Red-Light-Activatable AND-Gated Antitumor Immunosuppressant
Abstract
:1. Introduction
2. Experimental Methods
2.1. General
2.2. Organic Synthesis
2.3. Ethical Statement
2.4. Generation of Xenograft Mouse Model
2.5. Red Light AND-Gated Suppression of Tumor Growth In Vivo
3. Results and Discussion
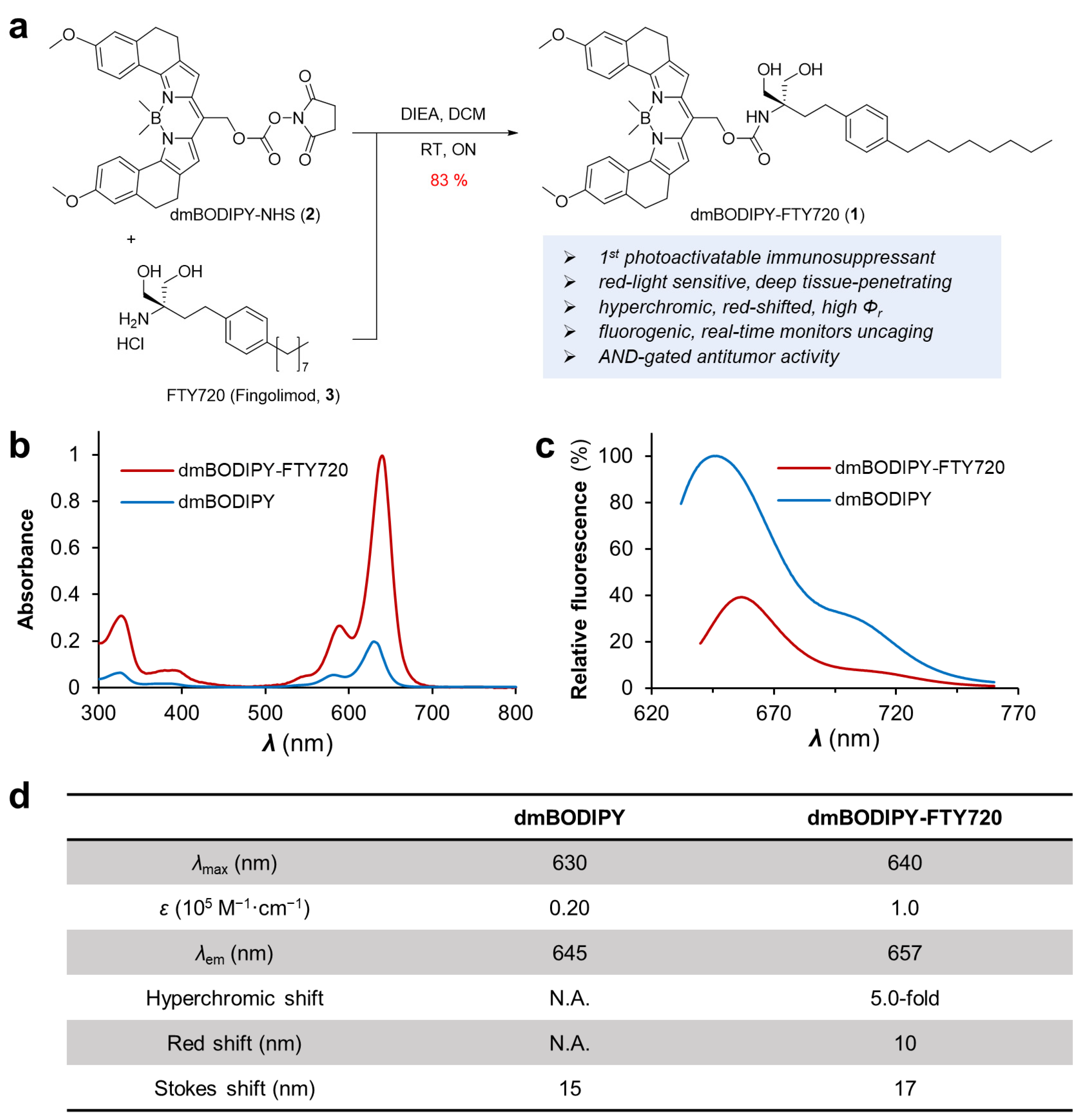
3.1. Design, Preparation and Spectroscopic Characterization of Red Light Activatable FTY720, dmBODIPY-FTY720
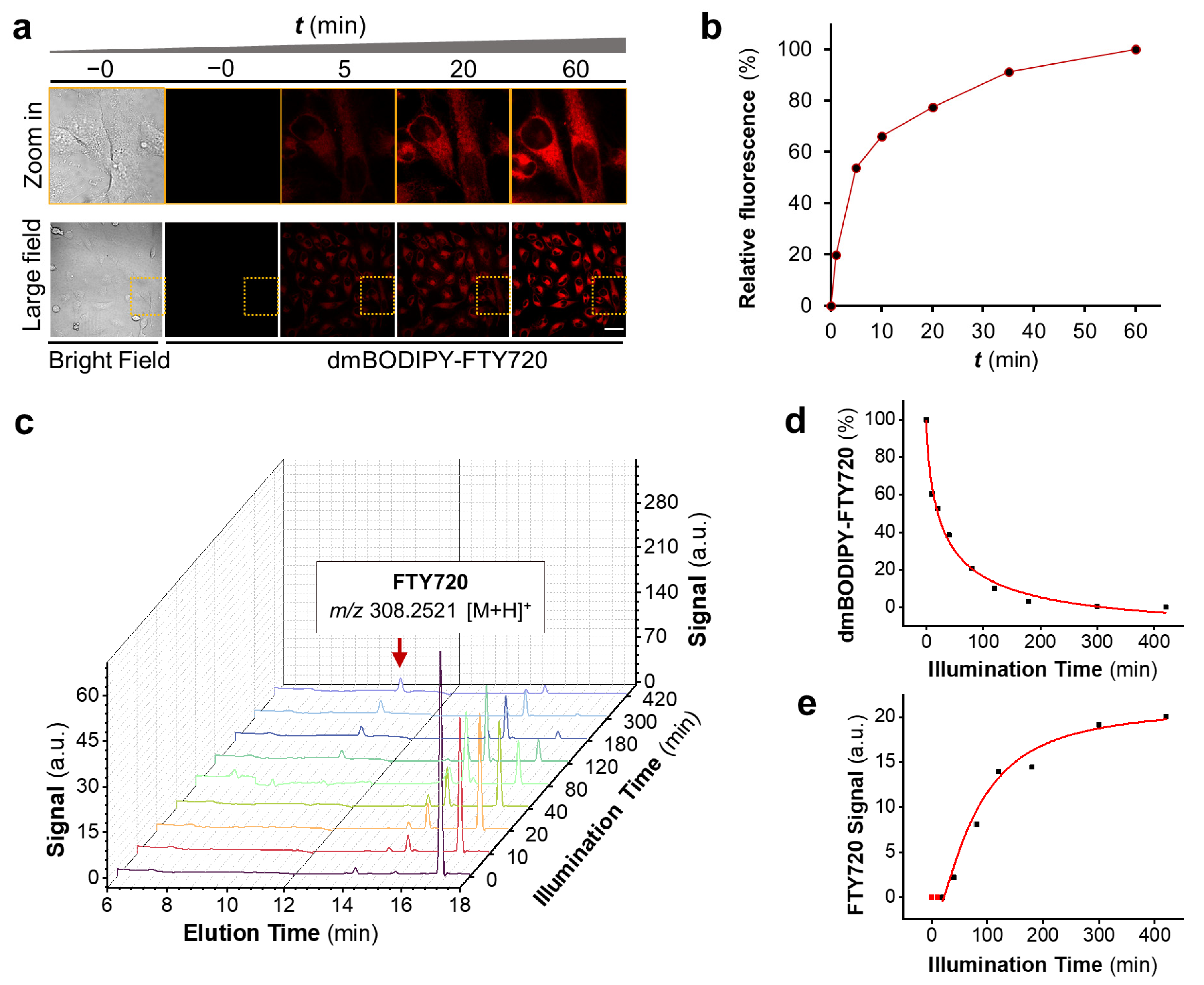
3.2. dmBODIPY-FTY720 (1) Is Readily Cell-Permeable and Releases FTY720 upon Deep Red-Light Illumination
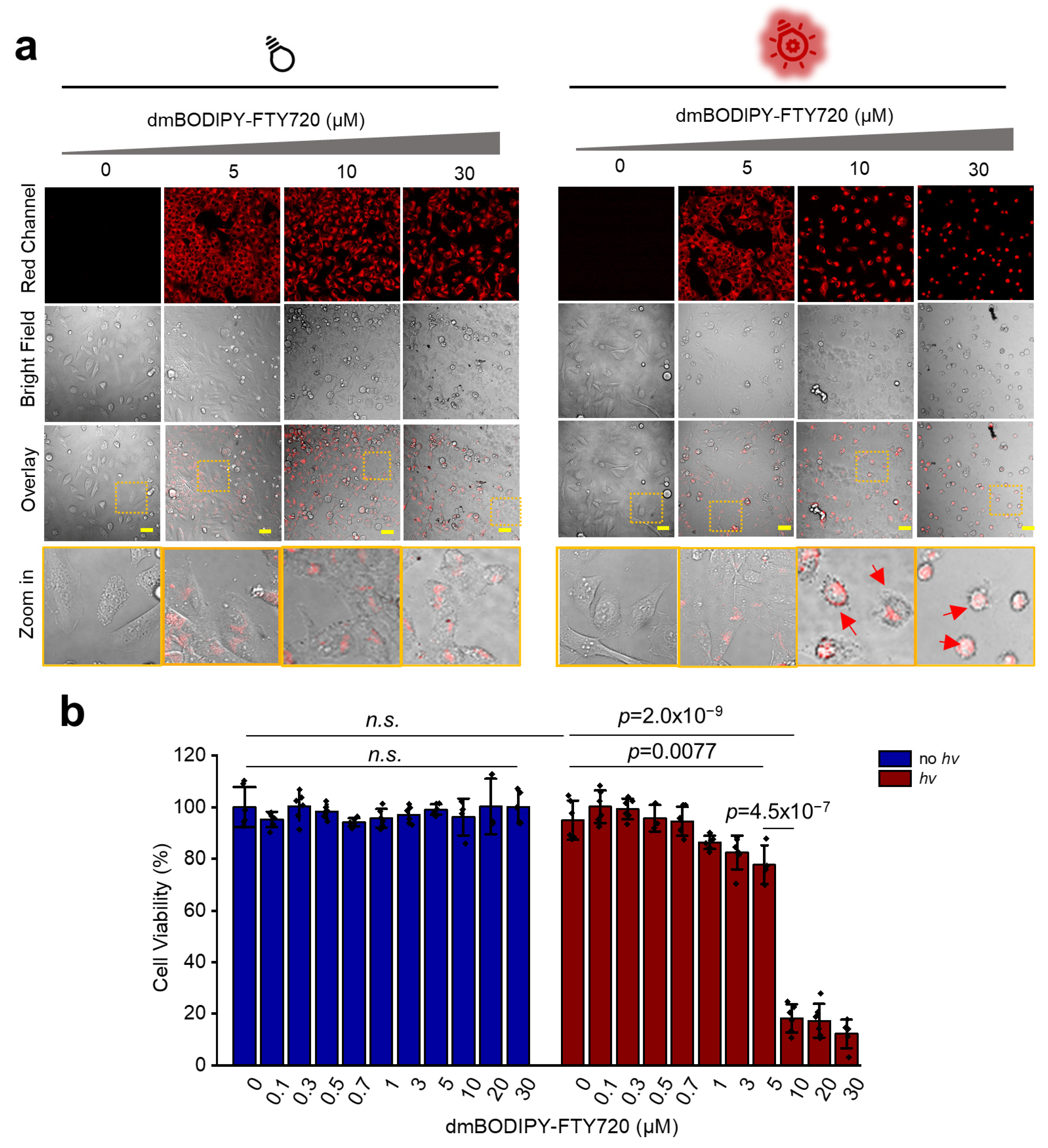
3.3. dmBODIPY-FTY720 (1) Kills Tumor Cells Only upon Red Light Illumination
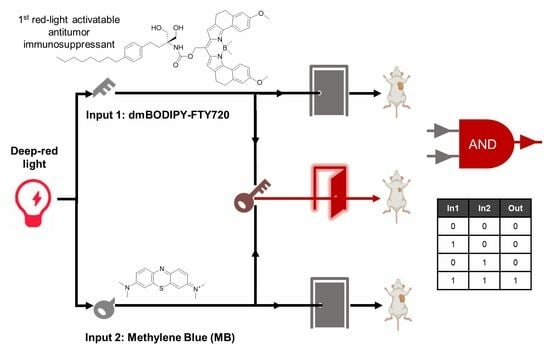
