Ferritin Is Secreted from Primary Cultured Astrocyte in Response to Iron Treatment via TRPML1-Mediated Exocytosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
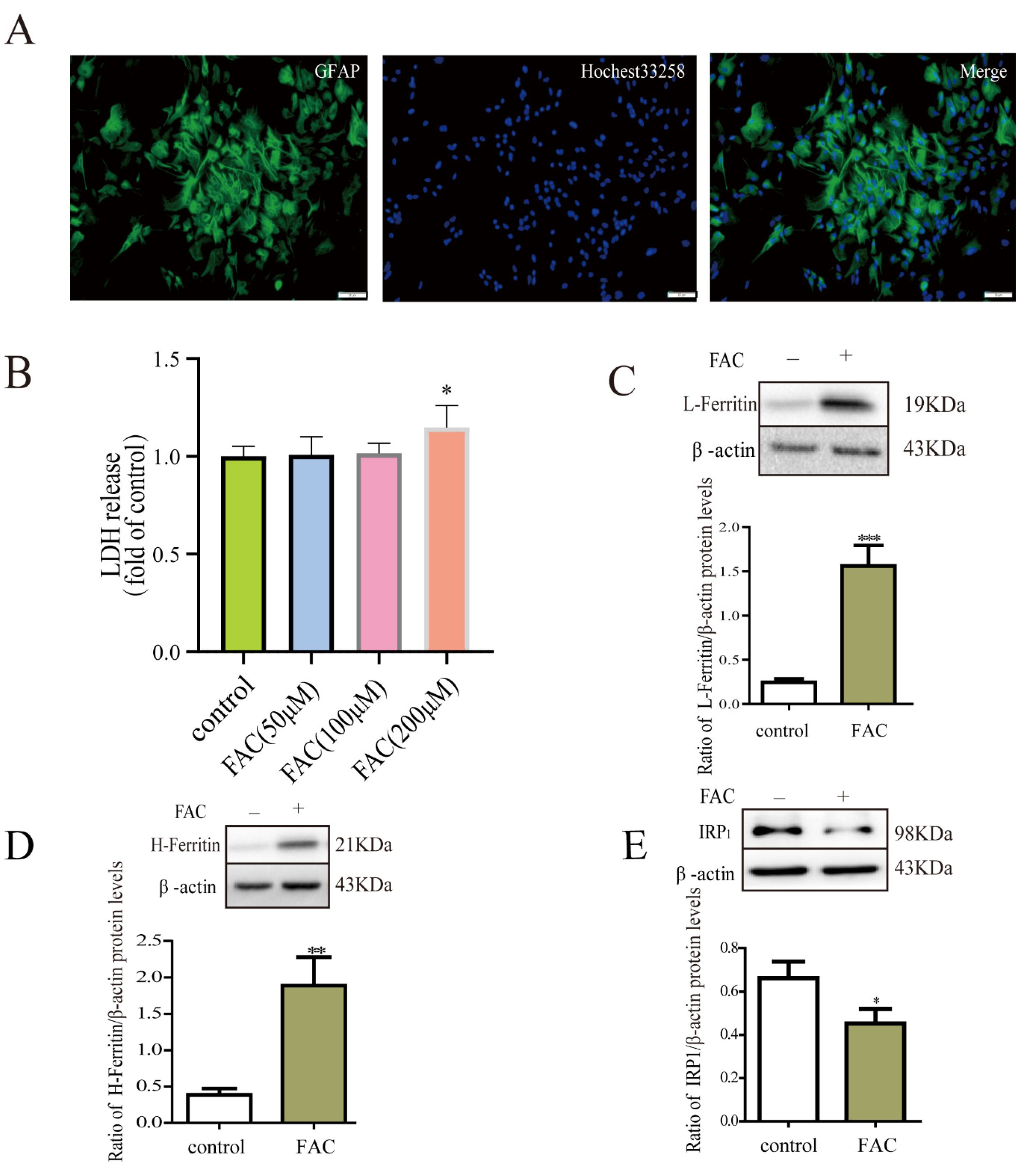
2.2. Detection of Cell Viability by LDH Assay Kit
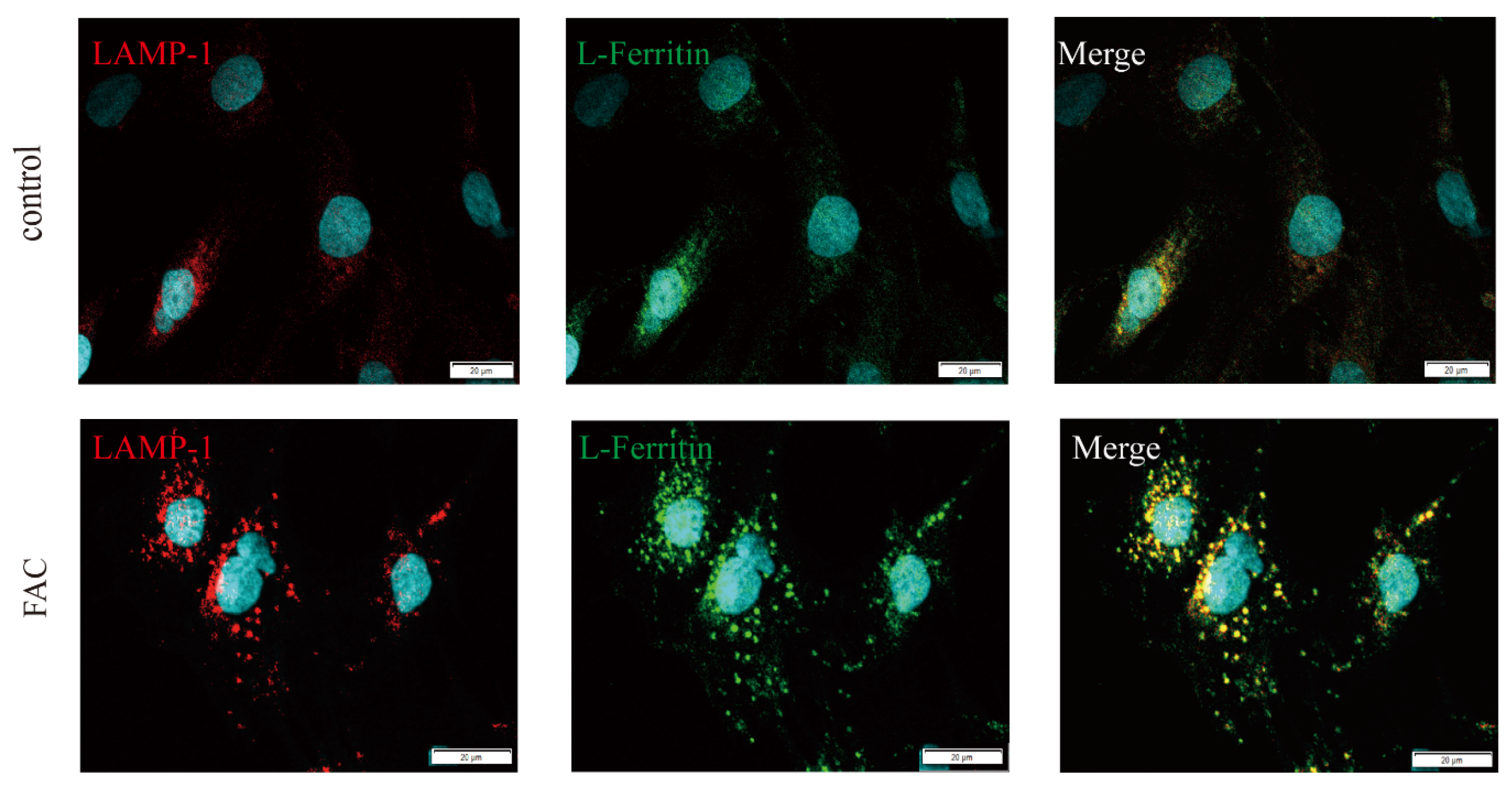
2.3. Immunofluorescence Staining
2.4. Protein Extraction
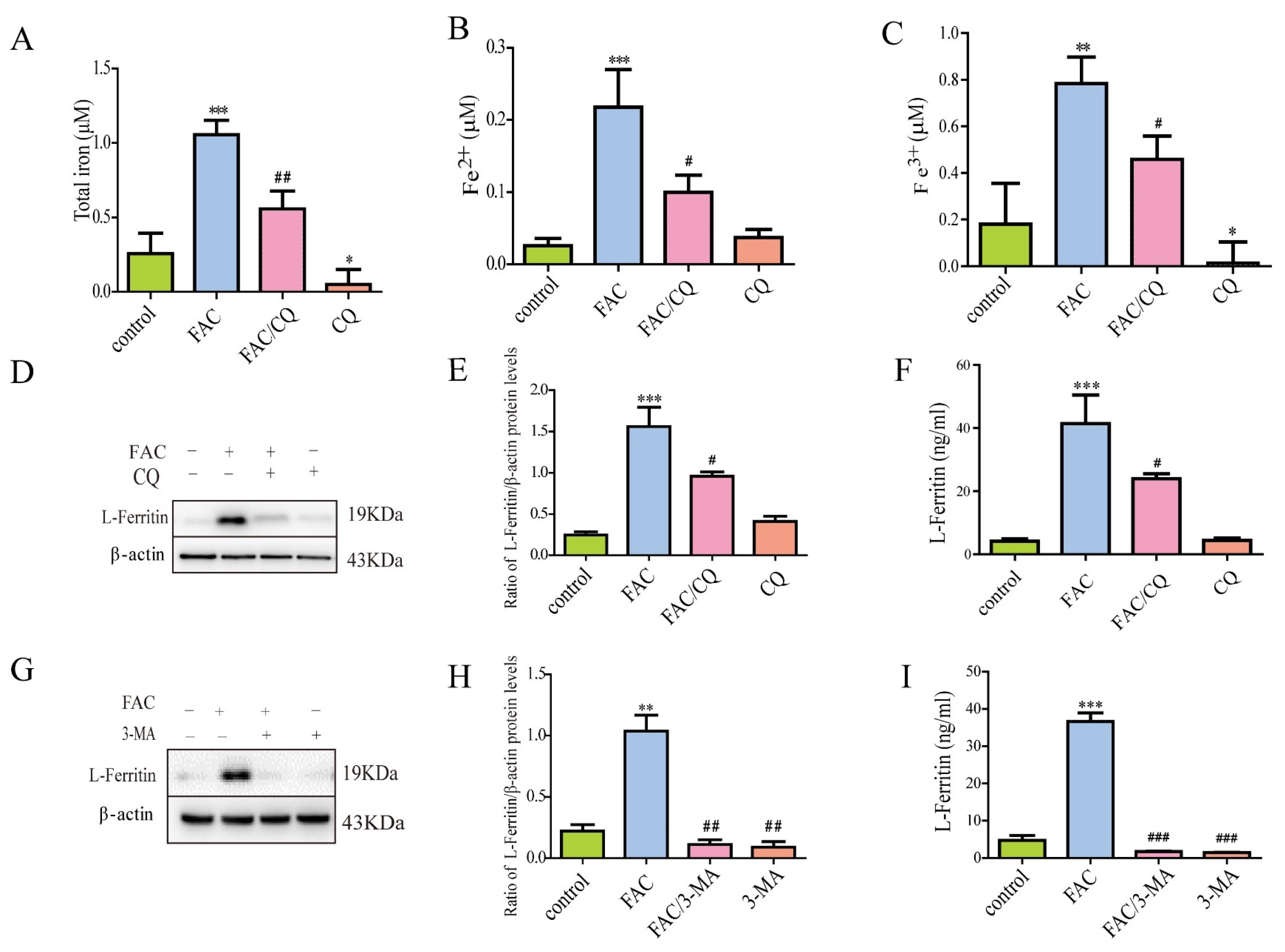
2.5. Western Blots
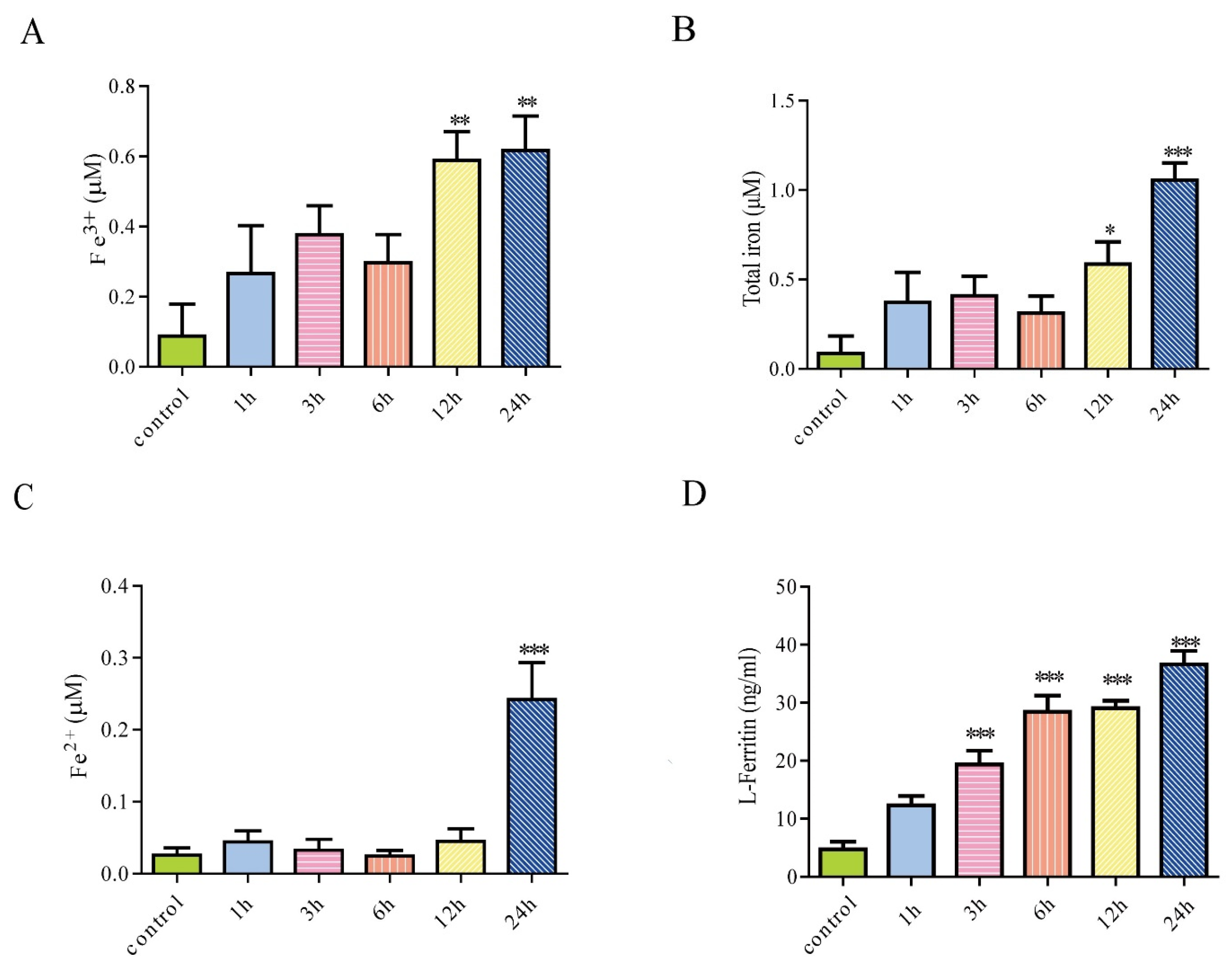
2.6. Iron Assay Kit
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Knockdown of Rab27a and TRPML1
2.9. Real-Time PCR
2.10. Statistical Analysis
3. Results
3.1. Iron Treatment Enhanced the Expression of Ferritin via IRP1 in Astrocytes
3.2. Iron Treatment Increased Extracellular Iron and Enhanced Secretion of Ferritin from Astrocytes
3.3. Lysosomal Secretory Pathway Might Be Involved in Iron-Induced Secretion of Ferritin
3.4. Ferritin Colocalized with LAMP1 in Iron-Loaded Astrocytes
3.5. Lentivirus-Mediated TRPML1 Gene Knockdown Antagonized Iron-Induced Ferritin Secretion
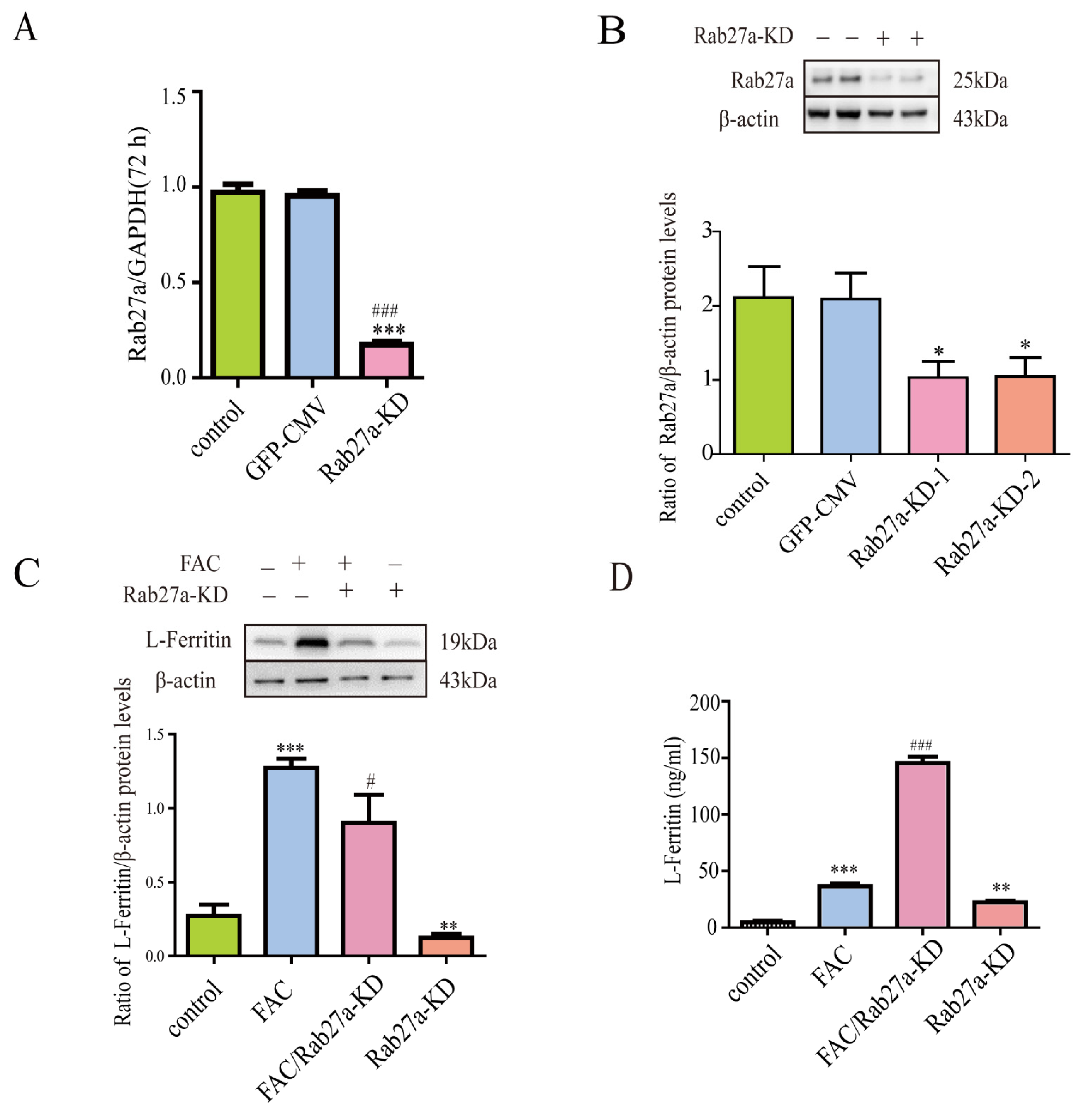
3.6. Lentivirus-Mediated Rab27a Gene Knockdown Further Enhanced Ferritin Secretion
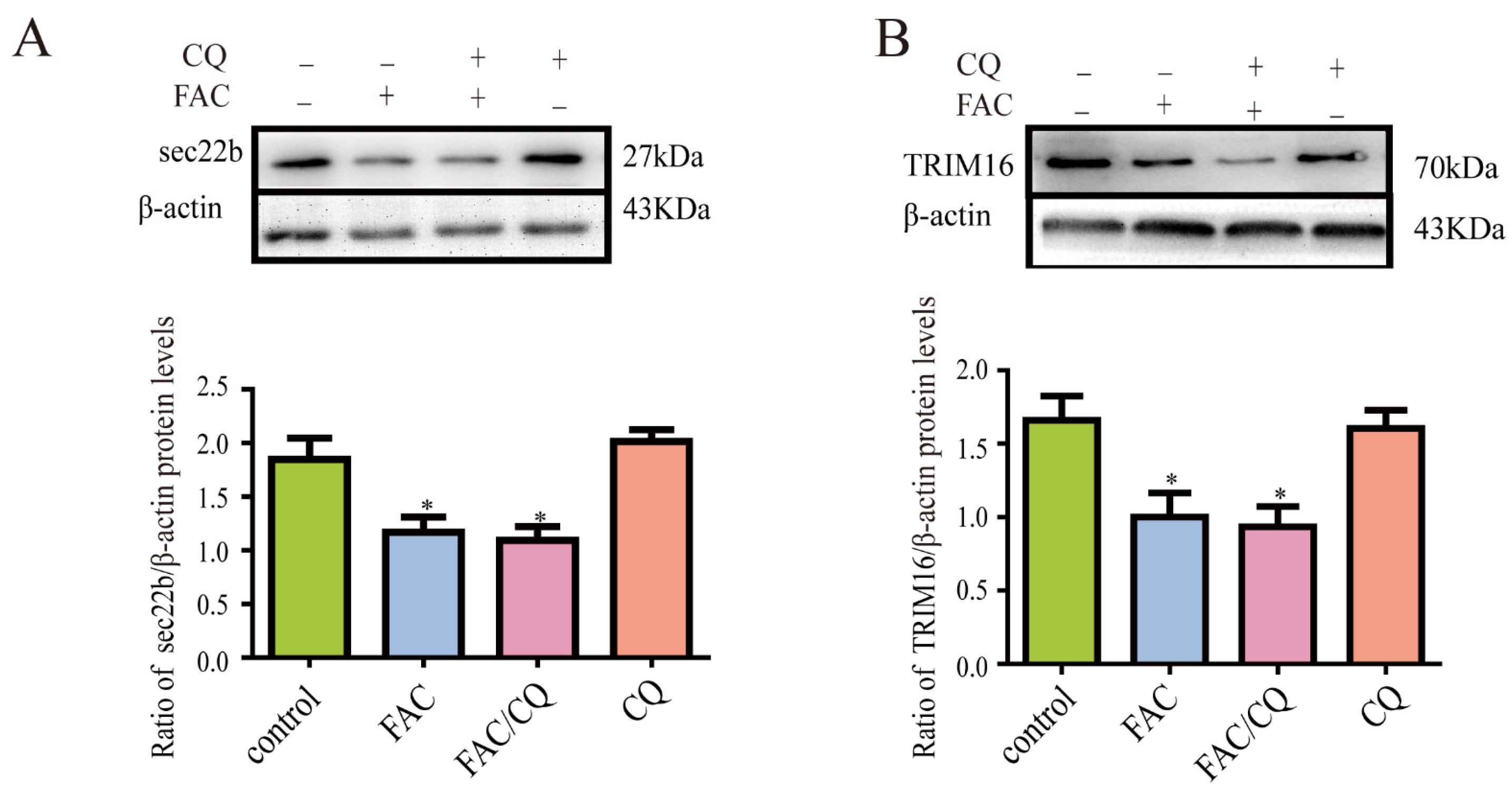
3.7. Secretory Autophagy Protein TRIM16 and sec22b Decreased in Iron-Treated Astrocytes
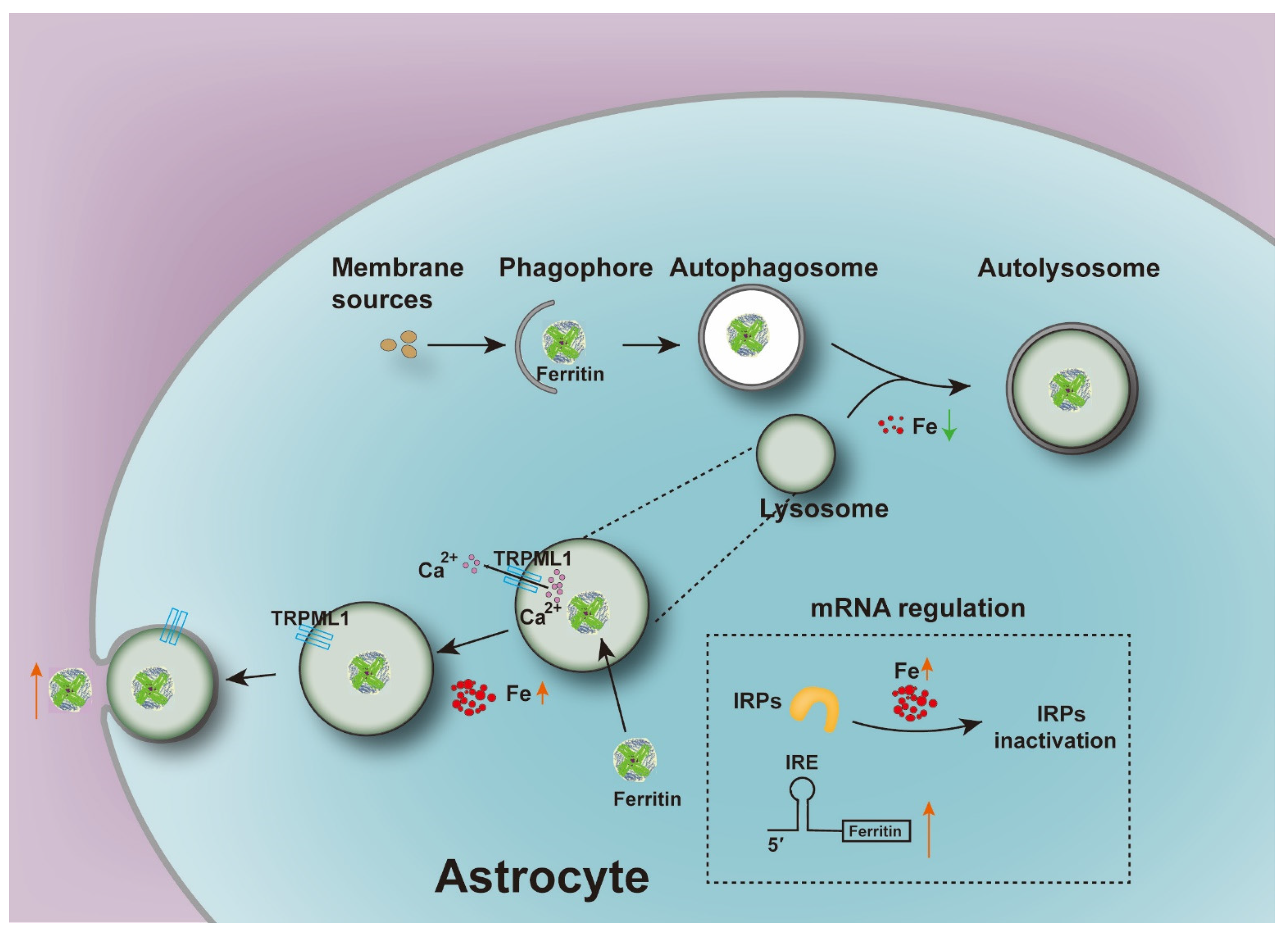
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dexter, D.T.; Sian, J.; Jenner, P.; Marsden, C.D. Implications of alterations in trace element levels in brain in Parkinson’s disease and other neurological disorders affecting the basal ganglia. Adv. Neurol. 1993, 60, 273–281. [Google Scholar]
