Principle Superiority and Clinical Extensibility of 2D and 3D Charged Nanoprobe Detection Platform Based on Electrophysiological Characteristics of Circulating Tumor Cells
Abstract
1. Introduction
2. Results
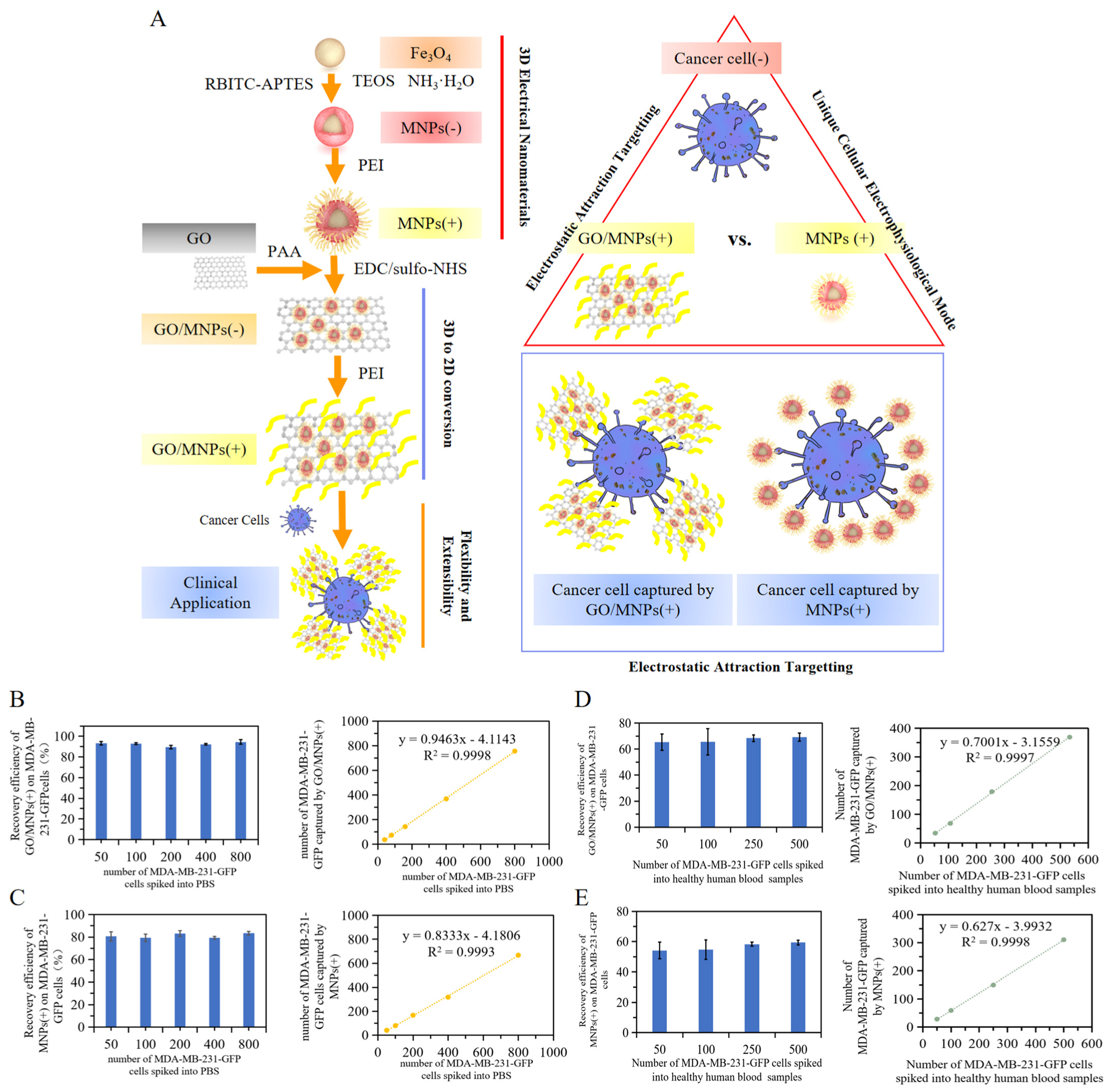
2.1. Unique Electrophysiological Pattern of Cancer Cells and Design of Electrophysiological Nanoprobes for Monitoring
2.2. Characterization of Electrophysiological Nanoprobes
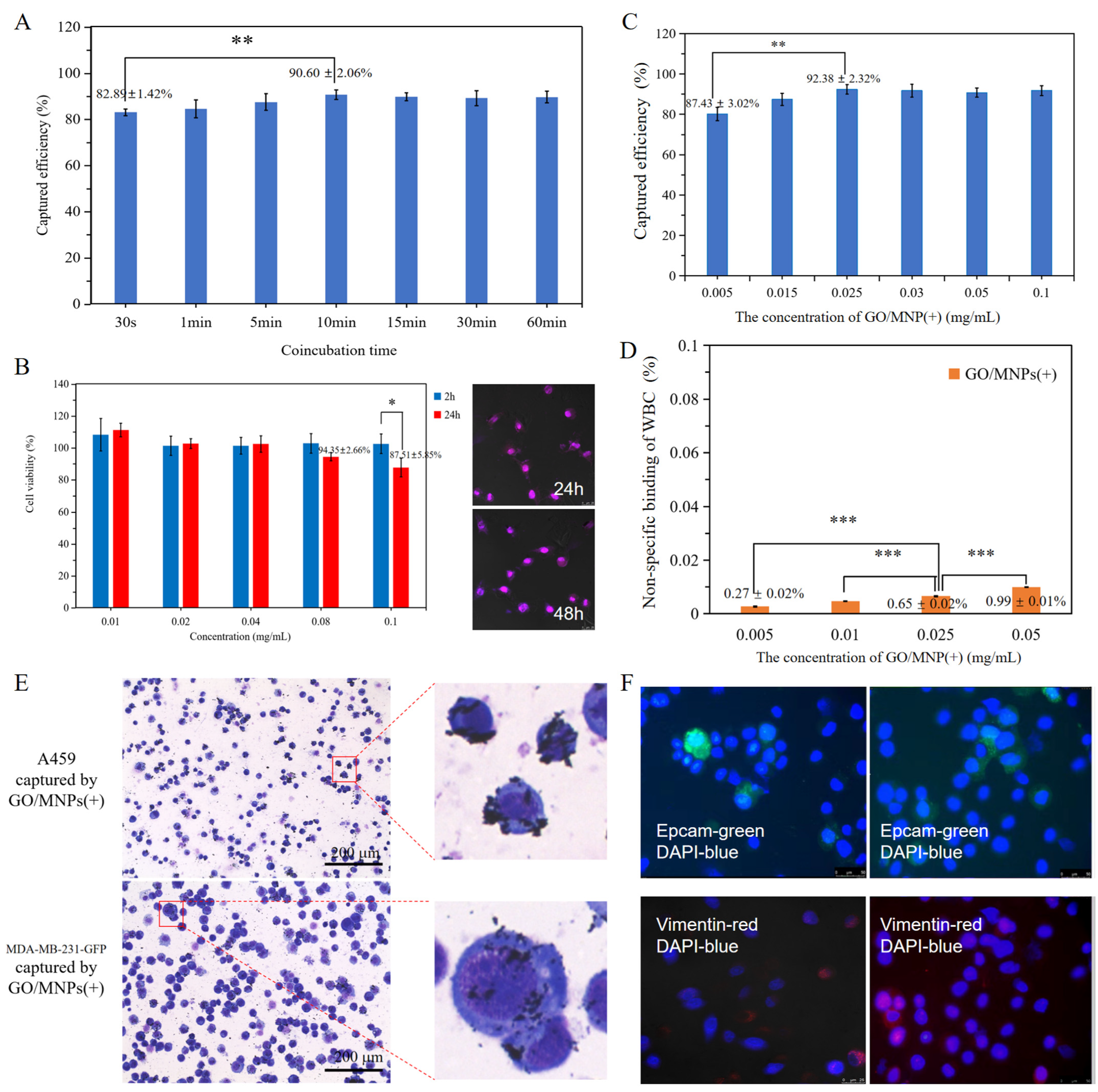
2.3. Specific Targeting of Cancer Cells Based on the Cellular Electrophysiological Characteristics
2.4. Analysis of the Effect of 2D and 3D Nanoprobe Spatial Specific-Surface-Area on Electrostatic Attraction Binding
2.5. Clinical Flexibility Based on Electrophysiological Pattern of Circulating Tumor Cells Detection
2.6. Clinical Validation of Electrophysiological Nanoprobes
3. Materials and Methods
3.1. Synthesis of 2D and 3D Nanoprobe Transition Interface
3.2. Synthesis of Nanoprobes with Opposing Electrical Modes
3.3. Characterization of the 2D and 3D Electrophysiological Nanoprobes
3.4. Validation of Nanoprobe Targeting Recovery by Spiked Cells
3.5. Cytotoxicity Detection of Nanoprobes
3.6. Clinical Blood Specimen Collection
3.7. Detection of CTCs
3.8. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trujillo, B.; Wu, A.; Wetterskog, D.; Attard, G. Blood-based liquid biopsies for prostate cancer: Clinical opportunities and challenges. Br. J. Cancer 2022, 127, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Dathathri, E.; Isebia, K.T.; Abali, F.; Lolkema, M.P.; Martens, J.W.M.; Terstappen, L.W.M.M.; Bansal, R. Liquid Biopsy Based Circulating Biomarkers in Metastatic Prostate Cancer. Front. Oncol. 2022, 12, 863472. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, Y.; Zhou, Y.; Zhao, H.; Lan, Y.; Yu, Z.; Jia, C.; Cong, H.; Zhao, J. The Discovery of Novel Circulating Cancer-Related Cells in Circulation Poses New Challenges to Microfluidic Devices for Enrichment and Detection. Small Methods 2022, 6, 2200226. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huo, F.; Zhang, Y.; Ma, K.; Chao, J.; Yin, C. Spectrochim. A novel strategy: A consecutive reaction was used to distinguish in the presence of statins between normal cells and cancer cells. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117987. [Google Scholar] [CrossRef]
