Cell Surface Charge Mapping Using a Microelectrode Array on ITO Substrate
Abstract
1. Introduction
2. Materials and Methods
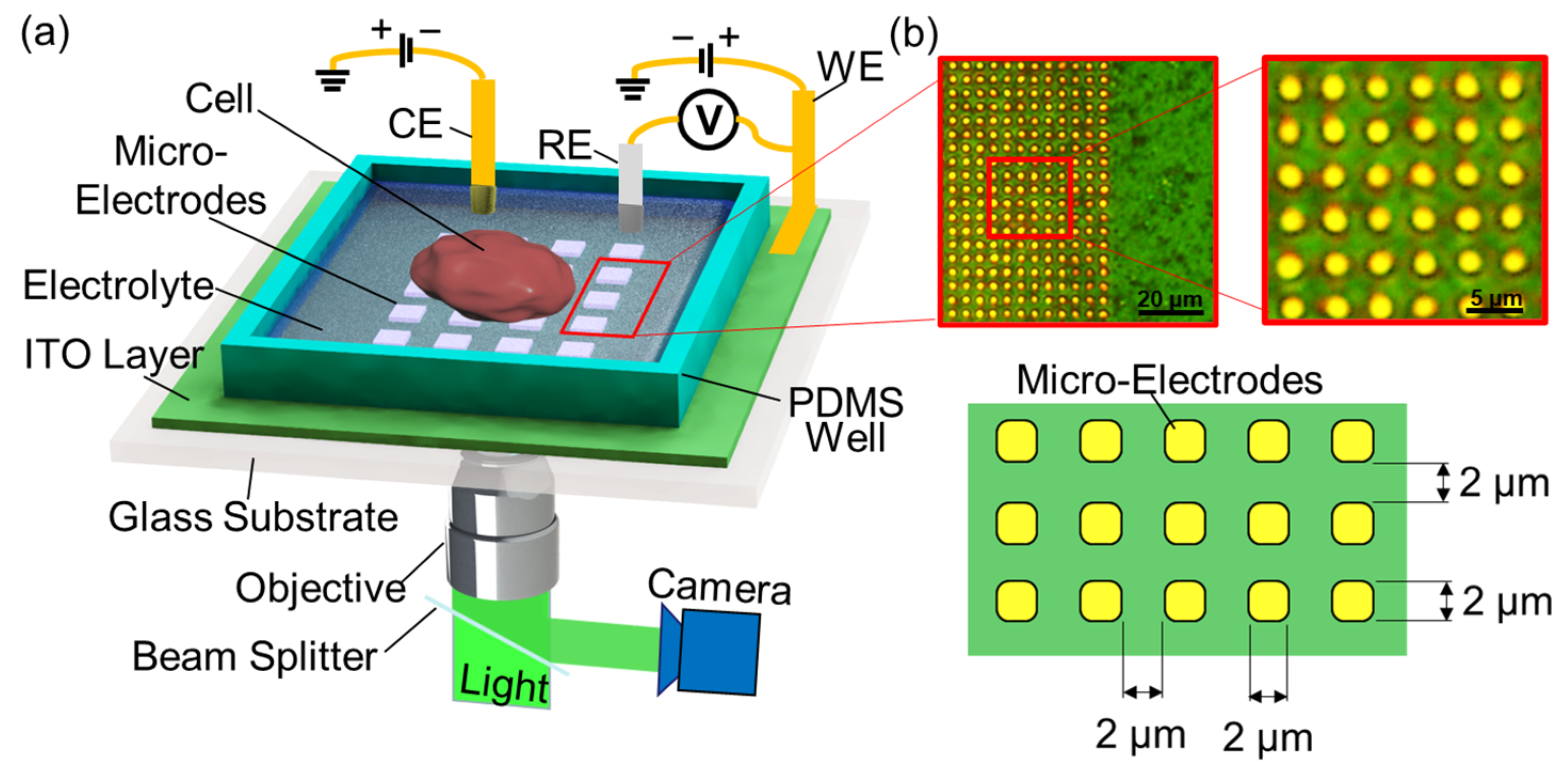
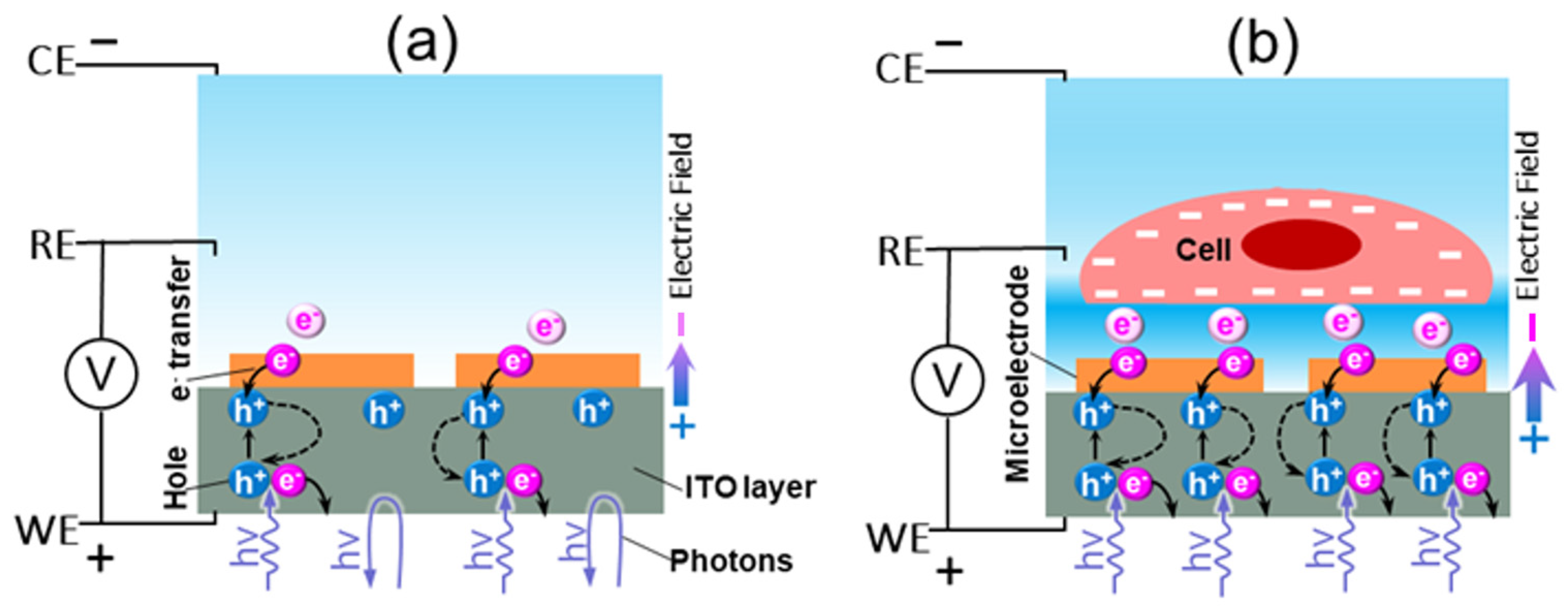
2.1. Detection Principle and Measurement Setup
2.2. Microelectrode Fabrication
2.3. Cell Culture
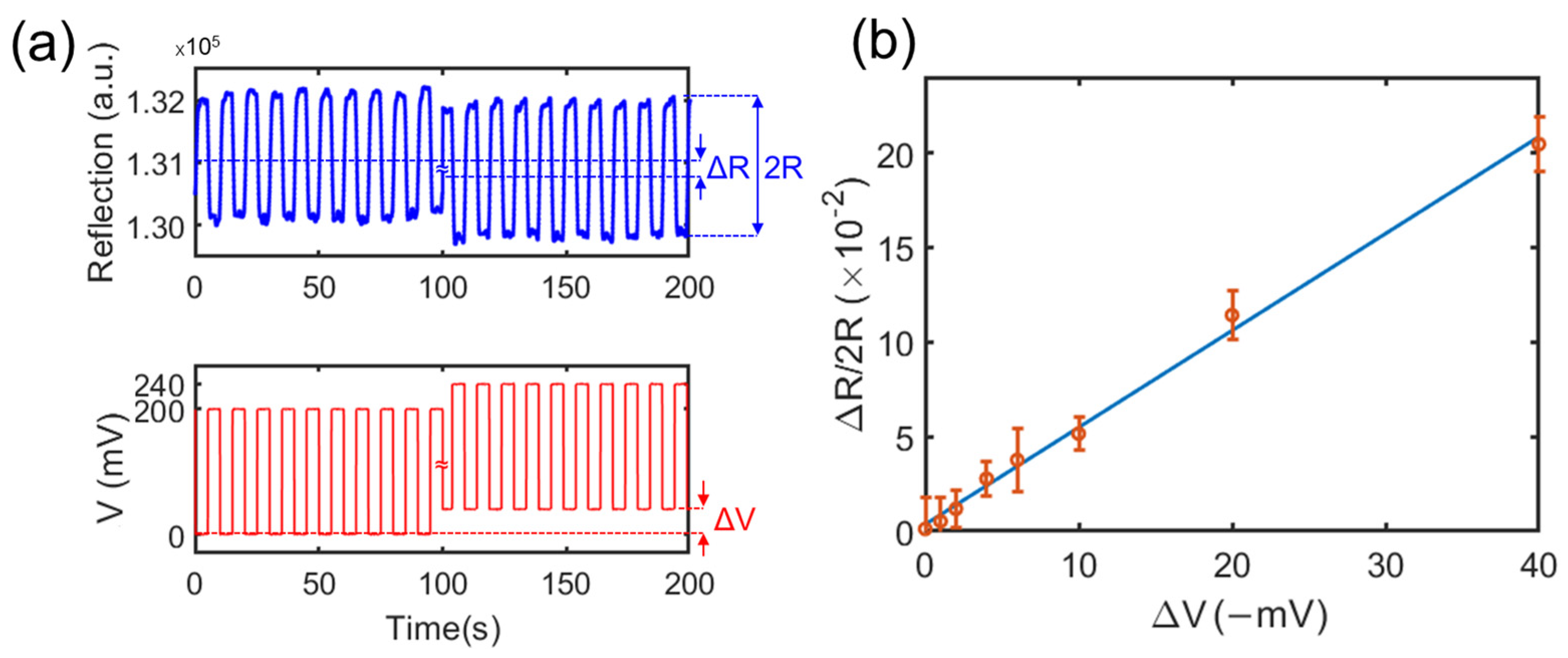
2.4. Calibration of the Relation between the Potential and the Light Intensity
3. Results
3.1. Validation of the Method via Charged Microparticles
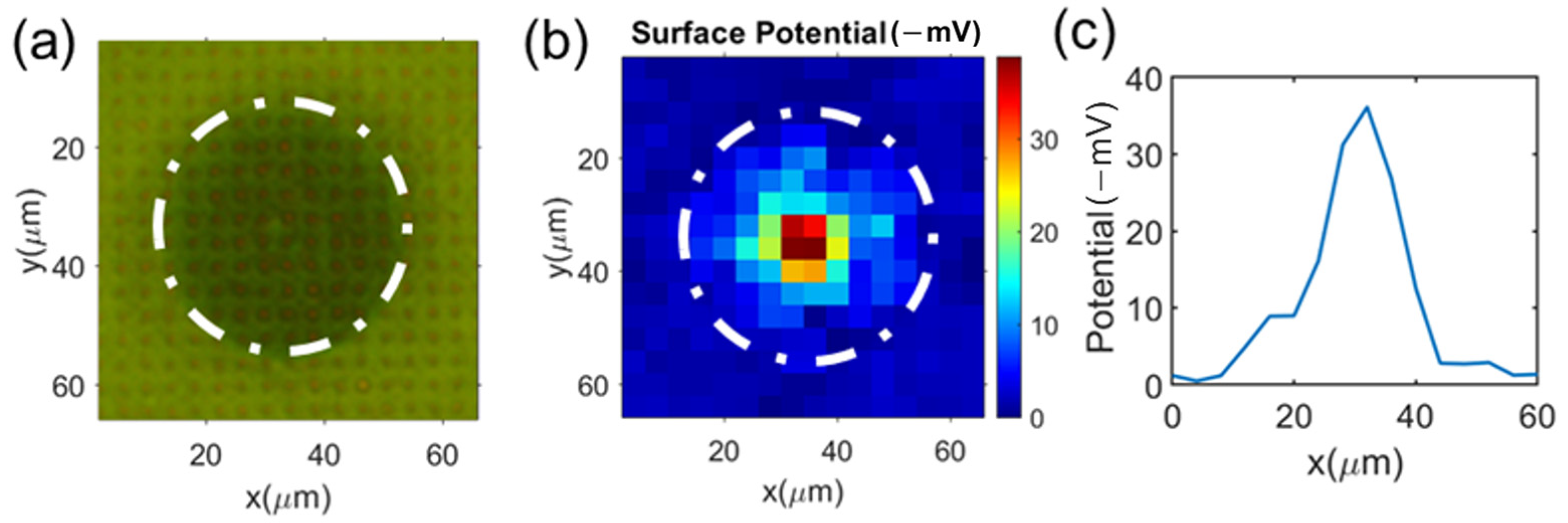
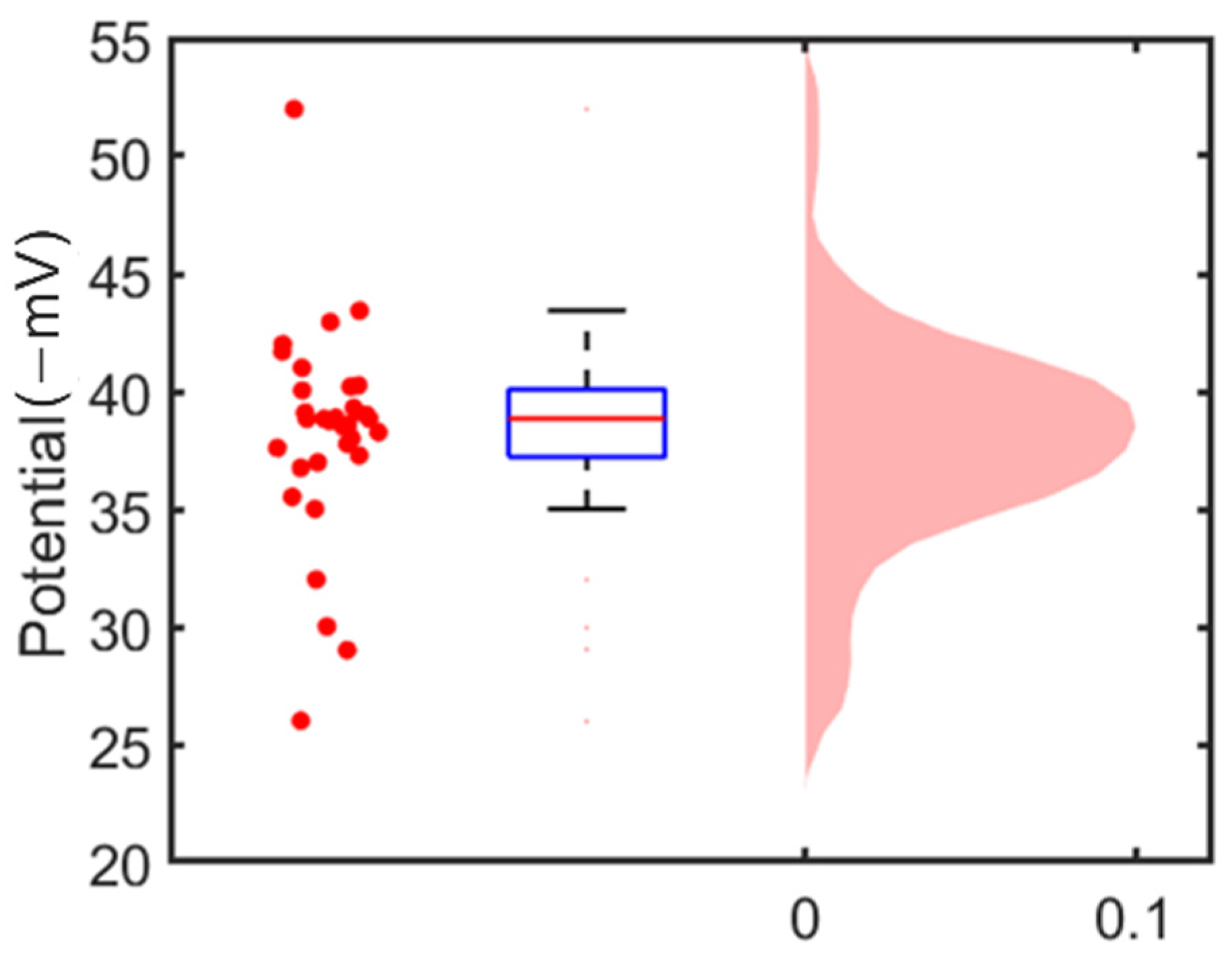
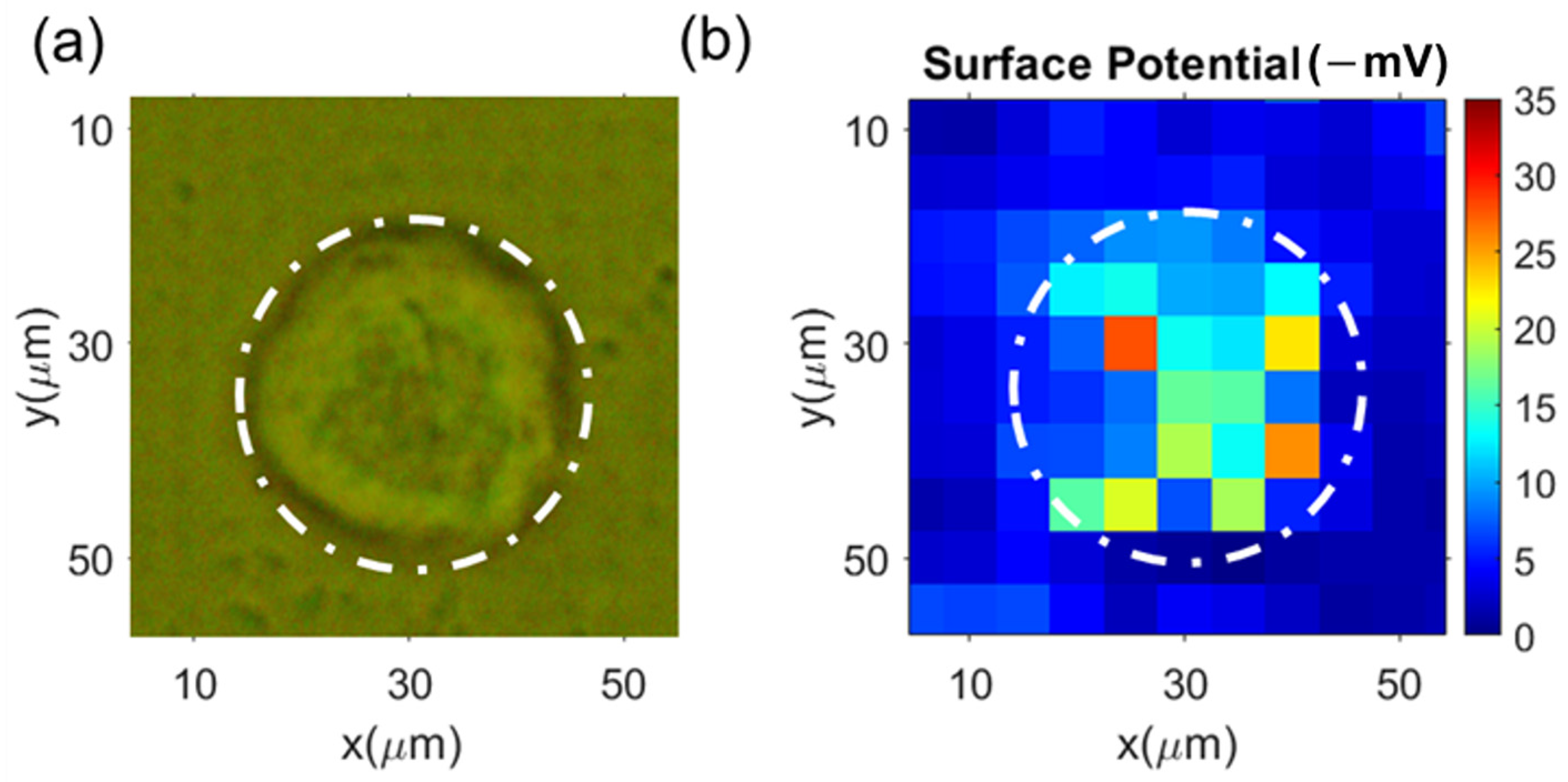
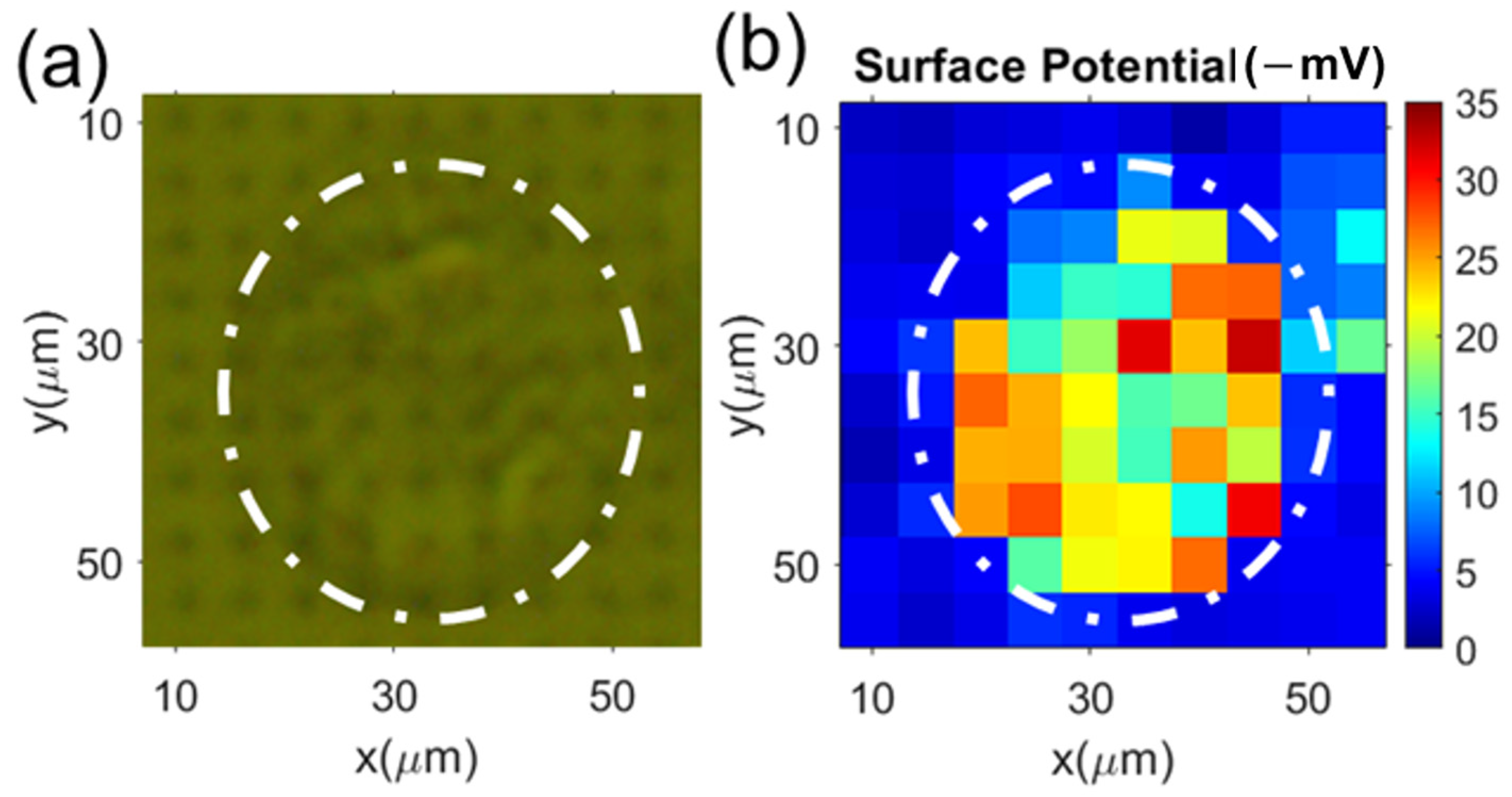
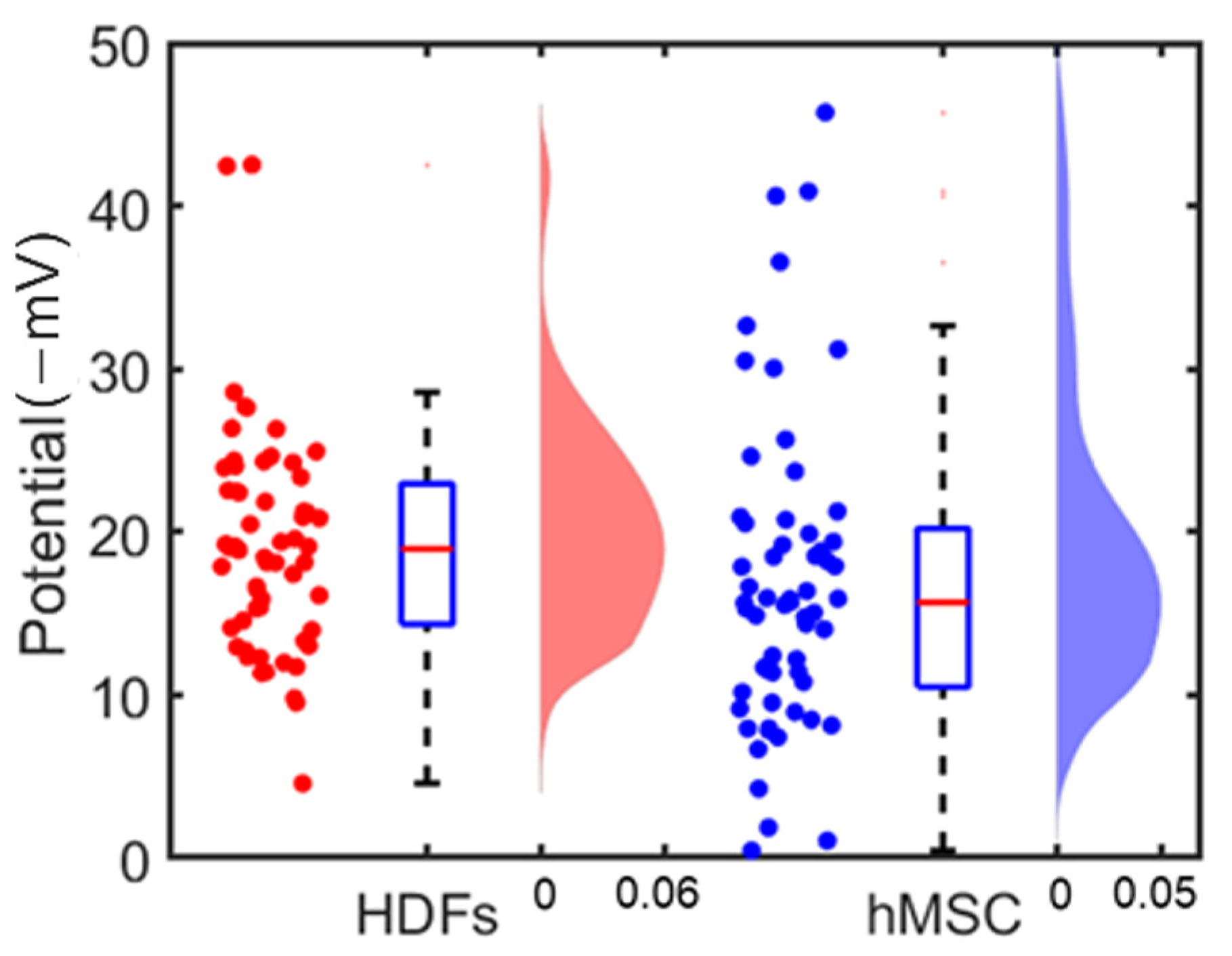
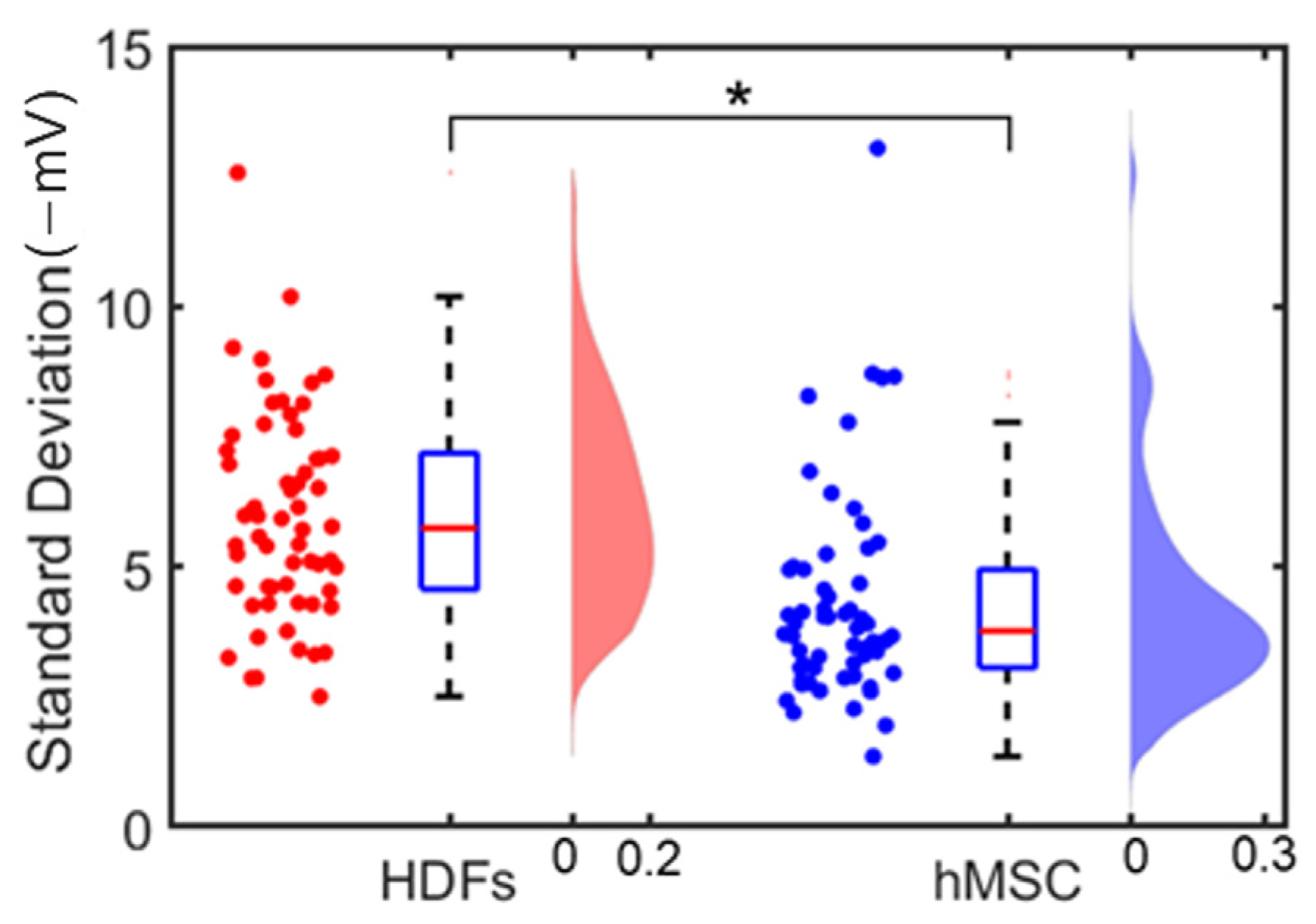
3.2. Single Cell’s Surface Charge Mapping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakhti, M.; Snaidero, N.; Schneider, D.; Aggarwal, S.; Mobius, W.; Janshoff, A.; Eckhardt, M.; Nave, K.A.; Simons, M. Loss of electrostatic cell-surface repulsion mediates myelin membrane adhesion and compaction in the central nervous system. Proc. Natl. Acad Sci. USA 2013, 110, 3143–3148. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Bi, Y.; Yang, W.; Guo, X.; Jiang, Y.; Wan, C.; Li, L.; Bai, Y.; Guo, J.; Wang, Y.; et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature 2013, 493, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-D.; Hong, K.; Papahadjopoulos, D. Recognition of liposomes by cells: In vitro binding and endocytosis mediated by specific lipid headgroups and surface charge density. Biochim. Biophys. Acta (BBA)-Biomembr. 1992, 1103, 185–197. [Google Scholar] [CrossRef]
- Chou, Y.S.; Lu, J.N.; Li, Y.C.; Wang, J.H.; Young, T.H. The Surface Potential Variation of Neural Stem/Progenitor Cells during Differentiation Process. J. Neurol. Neurosci. 2015, 6, 3. [Google Scholar] [CrossRef]
- Ma, Y.; Poole, K.; Goyette, J.; Gaus, K. Introducing Membrane Charge and Membrane Potential to T Cell Signaling. Front. Immunol. 2017, 8, 1513. [Google Scholar] [CrossRef]
- Eisenberg, S.; Haimov, E.; Walpole, G.F.W.; Plumb, J.; Kozlov, M.M.; Grinstein, S. Mapping the electrostatic profiles of cellular membranes. Mol. Biol. Cell 2021, 32, 301–310. [Google Scholar] [CrossRef]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S.I. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar] [CrossRef]
