Sperm-Induced Ca2+ Release in Mammalian Eggs: The Roles of PLCζ, InsP3, and ATP
Abstract
1. Introduction
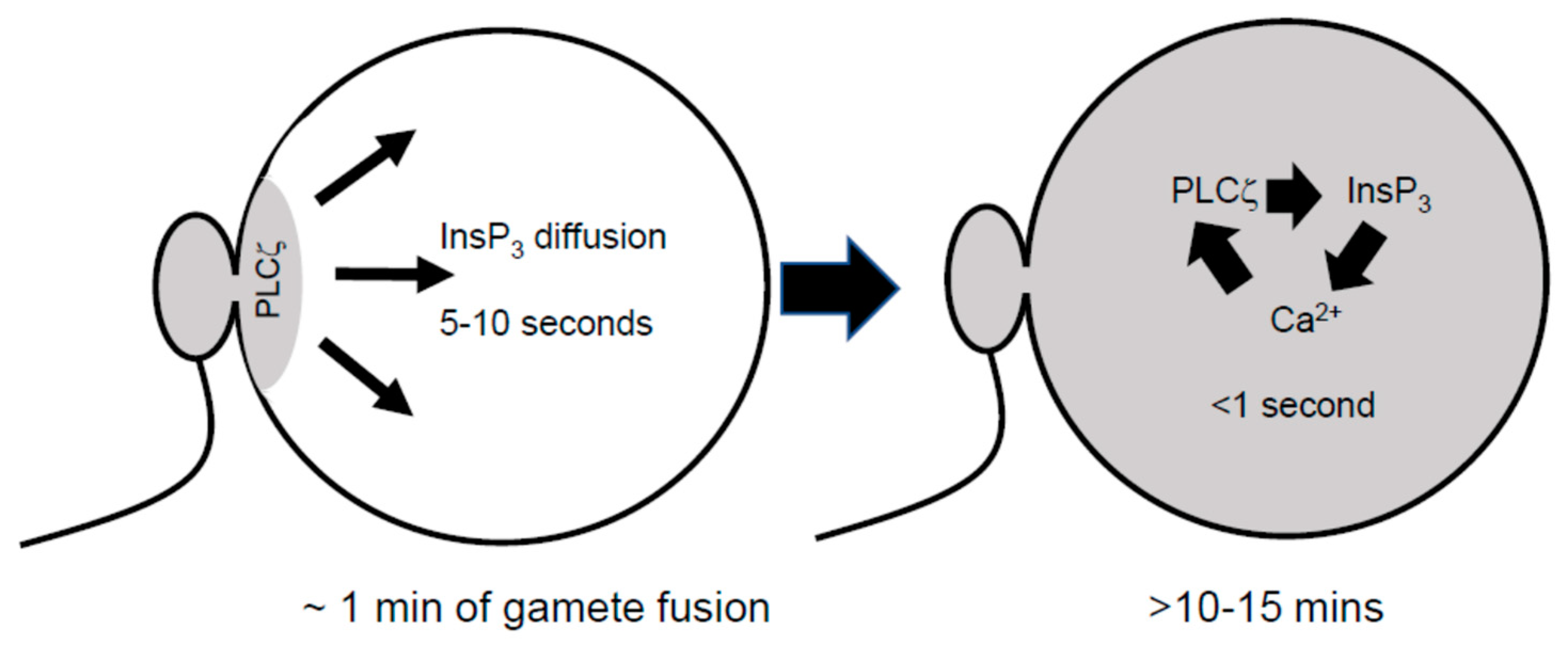
2. Diffusion of PLCζ and InsP3 Can Explain PIP2 Distribution and Ca2+ Waves
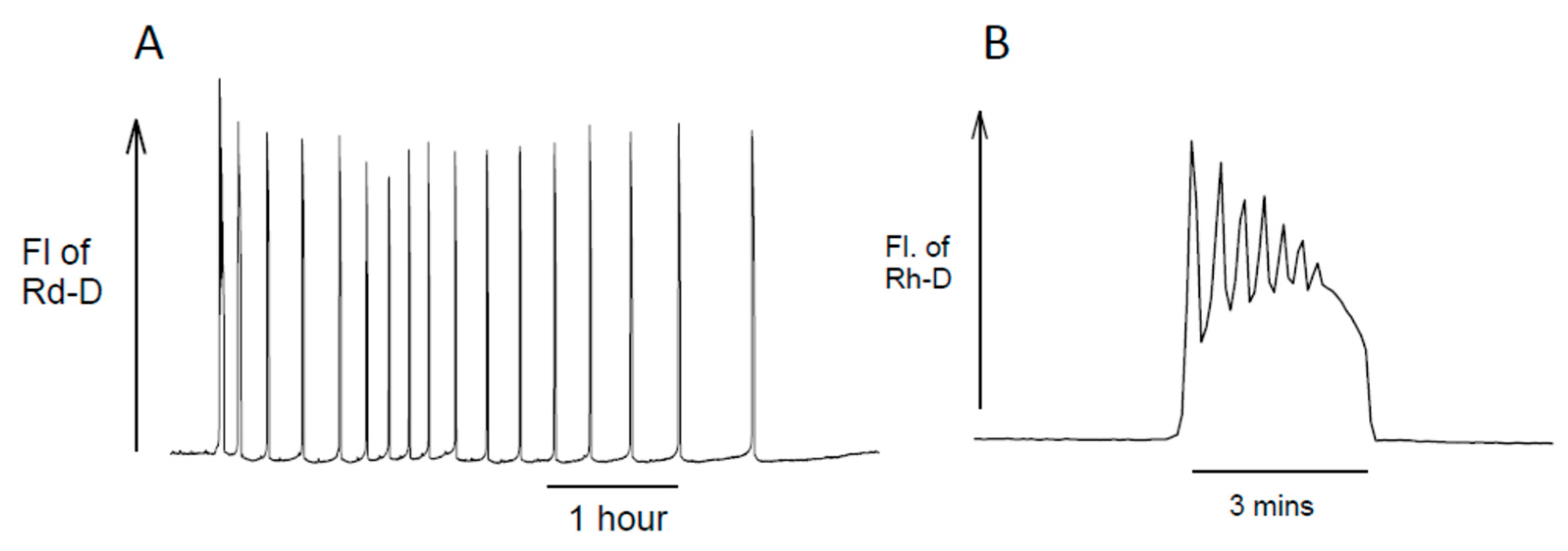
3. The Dynamics of InsP3 at Fertilization
4. Models of Ca2+ Oscillations in Eggs
5. PLCζ and Other Sperm Factors
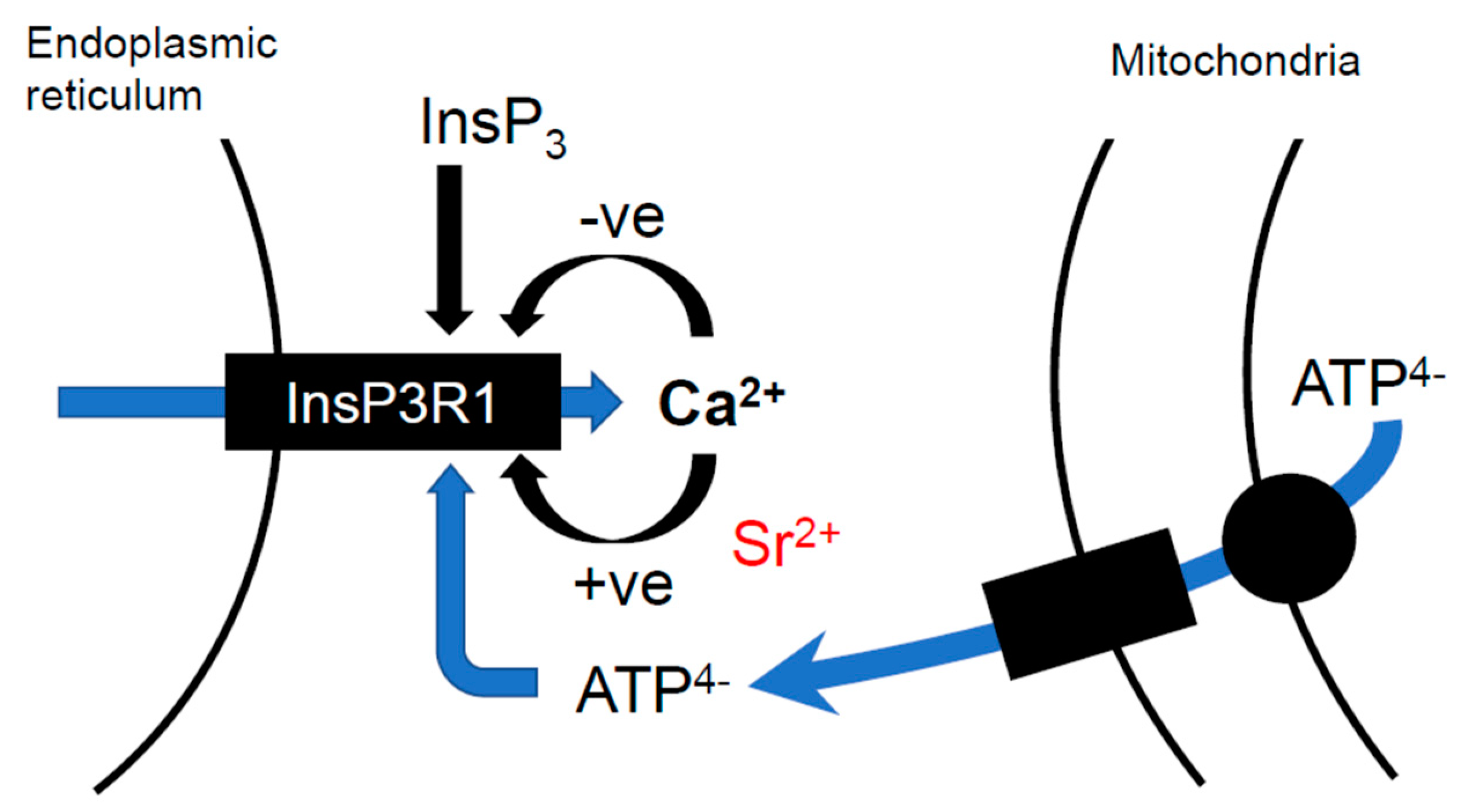
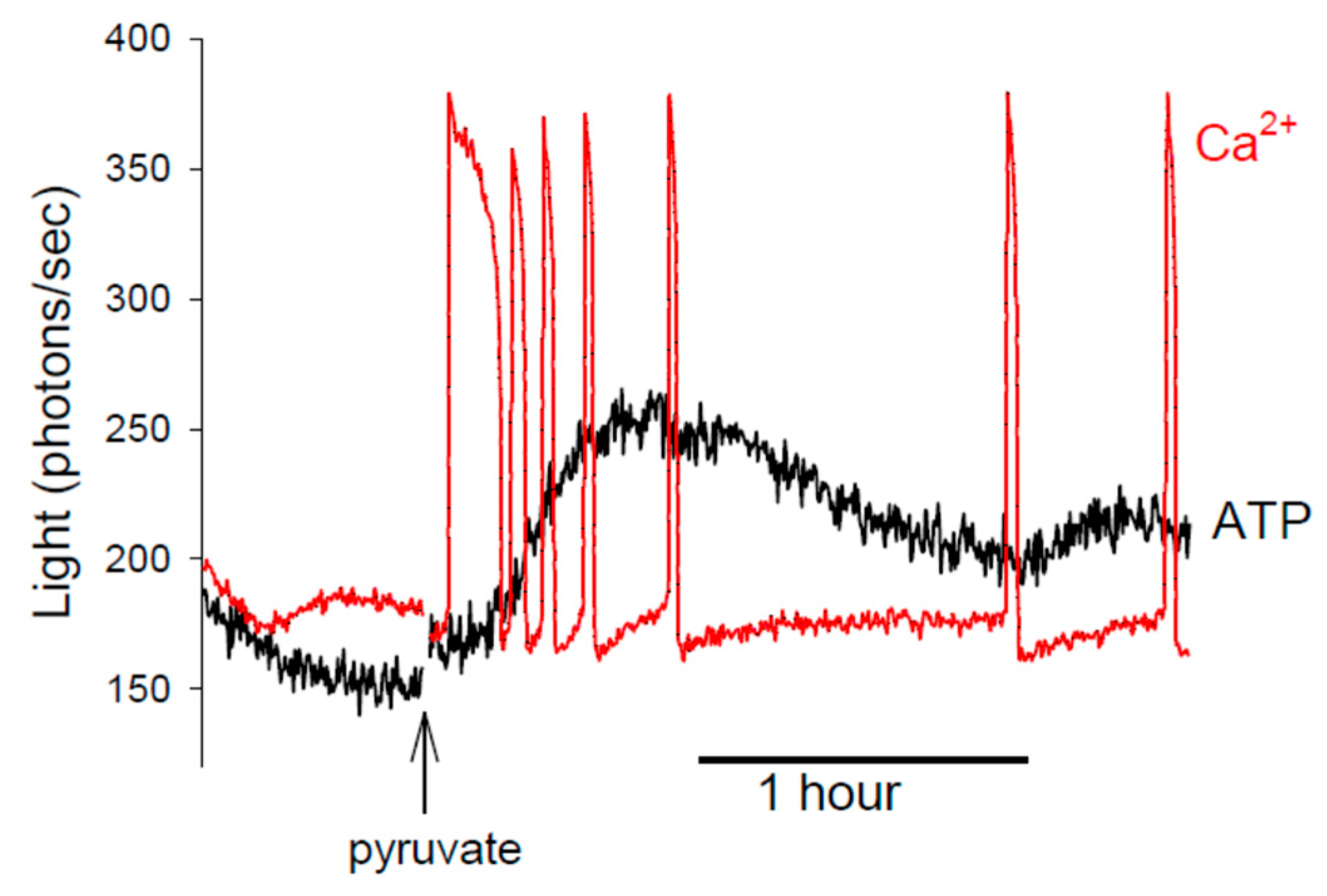
6. ATP and the Sensitivity of Ca2+ Release in Eggs
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuthbertson, K.S.; Cobbold, P.H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature 1985, 316, 541–542. [Google Scholar] [CrossRef]
- Igusa, Y.; Miyazaki, S. Periodic increase of cytoplasmic free calcium in fertilized hamster eggs measured with calcium-sensitive electrodes. J. Physiol. 1986, 377, 193–205. [Google Scholar] [CrossRef]
- Miyazaki, S.; Hashimoto, N.; Yoshimoto, Y.; Kishimoto, T.; Igusa, Y.; Hiramoto, Y. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev. Biol. 1986, 118, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.; Kline, J.T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 1992, 149, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S. Intracellular calcium oscillations in mammalian eggs at fertilization. J. Physiol. 2007, 584, 713–714. [Google Scholar] [CrossRef]
- Swann, K.; Lai, F.A. Egg Activation at Fertilization by a Soluble Sperm Protein. Physiol. Rev. 2016, 96, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLC zeta: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef]
- Sato, M.S.; Yoshitomo, M.; Mohri, T.; Miyazaki, S. Spatiotemporal analysis of [Ca2+]i rises in mouse eggs after intracytoplasmic sperm injection (ICSI). Cell Calcium 1999, 26, 49–58. [Google Scholar] [CrossRef]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci. Rep. 2018, 8, 1315. [Google Scholar] [CrossRef]