- Al Mazid, M.F.; Park, S.B.; Cheekatla, S.R.; Murale, D.P.; Shin, K.H.; Lee, J. Chemical Probes and Activity-Based Protein Profiling for Cancer Research. Int. J. Mol. Sci. 2022, 23, 5936. [Google Scholar] [CrossRef]
- Ahn, J.C.; Teng, P.C.; Chen, P.J.; Posadas, E.; Tseng, H.R.; Lu, S.C.; Yang, J.D. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021, 73, 422–436. [Google Scholar] [CrossRef]
- Teng, P.C.; Agopian, V.G.; Lin, T.Y.; You, S.; Zhu, Y.; Tseng, H.R.; Yang, J.D. Circulating tumor cells: A step toward precision medicine in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2022, 37, 1179–1190. [Google Scholar] [CrossRef]
- Patel, D.A.; Blay, J. Seeding metastases: The role and clinical utility of circulating tumour cells. Tumor Biol. 2021, 43, 285–306. [Google Scholar] [CrossRef]
- Modlin, I.M.; Bodei, L.; Kidd, M. Neuroendocrine tumor biomarkers: From monoanalytes to transcripts and algorithms. Best Pract. Res. Clin. Endocrinol. 2016, 30, 59–77. [Google Scholar] [CrossRef]
- Van der Laan, P.; van Houdt, W.J.; van den Broek, D.; Steeghs, N.; van der Graaf, W.T.A. Liquid Biopsies in Sarcoma Clinical Practice: Where Do We Stand? Biomedicines 2021, 9, 1315. [Google Scholar] [CrossRef]
- Ni, L.; Shaik, R.; Xu, R.; Zhang, G.; Zhe, J. A Microfluidic Sensor for Continuous, in Situ Surface Charge Measurement of Single Cells. ACS Sens. 2020, 5, 527–534. [Google Scholar] [CrossRef]
- Pouysségur, J.; Marchiq, I.; Parks, S.K.; Durivault, J.; Ždralević, M.; Vucetic, M. ‘Warburg effect’ controls tumor growth, bacterial, viral infections and immunity-Genetic deconstruction and therapeutic perspectives. Semin. Cancer Biol. 2022, 86, 334–346. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; Yang, P.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Cui, Z.; Shi, D. Bioelectricity, Its Fundamentals, Characterization Methodology, and Applications in Nano-Bioprobing and Cancer Diagnosis. Adv. Biosyst. 2019, 3, 1900101. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Si, W.; Liu, Y.; Guo, Z.; Zhou, W.; Zhou, H.; Liang, X.; Wang, C.J. Strong electrostatic repulsive interaction used for fast and effective alkaloid enrichment from plants. Chromatogr. B 2019, 1105, 148–155. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Daniyal, M.; Liu, B.; Wang, W. Comprehensive Review on Graphene Oxide for Use in Drug Delivery System. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- Khoja, L.; Backen, A.; Sloane, R.; Menasce, L.; Ryder, D.; Krebs, M.; Board, R.; Clack, G.; Hughes, A.; Blackhall, F.; et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br. J. Cancer 2012, 106, 508–516. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Feng, W.; Qian, X.; Yu, L.; Chen, Y.; Li, Y. Emerging Nanomedicine-Enabled/Enhanced Nanodynamic Therapies beyond Traditional Photodynamics. Adv. Mater. 2021, 33, 2005062. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tian, Q.; Pan, D.; Hui, L.; Chen, Y.; Zhang, Q.; Tian, W.; Yu, J.; Hu, S.; Gao, Y.; et al. Antibody-Functional Microsphere-Integrated Filter Chip with Inertial Microflow for Size–Immune-Capturing and Digital Detection of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 29569–29578. [Google Scholar]
- Li, C.; He, W.; Wang, N.; Xi, Z.; Deng, R.; Liu, X.; Kang, R.; Xie, L.; Liu, X. Application of Microfluidics in Detection of Circulating Tumor Cells. Front. Bioeng. Biotechnol. 2022, 10, 907232. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Zhu, P.; McCarty, G.; Meyer, C.F.; Pratilas, C.A.; Levin, A.; Morris, C.D.; Albert, C.M.; Jackson, K.W.; Tang, C.; et al. Size-based detection of sarcoma circulating tumor cells and cell clusters. Oncotarget 2017, 8, 78965–78977. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Abate, M.F.; Song, Y.; Zhu, Z.; Yan, F.; Xu, Y.; Wang, X.; Li, Q.; Yang, C. Isolation, Detection, and Antigen-Based Profiling of Circulating Tumor Cells Using a Size-Dictated Immunocapture Chip. Angew. Chem. Int. Ed. 2017, 56, 10681–10685. [Google Scholar] [CrossRef]
- Consiglio, G.; Di Pietro, P.; D’Urso, L.; Forte, G.; Grasso, G.; Sgarlata, C.; Cossement, D.; Snyders, R.; Satriano, C. Surface tailoring of polyacrylate-grafted graphene oxide for controlled interactions at the biointerface. J. Colloid Interface Sci. 2017, 506, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, V.M.L.; Carvalho, L. Heterogeneity in Lung Cancer. Pathobiology 2018, 85, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Sung, W.; Chow, J.; Cho, W. Tumor mutational burden as a tissue-agnostic biomarker for cancer immunotherapy. Expert Rev. Clin. Pharmacol. 2021, 14, 141–143. [Google Scholar] [CrossRef]