- Le, W.; Chen, B.; Cui, Z.; Liu, Z.; Shi, D. Detection of cancer cells based on glycolytic-regulated surface electrical charges. Biophys. Rep. 2019, 5, 10–18. [Google Scholar] [CrossRef]
- Jachimska, B.; Wasilewska, M.; Adamczyk, Z. Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements. Langmuir 2008, 24, 6866–6872. [Google Scholar] [CrossRef]
- Ni, L.; Shaik, R.; Xu, R.; Zhang, G.; Zhe, J. A Microfluidic Sensor for Continuous, in Situ Surface Charge Measurement of Single Cells. ACS Sens. 2020, 5, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, G.; Tezcan, G.; Meloni, G.N.; Unwin, P.R.; Kranz, C. Probing and Visualizing Interfacial Charge at Surfaces in Aqueous Solution. Annu. Rev. Anal. Chem. 2022, 15, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Heinz, W.F.; Hoh, J.H. Relative Surface Charge Density Mapping with the Atomic Force Microscope. Biophys. J. 1999, 76, 528–538. [Google Scholar] [CrossRef]
- Kumar, N.; Zhao, C.; Klaassen, A.; van den Ende, D.; Mugele, F.; Siretanu, I. Characterization of the surface charge distribution on kaolinite particles using high resolution atomic force microscopy. Geochim. Cosmochim. Acta 2016, 175, 100–112. [Google Scholar] [CrossRef]