- Stein, P.; Savy, V.; Williams, A.M.; Williams, C.J. Modulators of calcium signalling at fertilization. Open Biol. 2020, 10, 200118. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Mehregan, A.; Fissore, R.A. Ca2+ Signaling and Homeostasis in Mammalian Oocytes and Eggs. Cold Spring Harb. Perspect. Biol. 2019, 11, a035162. [Google Scholar] [CrossRef]
- Ducibella, T.; Schultz, R.M.; Ozil, J.P. Role of calcium signals in early development. Semin. Cell Dev. Biol. 2006, 17, 324–332. [Google Scholar] [CrossRef]
- Jones, K.T. Intracellular calcium in the fertilization and development of mammalian eggs. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Swann, K. Ca2+ oscillations stimulate an ATP increase during fertilization of mouse eggs. Dev. Biol. 2006, 298, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, R.; Shirakawa, H.; Oda, S.; Mohri, T.; Miyazaki, S. Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal-vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev. Biol. 2000, 218, 299–313. [Google Scholar] [CrossRef]
- Swann, K. Soluble sperm factors and Ca2+ release in eggs at fertilization. Rev. Reprod. 1996, 1, 33–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Y.; Nomikos, M.; Theodoridou, M.; Nounesis, G.; Lai, F.A.; Swann, K. PLCζ causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P2. Mol. Biol. Cell 2012, 23, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.R.; Ashley, B.; Moon, A.; Woolley, T.E.; Swann, K. PLCζ Induced Ca2+ Oscillations in Mouse Eggs Involve a Positive Feedback Cycle of Ca2+ Induced InsP3 Formation From Cytoplasmic PIP2. Front. Cell Dev. Biol. 2018, 6, 36. [Google Scholar] [CrossRef]
- Sneyd, J.; Tsaneva-Atanasova, K.; Reznikov, V.; Bai, Y.; Sanderson, M.J.; Yule, D.I. A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc. Natl. Acad. Sci. USA 2006, 103, 1675–1680. [Google Scholar] [CrossRef]
- Politi, A.; Gaspers, L.D.; Thomas, A.P.; Höfer, T. Models of IP3 and Ca2+ oscillations: Frequency encoding and identification of underlying feedbacks. Biophys. J. 2006, 90, 3120–3133. [Google Scholar] [CrossRef]