- Dexter, D.T.; Wells, F.R.; Lees, A.J.; Agid, F.; Agid, Y.; Jenner, P.; Marsden, C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J. Neurochem. 1989, 52, 1830–1836. [Google Scholar] [CrossRef]
- Helie, S.; Fansher, M. Categorization system-switching deficits in typical aging and Parkinson’s disease. Neuropsychology 2018, 32, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.; Huddleston, D.E.; Sedlacik, J.; Boelmans, K.; Hu, X.P. Parkinson’s disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov. Disord. 2017, 32, 441–449. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, I.; Petragnano, F.; Aloisi, G.; Marampon, F.; Rossi, M.; Coppolino, M.F.; Rossi, R.; Longoni, B.; Scarselli, M.; Maggio, R. A New Threat to Dopamine Neurons: The Downside of Artificial Light. Neuroscience 2020, 432, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B. Update on the pathogenesis of Parkinson’s disease. J. Neurol. 2008, 255 (Suppl. 5), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Apfeld, J.; Fontana, W. Age-Dependence and Aging-Dependence: Neuronal Loss and Lifespan in a C. elegans Model of Parkinson’s Disease. Biology 2017, 7, 1. [Google Scholar] [CrossRef]
- Nassif, D.V.; Pereira, J.S. Fatigue in Parkinson’s disease: Concepts and clinical approach. Psychogeriatrics 2018, 18, 143–150. [Google Scholar] [CrossRef]
- Yu, S.Y.; Cao, C.J.; Zuo, L.J.; Chen, Z.J.; Lian, T.H.; Wang, F.; Hu, Y.; Piao, Y.S.; Li, L.X.; Guo, P.; et al. Clinical features and dysfunctions of iron metabolism in Parkinson disease patients with hyper echogenicity in substantia nigra: A cross-sectional study. BMC Neurol. 2018, 18, 9. [Google Scholar] [CrossRef]
- Amara, A.W.; Memon, A.A. Effects of Exercise on Non-motor Symptoms in Parkinson’s Disease. Clin. Ther. 2018, 40, 8–15. [Google Scholar] [CrossRef]