- Page, A.; Perry, D.; Young, P.; Mitchell, D.; Frenguelli, B.G.; Unwin, P.R. Fast Nanoscale Surface Charge Mapping with Pulsed-Potential Scanning Ion Conductance Microscopy. Anal. Chem. 2016, 88, 10854–10859. [Google Scholar] [CrossRef]
- Cremin, K.; Jones, B.A.; Teahan, J.; Meloni, G.N.; Perry, D.; Zerfass, C.; Asally, M.; Soyer, O.S.; Unwin, P.R. Scanning Ion Conductance Microscopy Reveals Differences in the Ionic Environments of Gram-Positive and Negative Bacteria. Anal. Chem. 2020, 92, 16024–16032. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Moritz, W.; Talabani, H.; Xu, M.; Sabot, A.; Ensell, G. Scanning Photo-Induced Impedance Microscopy—Resolution studies and polymer characterization. Electrochim. Acta 2006, 51, 1423–1430. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, B.; Wang, J.; Zhong, M.; Das, A.; Watkinson, M.; Hing, K.; Zhang, D.W.; Krause, S. Photoelectrochemical Imaging System for the Mapping of Cell Surface Charges. Anal. Chem. 2019, 91, 5896–5903. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, F.; Wang, H.; Lu, Z.; Chen, H.Y.; Li, J.; Tao, N. Optical Imaging of Charges with Atomically Thin Molybdenum Disulfide. ACS Nano 2019, 13, 2298–2306. [Google Scholar] [CrossRef]