- Miyazaki, S.; Yuzaki, M.; Nakada, K.; Shirakawa, H.; Nakanishi, S.; Nakade, S.; Mikoshiba, K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 1992, 257, 251–255. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, S.Y.; Malcuit, C.; Parys, J.B.; Fissore, R.A. Inositol 1,4,5-trisphosphate receptor 1 degradation in mouse eggs and impact on [Ca2+]i oscillations. J. Cell Physiol. 2010, 222, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; White, C.; Cheung, K.H.; Mak, D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef] [PubMed]
- Kouchi, Z.; Fukami, K.; Shikano, T.; Oda, S.; Nakamura, Y.; Takenawa, T.; Miyazaki, S. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 2004, 279, 10408–10412. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Blayney, L.M.; Larman, M.G.; Campbell, K.; Rossbach, A.; Saunders, C.M.; Swann, K.; Lai, F.A. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J. Biol. Chem. 2005, 280, 31011–31018. [Google Scholar] [CrossRef] [PubMed]
- Mak, D.O.; McBride, S.; Foskett, J.K. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA 1998, 95, 15821–15825. [Google Scholar] [CrossRef] [PubMed]
- Sienaert, I.; Missiaen, L.; De Smedt, H.; Parys, J.B.; Sipma, H.; Casteels, R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1997, 272, 25899–25906. [Google Scholar] [CrossRef] [PubMed]
- Ornelas-Guevara, R.; Gil, D.; Voorsluijs, V.; Dupont, G. Computational investigation of IP3 diffusion. Sci. Rep. 2023, 13, 2922. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Ito, M.; Sato, M.; Umezawa, Y.; Miyazaki, S. Measurement of intracellular IP3 during Ca2+ oscillations in mouse eggs with GFP-based FRET probe. Biochem. Biophys. Res. Commun. 2006, 345, 781–788. [Google Scholar] [CrossRef]
- Matsu-Ura, T.; Shirakawa, H.; Suzuki, K.G.N.; Miyamoto, A.; Sugiura, K.; Michikawa, T.; Kusumi, A.; Mikoshiba, K. Dual-FRET imaging of IP3 and Ca2+ revealed Ca2+-induced IP3 production maintains long lasting Ca2+ oscillations in fertilized mouse eggs. Sci. Rep. 2019, 9, 4829. [Google Scholar] [CrossRef]