- Li, K.; Reichmann, H. Role of iron in neurodegenerative diseases. J. Neural. Transm. Vienna 2016, 123, 389–399. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef]
- Cookson, M.R. The biochemistry of Parkinson’s disease. Annu. Rev. Biochem. 2005, 74, 29–52. [Google Scholar] [CrossRef]
- De Farias, C.C.; Maes, M.; Bonifacio, K.L.; Matsumoto, A.K.; Bortolasci, C.C.; Nogueira, A.S.; Brinholi, F.F.; Morimoto, H.K.; de Melo, L.B.; Moreira, E.G.; et al. Parkinson’s Disease is Accompanied by Intertwined Alterations in Iron Metabolism and Activated Immune-inflammatory and Oxidative Stress Pathways. CNS Neurol. Disord. Drug Targets 2017, 16, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Rogers, J.; Xie, J. Brain Iron Metabolism Dysfunction in Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 3078–3101. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M. Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. Curr. Environ. Health Rep. 2017, 4, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta 2010, 1800, 719–731. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Bradley, J.M.; Moore, G.R.; Le Brun, N.E. Diversity of Fe(2+) entry and oxidation in ferritins. Curr. Opin. Chem. Biol. 2017, 37, 122–128. [Google Scholar] [CrossRef]
- Koorts, A.M.; Viljoen, M. Ferritin and ferritin isoforms II: Protection against uncontrolled cellular proliferation, oxidative damage and inflammatory processes. Arch. Physiol. Biochem. 2007, 113, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Chu, M.; Ai, H.; Hu, C.; Ding, W. Association of serum IL-18 with protein-energy wasting in end-stage renal disease patients on haemodialysis. Int. Urol. Nephrol. 2019, 51, 1271–1278. [Google Scholar] [CrossRef]
