Circulating Tumour DNA as Biomarker for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction and Quality Assessment
2.2. Quantitative Assessment
3. Results
3.1. Literature Search
3.2. Overall Study Characteristics
3.3. Studies Using a Tumour-Informed ctDNA Approach
3.4. Studies Using Single Gene ctDNA Status
3.5. Studies Using a Tumour-Agnostic ctDNA Approach
3.6. Studies Investigating Only Preoperative ctDNA Samples
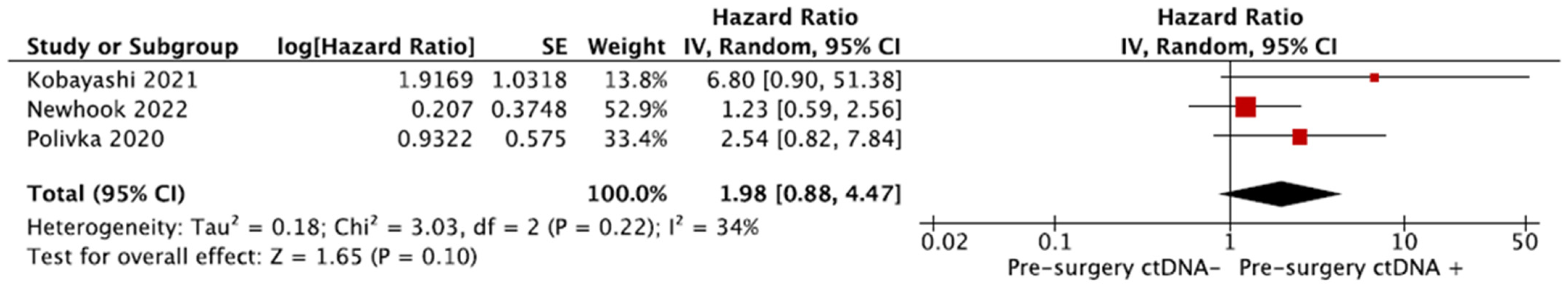
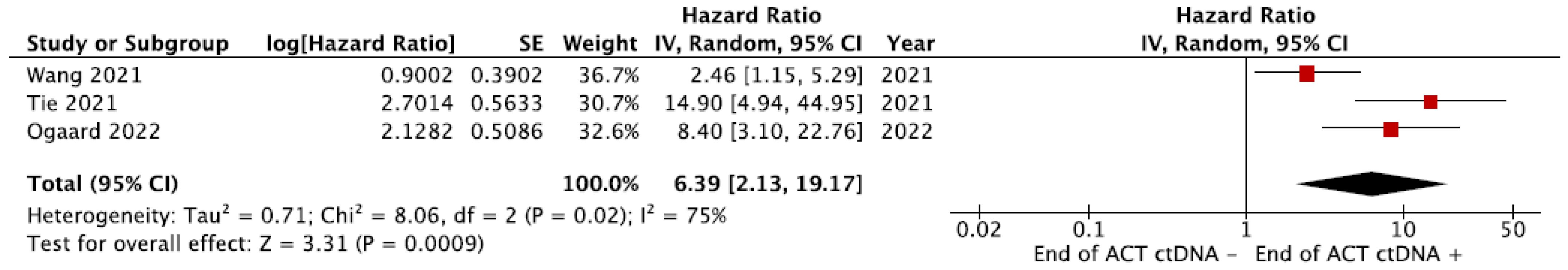
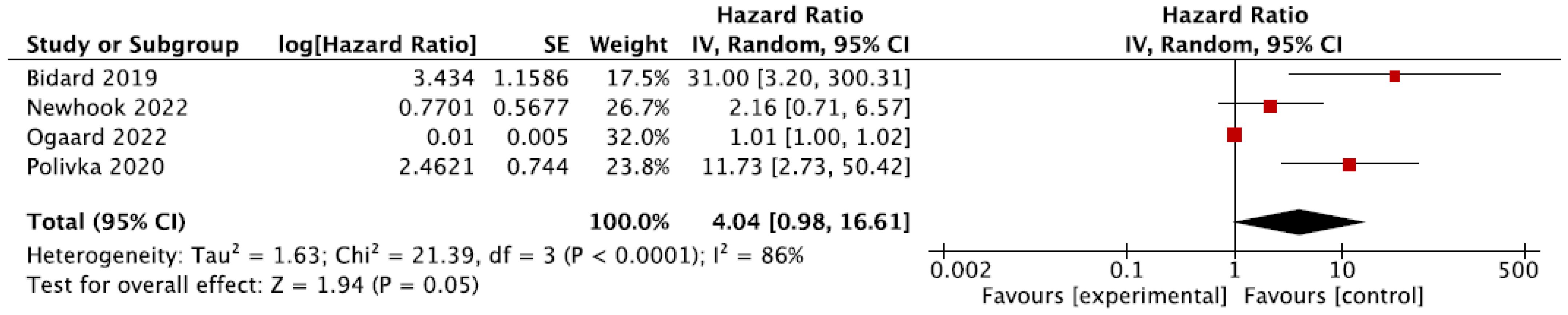
3.7. Meta-Analyses
3.7.1. Recurrence-Free Survival (RFS)
3.7.2. Overall Survival (OS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Geest, L.G.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.; de Wilt, J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef]
- Meyer, Y.; Olthof, P.B.; Grünhagen, D.J.; de Hingh, I.; de Wilt, J.H.W.; Verhoef, C.; Elferink, M.A.G. Treatment of metachronous colorectal cancer metastases in the Netherlands: A population-based study. Eur. J. Surg. Oncol. 2022, 48, 1104–1109. [Google Scholar] [CrossRef]
- Buisman, F.E.; Giardiello, D.; Kemeny, N.E.; Steyerberg, E.W.; Höppener, D.J.; Galjart, B.; Nierop, P.M.H.; Balachandran, V.P.; Cercek, A.; Drebin, J.A.; et al. Predicting 10-year survival after resection of colorectal liver metastases; an international study including biomarkers and perioperative treatment. Eur. J. Cancer 2022, 168, 25–33. [Google Scholar] [CrossRef] [PubMed]
- D’angelica, M.; Kornprat, P.; Gonen, M.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann. Surg. Oncol. 2011, 18, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, F.; Hayashi, M.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Uchiyama, K. Early Recurrence After Initial Hepatectomy for Colorectal Liver Metastases. Int. Surg. 2021, 104, 375–382. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, N.; Brennan, M.F.; Blumgart, L.H.; D’Angelica, M.; et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Keller, L.; Belloum, Y.; Wikman, H.; Pantel, K. Clinical relevance of blood-based ctDNA analysis: Mutation detection and beyond. Br. J. Cancer 2021, 124, 345–358. [Google Scholar] [CrossRef]
- Kaifi, J.T.; Kunkel, M.; Dicker, D.T.; Joude, J.; Allen, J.E.; Das, A.; Zhu, J.; Yang, Z.; Sarwani, N.E.; Li, G.; et al. Circulating tumor cell levels are elevated in colorectal cancer patients with high tumor burden in the liver. Cancer Biol. Ther. 2015, 16, 690–698. [Google Scholar] [CrossRef]
- Litvak, A.; Cercek, A.; Segal, N.; Reidy-Lagunes, D.; Stadler, Z.K.; Yaeger, R.D.; Kemeny, N.E.; Weiser, M.R.; Pessin, M.S.; Saltz, L. False-positive elevations of carcinoembryonic antigen in patients with a history of resected colorectal cancer. J. Natl. Compr. Cancer Netw. 2014, 12, 907–913. [Google Scholar] [CrossRef]