- Herzberg, M.; Dobberschütz, S.; Okhrimenko, D.; Bovet, N.E.; Andersson, M.P.; Stipp, S.L.S.; Hassenkam, T. Comparison of atomic force microscopy and zeta potential derived surface charge density. Europhys. Lett. 2020, 130, 36001. [Google Scholar] [CrossRef]
- Wagner, T.; Shigiahara, N.; Miyamoto, K.; Suzurikawa, J.; Finger, F.; Schöning, M.J.; Yoshinobu, T. Light-addressable Potentiometric Sensors and Light–addressable Electrodes as a Combined Sensor-and-manipulator Microsystem with High Flexibility. Procedia Eng. 2012, 47, 890–893. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Batool, F.; Ali, R.; Zahra, Q.u.A.; Wang, W.; Li, S.; Wang, G.; Liu, L.; Khan, S.U.; Mansoor, M.; et al. Tailoring radiotherapies and nanotechnology for targeted treatment of solid tumors. Coord. Chem. Rev. 2022, 472, 214757. [Google Scholar] [CrossRef]
- Ouyang, L.; Shaik, R.; Xu, R.; Zhang, G.; Zhe, J. Mapping Surface Charge Distribution of Single-Cell via Charged Nanoparticle. Cells 2021, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Sablon, K.A.; Little, J.W.; Mitin, V.; Sergeev, A.; Vagidov, N.; Reinhardt, K. Strong