- Faucheux, B.A.; Martin, M.E.; Beaumont, C.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J. Neurochem. 2003, 86, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, B.A.; Martin, M.E.; Beaumont, C.; Hunot, S.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson’s disease. J. Neurochem. 2002, 83, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.A.; Gutierrez, L.; Weiss, A.; Leichtmann-Bardoogo, Y.; Zhang, D.L.; Crooks, D.R.; Sougrat, R.; Morgenstern, A.; Galy, B.; Hentze, M.W.; et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010, 116, 1574–1584. [Google Scholar] [CrossRef]
- Ponpuak, M.; Mandell, M.A.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory autophagy. Curr. Opin. Cell Biol. 2015, 35, 106–116. [Google Scholar] [CrossRef]
- Truman-Rosentsvit, M.; Berenbaum, D.; Spektor, L.; Cohen, L.A.; Belizowsky-Moshe, S.; Lifshitz, L.; Ma, J.; Li, W.; Kesselman, E.; Abutbul-Ionita, I.; et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 2018, 131, 342–352. [Google Scholar] [CrossRef]
- Moos, T.; Trinder, D.; Morgan, E.H. Cellular distribution of ferric iron, ferritin, transferrin and divalent metal transporter 1 (DMT1) in substantia nigra and basal ganglia of normal and beta2-microglobulin deficient mouse brain. Cell Mol. Biol. 2000, 46, 549–561. [Google Scholar]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef]