- Sørensen, C.G.; Karlsson, W.K.; Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence—A systematic review. Int. J. Surg. 2016, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the Quality of Prognosis Studies in Systematic Reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Øgaard, N.; Reinert, T.; Henriksen, T.V.; Frydendahl, A.; Aagaard, E.; Ørntoft, M.-B.W.; Larsen, M.; Knudsen, A.R.; Mortensen, F.V.; Andersen, C.L. Tumour-agnostic circulating tumour DNA analysis for improved recurrence surveillance after resection of colorectal liver metastases: A prospective cohort study. Eur. J. Cancer 2022, 163, 163–176. [Google Scholar] [CrossRef]
- Reinert, T.; Petersen, L.M.S.; Henriksen, T.V.; Larsen, M.; Rasmussen, M.H.; Johansen, A.F.B.; Øgaard, N.; Knudsen, M.; Nordentoft, I.; Vang, S.; et al. Circulating tumor DNA for prognosis assessment and postoperative management after curative-intent resection of colorectal liver metastases. Int. J. Cancer 2022, 150, 1537–1548. [Google Scholar] [CrossRef]
- Schøler, L.V.; Reinert, T.; Ørntoft, M.W.; Kassentoft, C.G.; Árnadóttir, S.S.; Vang, S.; Nordentoft, I.; Knudsen, M.; Lamy, P.; Andreasen, D.; et al. Clinical Implications of Monitoring Circulating Tumor DNA in Patients with Colorectal Cancer. Clin. Cancer Res. 2017, 23, 5437–5445. [Google Scholar] [CrossRef]
- Newhook, T.E.; Overman, M.J.; Chun, Y.S.; Dasari, A.; Tzeng, C.-W.D.; Cao, H.S.T.; Raymond, V.; Parseghian, C.; Johnson, B.; Nishioka, Y.; et al. Prospective Study of Perioperative Circulating Tumor DNA Dynamics in Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Ann. Surg. 2023, 277, 813–820. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakamura, Y.; Taniguchi, H.; Odegaard, J.I.; Nomura, S.; Kojima, M.; Sugimoto, M.; Konishi, M.; Gotohda, N.; Takahashi, S.; et al. Impact of Preoperative Circulating Tumor DNA Status on Survival Outcomes after Hepatectomy for Resectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2021, 28, 4744–4755. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ma, X.; Chen, K.; Liu, F.; Cai, S.; Han-Zhang, H.; Hou, T.; Xiang, J.; Peng, J. Perioperative Circulating Tumor DNA in Colorectal Liver Metastases: Concordance with Metastatic Tissue and Predictive Value for Tumor Burden and Prognosis. Cancer Manag. Res. 2020, 12, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.R.; Goldman, D.A.; Gonen, M.; Reichel, J.; Huberman, K.H.; Raj, S.; Viale, A.; Kemeny, N.E.; Allen, P.J.; Balachandran, V.P.; et al. Peripheral Circulating Tumor DNA Detection Predicts Poor Outcomes After Liver Resection for Metastatic Colorectal Cancer. Ann. Surg. Oncol. 2019, 26, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Yang, H.; Liu, X.-Y.; Chen, Z.-G.; Wang, Y.; Fong, W.P.; Hu, M.-T.; Zheng, Y.-C.; Zheng, Y.; Li, B.-K.; et al. Dynamic monitoring of circulating tumor DNA to predict prognosis and efficacy of adjuvant chemotherapy after resection of colorectal liver metastases. Theranostics 2021, 11, 7018–7028. [Google Scholar] [CrossRef]