- Igusa, Y.; Miyazaki, S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J. Physiol. 1983, 340, 611–632. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Zhang, N.; Vangheluwe, P.; Fissore, R.A. Regulation of endoplasmic reticulum Ca2+ oscillations in mammalian eggs. J. Cell Sci. 2013, 126, 5714–5724. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M.L.; Stein, P.; Carvacho, I.; Krapp, C.; Ardestani, G.; Mehregan, A.; Umbach, D.M.; Bartolomei, M.S.; Fissore, R.A.; Williams, C.J. TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization. Proc. Natl. Acad. Sci. USA 2018, 115, E10370–E10378. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 1981, 290, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Ferrer Buitrago, M.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; de Sutter, P.; et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef]
- Wu, A.T.; Sutovsky, P.; Manandhar, G.; Xu, W.; Katayama, M.; Day, B.N.; Park, K.W.; Yi, Y.J.; Xi, Y.W.; Prather, R.S.; et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J. Biol. Chem. 2007, 282, 12164–12175. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.; Balakier, H.; Bashar, S.; Moskovtsev, S.I.; Sutovsky, P.; Librach, C.L.; Oko, R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014, 28, 4434–4440. [Google Scholar] [CrossRef]
- Kang, W.; Harada, Y.; Yamatoya, K.; Kawano, N.; Kanai, S.; Miyamoto, Y.; Nakamura, A.; Miyado, M.; Hayashi, Y.; Kuroki, Y.; et al. Extra-mitochondrial citrate synthase initiates calcium oscillation and suppresses age-dependent sperm dysfunction. Lab. Investig. 2020, 100, 583–595. [Google Scholar] [CrossRef]
- Nomikos, M.; Sanders, J.R.; Kashir, J.; Sanusi, R.; Buntwal, L.; Love, D.; Ashley, P.; Sanders, D.; Knaggs, P.; Bunkheila, A.; et al. Functional disparity between human PAWP and PLCζ in the generation of Ca2+ oscillations for oocyte activation. Mol. Hum. Reprod. 2015, 21, 702–710. [Google Scholar] [CrossRef]