- Greco, T.M.; Seeholzer, S.H.; Mak, A.; Spruce, L.; Ischiropoulos, H. Quantitative mass spectrometry-based proteomics reveals the dynamic range of primary mouse astrocyte protein secretion. J. Proteome Res. 2010, 9, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Estevez, M.; Rolle, S.O.; Mampay, M.; Dev, K.K.; Sheridan, G.K. Piezo1 regulates calcium oscillations and cytokine release from astrocytes. Glia 2020, 68, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yu, X.; Song, L.; Xiao, Z.; Xie, J.; Xu, H. Ferritin confers protection against iron-mediated neurotoxicity and ferroptosis through iron chelating mechanisms in MPP(+)-induced MES23.5 dopaminergic cells. Free Radic. Biol. Med. 2022, 193 Pt 2, 751–763. [Google Scholar]
- Li, J.; Tian, M.; Hua, T.; Wang, H.; Yang, M.; Li, W.; Zhang, X.; Yuan, H. Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy 2021, 17, 4062–4082. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.; Zhang, Y.; Bai, Y.; Chen, X.; Huang, R.; Wu, F.; Leng, S.; Chao, J.; Zhang, J.H.; et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: Implications for cerebral ischemic stroke. Autophagy 2018, 14, 1164–1184. [Google Scholar] [CrossRef]
- Donaldson, J.G. Immunofluorescence Staining. Curr. Protoc. Cell Biol. 2015, 69, 431–437. [Google Scholar] [CrossRef]
- Kim, B. Western Blot Techniques. Methods Mol. Biol. 2017, 1606, 133–139. [Google Scholar]
- Singh, C.; Roy-Chowdhuri, S. Quantitative Real-Time PCR: Recent Advances. Methods Mol. Biol. 2016, 1392, 161–176. [Google Scholar]
- Andrejewski, N.; Punnonen, E.L.; Guhde, G.; Tanaka, Y.; Lullmann-Rauch, R.; Hartmann, D.; von Figura, K.; Saftig, P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J. Biol. Chem. 1999, 274, 12692–12701. [Google Scholar] [CrossRef]
- Li, G.; Huang, D.; Hong, J.; Bhat, O.M.; Yuan, X.; Li, P.L. Control of lysosomal TRPML1 channel activity and exosome release by acid ceramidase in mouse podocytes. Am. J. Physiol. Cell Physiol. 2019, 317, C481–C491. [Google Scholar] [CrossRef]