- Bolhuis, K.; van’t Erve, I.; Mijnals, C.; Delis-Van Diemen, P.M.; Huiskens, J.; Komurcu, A.; Lopez-Yurda, M.; van den Broek, D.; Swijnenburg, R.J.; Meijer, G.A.; et al. Postoperative circulating tumour DNA is associated with pathologic response and recurrence-free survival after resection of colorectal cancer liver metastases. EBioMedicine 2021, 70, 103498. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Kiavue, N.; Ychou, M.; Cabel, L.; Stern, M.H.; Madic, J.; Saliou, A.; Rampanou, A.; Decraene, C.; Bouché, O.; et al. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic Colorectal cancer: A prospective ancillary study to the Unicancer Prodige-14 Trial. Cells 2019, 8, 516. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Cohen, J.; Li, L.; Hong, W.; Christie, M.; Wong, H.L.; Kosmider, S.; Wong, R.; Thomson, B.; et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: A prospective cohort study. PLoS Med. 2021, 18, e1003620. [Google Scholar] [CrossRef]
- Nishioka, Y.; Chun, Y.S.; Overman, M.J.; Cao, H.S.T.; Tzeng, C.D.; Mason, M.C.; Kopetz, S.W.; Bauer, T.W.; Vauthey, J.N.; Newhook, T.E. Effect of Co-mutation of RAS and TP53 on Postoperative ctDNA Detection and Early Recurrence after Hepatectomy for Colorectal Liver Metastases. J. Am. Coll Surg. 2022, 234, 474–483. [Google Scholar] [CrossRef]
- Polivka, J.; Windrichova, J.; Pesta, M.; Houfkova, K.; Rezackova, H.; Macanova, T.; Vycital, O.; Kucera, R.; Slouka, D.; Topolcan, O. The level of preoperative plasma kras mutations and cea predict survival of patients undergoing surgery for colorectal cancer liver metastases. Cancers 2020, 12, 2434. [Google Scholar] [CrossRef]
- van Rees, J.M.; Wullaert, L.; Grüter, A.A.J.; Derraze, Y.; Tanis, P.J.; Verheul, H.M.W.; Martens, J.W.M.; Wilting, S.M.; Vink, G.; van Vugt, J.L.A.; et al. Circulating tumour DNA as biomarker for rectal cancer: A systematic review and meta-analyses. Front. Oncol. 2023, 13, 1083285. [Google Scholar] [CrossRef]
- Faulkner, L.G.; Howells, L.M.; Pepper, C.; Shaw, J.A.; Thomas, A.L. The utility of ctDNA in detecting minimal residual disease following curative surgery in colorectal cancer: A systematic review and meta-analysis. Br. J. Cancer 2022, 128, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Pugh, S.A.; Graham, J.; Primrose, J.N.; Barriuso, J. Circulating tumour DNA as a biomarker in resectable and irresectable stage IV colorectal cancer; a systematic review and meta-analysis. Eur. J. Cancer 2021, 144, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Lo, S.N.; Wang, Y.; Li, L.; Christie, M.; Lee, M.; Wong, R.; Kosmider, S.; Skinner, I.; et al. Prognostic significance of postsurgery circulating tumor DNA in nonmetastatic colorectal cancer: Individual patient pooled analysis of three cohort studies. Int. J. Cancer 2021, 148, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Zhang, R.; Han, J.-H.; Chen, S.-Q.; Zhao, F.-L.; Chen, H.; Lin, J.; Fan, J.; Zhu, W.W.; Lu, L.; et al. Early Circulating Tumor DNA Dynamics Predict Neoadjuvant Therapy Response and Recurrence in Colorectal Liver Metastases: A Prospective Study. Ann. Surg. Oncol. 2023, 30, 5252–5263. [Google Scholar] [CrossRef] [PubMed]