enhancement of solar cell efficiency due to quantum dots with built-in charge. Nano Lett. 2011, 11, 2311–2317. [Google Scholar] [CrossRef]
- Tan, S.-y.; Perry, D.; Unwin, P.R. Double layer effects in voltammetric measurements with scanning electrochemical microscopy (SECM). J. Electroanal. Chem. 2018, 819, 240–250. [Google Scholar] [CrossRef]
- Turiv, T.; Krieger, J.; Babakhanova, G.; Yu, H.; Shiyanovskii, S.V.; Wei, Q.H.; Kim, M.H.; Lavrentovich, O.D. Topology control of human fibroblast cells monolayer by liquid crystal elastomer. Sci. Adv. 2020, 6, eaaz6485. [Google Scholar] [CrossRef]
- Oki, Y.; Harano, K.; Hara, Y.; Sasajima, Y.; Sasaki, R.; Ito, T.; Fujishiro, M.; Ito, T. Cationic surface charge effect on proliferation and protein production of human dental pulp stem cells cultured on diethylaminoethyl-modified cellulose porous beads. Biochem. Eng. J. 2021, 176, 108217. [Google Scholar] [CrossRef]
- De Luca, I.; Di Salle, A.; Alessio, N.; Margarucci, S.; Simeone, M.; Galderisi, U.; Calarco, A.; Peluso, G. Positively charged polymers modulate the fate of human mesenchymal stromal cells via ephrinB2/EphB4 signaling. Stem Cell Res. 2016, 17, 248–255. [Google Scholar] [CrossRef]