- Lathoria, K.; Gowda, P.; Umdor, S.B.; Patrick, S.; Suri, V.; Sen, E. PRMT1 driven PTX3 regulates ferritinophagy in glioma. Autophagy 2023, 19, 1997–2014. [Google Scholar] [CrossRef]
- Sanchez, M.; Galy, B.; Schwanhaeusser, B.; Blake, J.; Bahr-Ivacevic, T.; Benes, V.; Selbach, M.; Muckenthaler, M.U.; Hentze, M.W. Iron regulatory protein-1 and -2: Transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood 2011, 118, e168–e179. [Google Scholar] [CrossRef]
- Kato, J.; Fujikawa, K.; Kanda, M.; Fukuda, N.; Sasaki, K.; Takayama, T.; Kobune, M.; Takada, K.; Takimoto, R.; Hamada, H.; et al. A mutation, in the iron-responsive element of H ferritin mRNA, causing autosomal dominant iron treatment. Am. J. Hum. Genet. 2001, 69, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.R.; Lachlan, K.L.; Mumford, A.D.; Temple, I.K.; Hodgkins, P.R. Hereditary hyperferritinemia cataract syndrome: Ocular, genetic, and biochemical findings. Eur. J. Ophthalmol. 2006, 16, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Theil, E.C. Regulation of ferritin and transferrin receptor mRNAs. J. Biol. Chem. 1990, 265, 4771–4774. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef]
- Luzio, J.P.; Hackmann, Y.; Dieckmann, N.M.; Griffiths, G.M. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol 2014, 6, a016840. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Lee, M. Autophagy inhibition in 3T3-L1 adipocytes breaks the crosstalk with tumor cells by suppression of adipokine production. Anim. Cells Syst. Seoul 2020, 24, 17–25. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F.; et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; She, J.; Zeng, W.; Guo, J.; Xu, H.; Bai, X.C.; Jiang, Y. Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature 2017, 550, 415–418. [Google Scholar] [CrossRef]