- Zviran, A.; Schulman, R.C.; Shah, M.; Hill, S.T.K.; Deochand, S.; Khamnei, C.C.; Maloney, D.; Patel, K.; Liao, W.; Widman, A.J.; et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat. Med. 2020, 26, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, X.; Bao, H.; Xia, F.; Wan, J.; Shen, L.; Wang, Y.; Zhang, H.; Wei, Y.; Wu, X.; et al. Utility of Circulating Free DNA Fragmentomics in the Prediction of Pathological Response after Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Chem. 2023, 69, 88–99. [Google Scholar] [CrossRef]
- Thierry, A.R. Circulating DNA fragmentomics and cancer screening. Cell Genom. 2023, 3, 100242. [Google Scholar] [CrossRef] [PubMed]
- Deger, T.; Boers, R.G.; de Weerd, V.; Angus, L.; van der Put, M.M.J.; Boers, J.B.; Azmani, Z.; van IJcken, W.F.; Grünhagen, D.J.; van Dessel, L.F.; et al. High-throughput and affordable genome-wide methylation profiling of circulating cell-free DNA by methylated DNA sequencing (MeD-seq) of LpnPI digested fragments. Clin. Epigenetics 2021, 13, 196. [Google Scholar] [CrossRef]
- Murahashi, S.; Akiyoshi, T.; Sano, T.; Fukunaga, Y.; Noda, T.; Ueno, M.; Zembutsu, H. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: Prediction of pathological response and postoperative recurrence. Br. J. Cancer 2020, 123, 803–810. [Google Scholar] [CrossRef]
- Parikh, A.R.; Van Seventer, E.E.; Siravegna, G.; Hartwig, A.V.; Jaimovich, A.; He, Y.; Kanter, K.; Fish, M.G.; Fosbenner, K.D.; Miao, B.; et al. Minimal Residual Disease Detection using a Plasma-only Circulating Tumor DNA Assay in Patients with Colorectal Cancer. Clin. Cancer Res. 2021, 27, 5586–5594. [Google Scholar] [CrossRef]
- Henriksen, T.V.; Reinert, T.; Christensen, E.; Sethi, H.; Birkenkamp-Demtröder, K.; Gögenur, M.; Gögenur, I.; Zimmermann, B.G.; Dyrskjøt, L.; Andersen, C.L.; et al. The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol. Oncol. 2020, 14, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.K.; Wu, H.-T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Slater, S.; Bryant, A.; Chen, H.-C.; Begum, R.; Rana, I.; Aresu, M.; Peckitt, C.; Zhitkov, O.; Lazaro-Alcausi, R.; Borja, V.; et al. ctDNA guided adjuvant chemotherapy versus standard of care adjuvant chemotherapy after curative surgery in patients with high risk stage II or stage III colorectal cancer: A multi-centre, prospective, randomised control trial (TRACC Part C). BMC Cancer 2023, 23, 257. [Google Scholar] [CrossRef]



| Author, Year | Study Design | Patients | Assay Type | NSG/PCR | Tumour-Informed/-Agnostic | Time Points (s) | Outcome | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Newhook et al., 2022 [20] (USA) | Prospective single centre | 48 | Guardant variant classifier (23 genes) | NGS | Tumour-agnostic; for 38 patients tumour informed | Pre- and post-surgery | Long-term (oncologic) survival | Low |
| Nishioka et al., 2022 [28] (USA) | Retrospective single centre | 105 | NGS panel of 70 cancer-related genes | NGS | Tumour-agnostic | Post-surgery | Long-term (oncologic) survival | Moderate |
| Ogaard et al., 2022 [17] (Denmark) | Prospective single centre | 96 | Methylation profile | ddPCR | Tumour-agnostic | Pre-surgery, post-surgery, multiple in follow-up | Long-term (oncologic) survival | Moderate |