- Nomikos, M.; Sanders, J.R.; Theodoridou, M.; Kashir, J.; Matthews, E.; Nounesis, G.; Lai, F.A.; Swann, K. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol. Hum. Reprod. 2014, 20, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Galione, A.; Swann, K.; Georgiou, P.; Whitaker, M. Regenerative and non-regenerative calcium transients in hamster eggs triggered by inositol 1,4,5-trisphosphate. J. Physiol. 1994, 480 Pt 3, 465–474. [Google Scholar] [CrossRef]
- Swann, K. Ca2+ oscillations and sensitization of Ca2+ release in unfertilized mouse eggs injected with a sperm factor. Cell Calcium 1994, 15, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Saunders, C.M.; Lai, F.A.; Swann, K. Preimplantation development of mouse oocytes activated by different levels of human phospholipase C zeta. Hum. Reprod. 2008, 23, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Reddy, R.; Ferrer Buitrago, M.; Vander Jeught, M.; Neupane, J.; De Vos, W.H.; Van den Abbeel, E.; Lierman, S.; De Sutter, P.; Heindryckx, B. Strontium fails to induce Ca2+ release and activation in human oocytes despite the presence of functional TRPV3 channels. Hum. Reprod. Open 2018, 2018, hoy005. [Google Scholar] [CrossRef]
- Tomashov-Matar, R.; Tchetchik, D.; Eldar, A.; Kaplan-Kraicer, R.; Oron, Y.; Shalgi, R. Strontium-induced rat egg activation. Reproduction 2005, 130, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Storey, A.; Elgmati, K.; Wang, Y.; Knaggs, P.; Swann, K. The role of ATP in the differential ability of Sr2+ to trigger Ca2+ oscillations in mouse and human eggs. Mol. Hum. Reprod. 2021, 27, gaaa086. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.S.; Lowther, K.M.; Mehlmann, L.M. Reorganization of the endoplasmic reticulum and development of Ca2+ release mechanisms during meiotic maturation of human oocytes. Biol. Reprod. 2010, 83, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Fissore, R.A.; Longo, F.J.; Anderson, E.; Parys, J.B.; Ducibella, T. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol. Reprod. 1999, 60, 49–57. [Google Scholar] [CrossRef]