- Kim, M.S.; Muallem, S.; Kim, S.H.; Kwon, K.B.; Kim, M.S. Exosomal release through TRPML1-mediated lysosomal exocytosis is required for adipogenesis. Biochem. Biophys. Res. Commun. 2019, 510, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Fukuda, M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J. Cell Sci. 2006, 119 Pt 11, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Nepal, A.; Wolfson, D.L.; Ahluwalia, B.S.; Jensen, I.; Jorgensen, J.; Iliev, D.B. Intracellular distribution and transcriptional regulation of Atlantic salmon (Salmo salar) Rab5c, 7a and 27a homologs by immune stimuli. Fish Shellfish Immunol. 2020, 99, 119–129. [Google Scholar] [CrossRef]
- Kornfeld, S.; Mellman, I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989, 5, 483–525. [Google Scholar] [CrossRef]
- Haddad, N.M.; Tilman, D.; Haarstad, J.; Ritchie, M.; Knops, J.M. Contrasting effects of plant richness and composition on insect communities: A field experiment. Am. Nat. 2001, 158, 17–35. [Google Scholar] [CrossRef]
- Stinchcombe, J.C.; Bossi, G.; Booth, S.; Griffiths, G.M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 2001, 15, 751–761. [Google Scholar] [CrossRef]
- Brzezinska, A.A.; Johnson, J.L.; Munafo, D.B.; Crozat, K.; Beutler, B.; Kiosses, W.B.; Ellis, B.A.; Catz, S.D. The Rab27a effectors JFC1/Slp1 and Munc13-4 regulate exocytosis of neutrophil granules. Traffic 2008, 9, 2151–2164. [Google Scholar] [CrossRef]
