | 13.2 | 24.9 |
| CD45- CD31- | CD45- CD31- group | [25] | 37 | 36.5 | 5.5 | 0.9 | 28.3 | 35.6 | 51.8 |
| CD45- CD31- CD34+ | Stromal fraction Adipose progenitors SVF progenitor cells Mesenchymal stem/stromal cells | [23] [25] [28] [29] | 37 | 32.8 | 6.5 | 1.1 | 20.9 | 30.9 | 48.3 |
| CD45- CD31- CD34+ CD146+ | Pericyte progenitors | [25] | 37 | 0.7 | 0.4 | 0.1 | 0.1 | 0.5 | 2.1 |
| CD45- CD31- CD34- | SVF nonprogenitors | [25] | 37 | 9.2 | 4.0 | 0.7 | 2.2 | 8.7 | 18.3 |
| CD45- CD31- CD34- CD146+ | Pericytes | [25] | 37 | 6.5 | 4.5 | 0.7 | 0.6 | 5.4 | 16.0 |
| CD45+ | CD45+ cell group | 37 | 42.0 | 7.7 | 1.3 | 27.9 | 42.4 | 57.9 | |
| CD45+ CD34+ | Leukocyte progenitors | [25] | 27 | 1.7 | 1.4 | 0.3 | 0.6 | 1.4 | 7.8 |
| CD45+ CD206+ | M2 macrophages | [25] | 37 | 16.4 | 4.3 | 0.7 | 8.1 | 16.0 | 26.1 |
| CD45+ CD4+ CD25- | Naïve T cells | [34] | 20 | 4.0 | 2.0 | 0.4 | 1.4 | 3.8 | 9.0 |
| CD45+ CD4+ CD25+ | Regulatory T cells | [35] | 20 | 4.3 | 1.9 | 0.4 | 0.7 | 4.8 | 8.6 |
| CD14+ | [29] | 37 | 18.9 | 5.8 | 1.0 | 8.1 | 18.4 | 31.8 | |
| CD31+ | [36] | 37 | 49.6 | 5.8 | 0.9 | 39.1 | 50.8 | 58.8 | |
| CD34+ | [36] | 37 | 56.0 | 7.3 | 1.2 | 39.0 | 56.7 | 70.6 | |
| CD73+ | [36] | 37 | 46.5 | 7.1 | 1.2 | 28.8 | 46.8 | 59.4 | |
| CD90+ | [36] | 37 | 56.6 | 8.0 | 1.3 | 40.6 | 57.8 | 74.8 | |
| CD105+ | [36] | 37 | 24.1 | 6.1 | 1.0 | 2.3 | 24.4 | 38.1 |
| Surface Markers | A | S | BMI | E | T | V2 | V4 |
|---|---|---|---|---|---|---|---|
| CD45− | 0.973 | 0.632 | 0.411 | 0.411 | 0.847 | 0.139 | 0.141 |
| CD45− CD73+ CD90+ | 0.100 | 0.002 | 0.373 | 0.402 | 0.483 | 0.617 | 0.060 |
| CD45− CD73+ CD90+ CD105+ | 0.858 | 0.547 | 0.769 | 0.762 | 0.464 | 0.156 | 0.295 |
| CD45− CD31+ | 0.306 | 0.494 | 0.469 | 0.648 | 0.659 | 0.025 | 0.343 |
| CD45− CD31 + CD34+ | 0.390 | 0.836 | 0.198 | 0.962 | 0.702 | 0.089 | 0.232 |
| CD45− CD31+ CD34+ CD146+ | 0.625 | 0.930 | 0.455 | 0.998 | 0.755 | 0.458 | 0.686 |
| CD45− CD31- | 0.157 | 0.914 | 0.918 | 0.638 | 0.508 | 0.656 | 0.386 |
| CD45− CD31− CD34+ | 0.049 | 0.908 | 0.462 | 0.397 | 0.742 | 0.186 | 0.819 |
| CD45− CD31− CD34+ CD146+ | 0.976 | 0.724 | 0.456 | 0.688 | 0.706 | 0.731 | 0.441 |
| CD45− CD31− CD34- | 0.099 | 0.719 | 0.507 | 0.339 | 0.852 | 0.035 | 0.129 |
| CD45− CD31− CD34− CD146+ | 0.145 | 0.680 | 0.114 | 0.179 | 0.578 | 0.037 | 0.545 |
| CD45+ | 0.969 | 0.625 | 0.406 | 0.437 | 0.859 | 0.139 | 0.148 |
| CD45+ CD34+ | 0.045 | --* | 0.186 | 0.600 | 0.073 | 0.521 | 0.976 |
| CD45+ CD206+ | 0.747 | 0.483 | 0.409 | 0.781 | 0.657 | 0.313 | 0.488 |
| CD45+ CD4+ CD25- | 0.390 | --* | 0.188 | 0.487 | 0.697 | 0.182 | 0.956 |
| CD45+ CD4+ CD25+ | 0.139 | --* | 0.572 | 0.278 | 0.645 | 0.108 | 0.501 |
| CD14+ | 0.919 | 0.794 | 0.181 | 0.292 | 0.709 | 0.440 | 0.952 |
| CD31+ | 0.041 | 0.931 | 0.757 | 0.372 | 0.454 | 0.362 | 0.327 |
| CD34+ | 0.008 | 0.826 | 0.035 | 0.030 | 0.820 | 0.274 | 0.478 |
| CD73+ | 0.017 | 0.671 | 0.553 | 0.073 | 0.934 | 0.293 | 0.617 |
| CD90+ | 0.273 | 0.245 | 0.351 | 0.368 | 0.671 | 0.941 | 0.356 |
| CD105+ | 0.590 | 0.903 | 0.601 | 0.610 | 0.854 | 0.664 | 0.267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz, C.; Alt, C.; Azares, A.R.; Pearce, D.A.; Facile, T.R.; Furia, J.P.; Maffulli, N.; Huang, C.; Alt, E.U. The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity. Cells 2023, 12, 30. https://doi.org/10.3390/cells12010030
Schmitz C, Alt C, Azares AR, Pearce DA, Facile TR, Furia JP, Maffulli N, Huang C, Alt EU. The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity. Cells. 2023; 12(1):30. https://doi.org/10.3390/cells12010030
Chicago/Turabian StyleSchmitz, Christoph, Christopher Alt, Alon R. Azares, David A. Pearce, Tiffany R. Facile, John P. Furia, Nicola Maffulli, Claire Huang, and Eckhard U. Alt. 2023. "The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity" Cells 12, no. 1: 30. https://doi.org/10.3390/cells12010030
APA StyleSchmitz, C., Alt, C., Azares, A. R., Pearce, D. A., Facile, T. R., Furia, J. P., Maffulli, N., Huang, C., & Alt, E. U. (2023). The Composition of Adipose-Derived Regenerative Cells Isolated from Lipoaspirate Using a Point of Care System Does Not Depend on the Subject’s Individual Age, Sex, Body Mass Index and Ethnicity. Cells, 12(1), 30. https://doi.org/10.3390/cells12010030