- Kummala, R.; Soto Veliz, D.; Fang, Z.; Xu, W.; Abitbol, T.; Xu, C.; Toivakka, M. Human Dermal Fibroblast Viability and Adhesion on Cellulose Nanomaterial Coatings: Influence of Surface Characteristics. Biomacromolecules 2020, 21, 1560–1567. [Google Scholar] [CrossRef]
- Dubiel, E.A.; Martin, Y.; Vermette, P. Bridging the gap between physicochemistry and interpretation prevalent in cell-surface interactions. Chem. Rev. 2011, 111, 2900–2936. [Google Scholar] [CrossRef]
- Waldchen, S.; Lehmann, J.; Klein, T.; van de Linde, S.; Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 2015, 5, 15348. [Google Scholar] [CrossRef] [PubMed]
- Filser, S.; Maier, T.L.; Nagel, R.D.; Schindler, W.; Lugli, P.; Becherer, M.; Krischer, K. Photoelectrochemical reactivity of well-defined mesoscale gold arrays on SiO2/Si substrates in CO2-saturated aqueous electrolyte. Electrochim. Acta 2018, 268, 546–553. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Dinesh, B.; Kang, S.M.; Krishnan, U.M.; Huh, Y.S.; Han, Y.K. Recent advances in molybdenum disulfide-based electrode materials for electroanalytical applications. Mikrochim. Acta 2019, 186, 203. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-Y.; Cai, Q.; Ma, J.-N.; Zhu, L.; Han, D.-D.; Zhang, Y.-L. Free-standing and flexible graphene supercapacitors of high areal capacitance fabricated by laser holography reduction of graphene oxide. Appl. Phys. Lett. 2021, 118, 071601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, L.; Shaik, R.; Xu, R.; Zhang, G.; Zhe, J. Cell Surface Charge Mapping Using a Microelectrode Array on ITO Substrate. Cells 2023, 12, 518. https://doi.org/10.3390/cells12040518
Ouyang L, Shaik R, Xu R, Zhang G, Zhe J. Cell Surface Charge Mapping Using a Microelectrode Array on ITO Substrate. Cells. 2023; 12(4):518. https://doi.org/10.3390/cells12040518
Chicago/Turabian StyleOuyang, Leixin, Rubia Shaik, Ruiting Xu, Ge Zhang, and Jiang Zhe. 2023. "Cell Surface Charge Mapping Using a Microelectrode Array on ITO Substrate" Cells 12, no. 4: 518. https://doi.org/10.3390/cells12040518
APA StyleOuyang, L., Shaik, R., Xu, R., Zhang, G., & Zhe, J. (2023). Cell Surface Charge Mapping Using a Microelectrode Array on ITO Substrate. Cells, 12(4), 518. https://doi.org/10.3390/cells12040518