- Nightingale, T.D.; Pattni, K.; Hume, A.N.; Seabra, M.C.; Cutler, D.F. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 2009, 113, 5010–5018. [Google Scholar] [CrossRef]
- Hume, A.N.; Collinson, L.M.; Rapak, A.; Gomes, A.Q.; Hopkins, C.R.; Seabra, M.C. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 2001, 152, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, S.; Han, X.; Jiang, Z.; Dong, L.; Liu, C.; Liang, X.; Dong, J.; Qiu, C.; Wang, Y.; et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 2019, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xiao, Z.; Zhang, N.; Yu, X.; Cui, W.; Xie, J.; Xu, H. Apoferritin improves motor deficits in MPTP-treated mice by regulating brain iron metabolism and ferroptosis. iScience 2021, 24, 102431. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xiao, Z.; Xie, J.; Xu, H. Ferritin Is Secreted from Primary Cultured Astrocyte in Response to Iron Treatment via TRPML1-Mediated Exocytosis. Cells 2023, 12, 2519. https://doi.org/10.3390/cells12212519
Yu X, Xiao Z, Xie J, Xu H. Ferritin Is Secreted from Primary Cultured Astrocyte in Response to Iron Treatment via TRPML1-Mediated Exocytosis. Cells. 2023; 12(21):2519. https://doi.org/10.3390/cells12212519
Chicago/Turabian StyleYu, Xiaoqi, Zhixin Xiao, Junxia Xie, and Huamin Xu. 2023. "Ferritin Is Secreted from Primary Cultured Astrocyte in Response to Iron Treatment via TRPML1-Mediated Exocytosis" Cells 12, no. 21: 2519. https://doi.org/10.3390/cells12212519
APA StyleYu, X., Xiao, Z., Xie, J., & Xu, H. (2023). Ferritin Is Secreted from Primary Cultured Astrocyte in Response to Iron Treatment via TRPML1-Mediated Exocytosis. Cells, 12(21), 2519. https://doi.org/10.3390/cells12212519