- Wu, S.; Gu, L.; Qin, J.; Zhang, L.; Sun, F.; Liu, Z.; Wang, Y.; Shi, D. Rapid Label-Free Isolation of Circulating Tumor Cells from Patients’ Peripheral Blood Using Electrically Charged Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4193–4203. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Gatenby, R.A. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metast. Rev. 2007, 26, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Han, K. Electrical Detection Method for Circulating Tumor Cells Using Graphene Nanoplates. Anal. Chem. 2015, 87, 10585–10592. [Google Scholar] [CrossRef] [PubMed]
- Kozminsky, M.; Fouladdel, S.; Chung, J.S.; Wang, Y.; Smith, D.C.; Alva, A.; Azizi, E.; Morgan, T.; Nagrath, S. Detection of CTC Clusters and a Dedifferentiated RNA-Expression Survival Signature in Prostate Cancer. Adv. Sci. 2019, 6, 1801254. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, D.; Zhou, C.; Zhu, Y.; Lin, C.; Guo, L.; Le, W.; Gu, Z.; Chen, B. Principle Superiority and Clinical Extensibility of 2D and 3D Charged Nanoprobe Detection Platform Based on Electrophysiological Characteristics of Circulating Tumor Cells. Cells 2023, 12, 305. https://doi.org/10.3390/cells12020305
Chen J, Li D, Zhou C, Zhu Y, Lin C, Guo L, Le W, Gu Z, Chen B. Principle Superiority and Clinical Extensibility of 2D and 3D Charged Nanoprobe Detection Platform Based on Electrophysiological Characteristics of Circulating Tumor Cells. Cells. 2023; 12(2):305. https://doi.org/10.3390/cells12020305
Chicago/Turabian StyleChen, Jingyao, Dan Li, Chenqi Zhou, Yuqian Zhu, Chenyu Lin, Liting Guo, Wenjun Le, Zhengrong Gu, and Bingdi Chen. 2023. "Principle Superiority and Clinical Extensibility of 2D and 3D Charged Nanoprobe Detection Platform Based on Electrophysiological Characteristics of Circulating Tumor Cells" Cells 12, no. 2: 305. https://doi.org/10.3390/cells12020305
APA StyleChen, J., Li, D., Zhou, C., Zhu, Y., Lin, C., Guo, L., Le, W., Gu, Z., & Chen, B. (2023). Principle Superiority and Clinical Extensibility of 2D and 3D Charged Nanoprobe Detection Platform Based on Electrophysiological Characteristics of Circulating Tumor Cells. Cells, 12(2), 305. https://doi.org/10.3390/cells12020305