- Tu, H.; Wang, Z.; Nosyreva, E.; De Smedt, H.; Bezprozvanny, I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys. J. 2005, 88, 1046–1055. [Google Scholar] [CrossRef]
- Mak, D.O.; McBride, S.; Foskett, J.K. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca2+ activation. J. Biol. Chem. 1999, 274, 22231–22237. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Ehrlich, B.E. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron 1993, 10, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M. Cytoplasmic free concentrations of Ca2+ and Mg2+ in skeletal muscle fibers at rest and during contraction. Jpn. J. Physiol. 1998, 48, 421–438. [Google Scholar] [CrossRef]
- Bers, D.M.; Patton, C.W.; Nuccitelli, R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994, 40, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Marshall, I.C.; Taylor, C.W. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem. J. 1994, 301 Pt 2, 591–598. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Davis, P.; Alexander, S. Inner mitochondrial membrane potential (DeltaPsim), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum. Reprod. 2003, 18, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W., Jr.; Castora, F.J. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef]
- Dumollard, R.; Campbell, K.; Halet, G.; Carroll, J.; Swann, K. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev. Biol. 2008, 316, 431–440. [Google Scholar] [CrossRef]
- Dumollard, R.; Marangos, P.; Fitzharris, G.; Swann, K.; Duchen, M.; Carroll, J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 2004, 131, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J. Metabolic implications of non-electrogenic ATP/ADP exchange in cancer cells: A mechanistic basis for the Warburg effect. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148410. [Google Scholar] [CrossRef]