| Bolhuis et al., 2021 [25] (The Netherlands) | Prospective multicentre | 23 | KRAS and NRAS mutational status | ddPCR | Tumour-informed (specific gene mutation status) | Before nACT, pre-surgery and post-surgery | Long-term (oncologic) survival, adjuvant treatment response | Moderate |
| Kobayashi et al., 2021 [21] (Japan) | Prospective single centre | 40 | Guardant360® | NGS | Tumour-agnostic | Pre-surgery | Long-term (oncologic) survival | High |
| Tie et al., 2021 [27] (Australia) | Prospective multicentre | 54 | Patient personalised assay | NGS | Tumour-informed (patient specific) | Before nACT, pre-surgery and post-surgery (up to 2 years) | Long-term (oncologic) survival, adjuvant treatment response | Low |
| Wang et al., 2021 [24] (China) | Prospective single centre | 91 | ctDNA 451-gene NGS panel | NGS | Tumour-agnostic | Before nACT, pre-surgery, post-surgery and post-ACT (+ at disease progression) | Long-term (oncologic) survival, adjuvant treatment response | Low |
| He et al., 2020 [22] (China) | Prospective single centre | 20 | Coloncore panel | NGS | Tumour-agnostic | Pre- and post-surgery | Long-term (oncologic) survival | High |
| Polivka et al., 2020 [29] (Czech Republic) | Retrospective single centre | 71 | KRAS mutational status | ddPCR | Tumour-informed (specific gene mutation status) | Pre- and post-surgery | Long-term (oncologic) survival | High |
| Bidard et al., 2019 [26] (France) | Prospective multicentre | 153 | KRAS exon 2 mutational status | ddPCR | Tumour-informed (specific gene mutation status) | Before nACT, after 1 month of chemotherapy and pre-surgery | Long-term (oncologic) survival | Moderate |
| Narayan et al., 2019 [23] (USA.) | Propsective single centre | 60 | TP53 and APC mutational status | NGS and PCR | Tumour-informed (specific gene mutation status) | Intra-operative, 3–4 weeks post-surgery | Long-term (oncologic) survival, adjuvant treatment response | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wullaert, L.; van Rees, J.M.; Martens, J.W.M.; Verheul, H.M.W.; Grünhagen, D.J.; Wilting, S.M.; Verhoef, C. Circulating Tumour DNA as Biomarker for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cells 2023, 12, 2520. https://doi.org/10.3390/cells12212520
Wullaert L, van Rees JM, Martens JWM, Verheul HMW, Grünhagen DJ, Wilting SM, Verhoef C. Circulating Tumour DNA as Biomarker for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cells. 2023; 12(21):2520. https://doi.org/10.3390/cells12212520
Chicago/Turabian StyleWullaert, Lissa, Jan M. van Rees, John W. M. Martens, Henk M. W. Verheul, Dirk J. Grünhagen, Saskia M. Wilting, and Cornelis Verhoef. 2023. "Circulating Tumour DNA as Biomarker for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis" Cells 12, no. 21: 2520. https://doi.org/10.3390/cells12212520
APA StyleWullaert, L., van Rees, J. M., Martens, J. W. M., Verheul, H. M. W., Grünhagen, D. J., Wilting, S. M., & Verhoef, C. (2023). Circulating Tumour DNA as Biomarker for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cells, 12(21), 2520. https://doi.org/10.3390/cells12212520