- Dumollard, R.; Carroll, J.; Duchen, M.R.; Campbell, K.; Swann, K. Mitochondrial function and redox state in mammalian embryos. Semin. Cell Dev. Biol. 2009, 20, 346–353. [Google Scholar] [CrossRef] [PubMed]
- de Paula, W.B.; Lucas, C.H.; Agip, A.N.; Vizcay-Barrena, G.; Allen, J.F. Energy, ageing, fidelity and sex: Oocyte mitochondrial DNA as a protected genetic template. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120263. [Google Scholar] [CrossRef] [PubMed]
- Snow, P.; Yim, D.L.; Leibow, J.D.; Saini, S.; Nuccitelli, R. Fertilization stimulates an increase in inositol trisphosphate and inositol lipid levels in Xenopus eggs. Dev. Biol. 1996, 180, 108–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rice, A.; Parrington, J.; Jones, K.T.; Swann, K. Mammalian sperm contain a Ca2+-sensitive phospholipase C activity that can generate InsP3 from PIP2 associated with intracellular organelles. Dev. Biol. 2000, 228, 125–135. [Google Scholar] [CrossRef]
- Dupont, G.; Dumollard, R. Simulation of calcium waves in ascidian eggs: Insights into the origin of the pacemaker sites and the possible nature of the sperm factor. J. Cell Sci. 2004, 117, 4313–4323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swann, K. Sperm-Induced Ca2+ Release in Mammalian Eggs: The Roles of PLCζ, InsP3, and ATP. Cells 2023, 12, 2809. https://doi.org/10.3390/cells12242809
Swann K. Sperm-Induced Ca2+ Release in Mammalian Eggs: The Roles of PLCζ, InsP3, and ATP. Cells. 2023; 12(24):2809. https://doi.org/10.3390/cells12242809
Chicago/Turabian StyleSwann, Karl. 2023. "Sperm-Induced Ca2+ Release in Mammalian Eggs: The Roles of PLCζ, InsP3, and ATP" Cells 12, no. 24: 2809. https://doi.org/10.3390/cells12242809
APA StyleSwann, K. (2023). Sperm-Induced Ca2+ Release in Mammalian Eggs: The Roles of PLCζ, InsP3, and ATP. Cells, 12(24), 2809. https://doi.org/10.3390/cells12242809