3.4. Design of AND-Gated System for Effective Killing of Cancer Cells Using Red Light at Further Reduced Drug Dosages
3.5. Mechanistic Study Reveals Synergistic Apoptosis Induction in Red-Light-Triggered AND-Gated Tumor Cell Death
3.6. Combination of dmBODIPY-FTY720 (BF, 1) and MB Efficiently Suppresses Tumor Growth In Vivo under Red Light Illumination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blagosklonny, M.V. Immunosuppressants in cancer prevention and therapy. Oncoimmunology 2013, 2, e26961. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, G.T.; Maceyka, M.; Milstien, S.; Spiegel, S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Law, B.K. Rapamycin: An anti-cancer immunosuppressant? Crit. Rev. Oncol. Hemat. 2005, 56, 47–60. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Alshaker, H.; Cooper, C.; Winkler, M.; Pchejetski, D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget 2016, 7, 23106–23127. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chander, R.; Sainis, K.B. Prodigiosins: A novel family of immunosuppressants with anti-cancer activity. Indian J. Biochem. Biophys. 2007, 44, 295–302. [Google Scholar] [PubMed]
- Zhang, S.; Wang, Z.L.; Fan, S.L.; Liu, T.; Yoshida, S.; Yang, S.; Liu, L.; Hou, W.; Cao, L.; Wang, J.X.; et al. Capecitabine can induce T cell apoptosis: A potential immunosuppressive agent with anti-cancer effect. Front. Immunol. 2021, 12, 737849. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy side-effects: Not all DNA damage Is equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Tan, J.X.; Dong, L.Q.; Ye, D.H.; Tang, Y.; Hu, T.Y.; Zhong, Z.X.; Tarun, P.; Xu, Y.C.; Qin, W. The efficacy and safety of immunosuppressive therapies in the treatment of IgA nephropathy: A network meta-analysis. Sci. Rep. 2020, 10, 6062. [Google Scholar] [CrossRef]
- Liu, T.T.; Wang, Y.Y.; Mao, H.M.; Yang, L.P.; Zhan, Y.L. Efficacy and safety of immunosuppressive therapies in the treatment of high-risk IgA nephropathy A network meta-analysis. Medicine 2021, 100, e24541. [Google Scholar] [CrossRef]
- Adachi, K.; Kohara, T.; Nakao, N.; Arita, M.; Chiba, K.; Mishina, T.; Sasaki, S.; Fujita, T. Design, synthesis, and structure-activity-relationships of 2-substituted-2-amino-1,3-propanediols-discovery of a novel immunosuppressant, FTY720. Bioorg. Med. Chem. Lett. 1995, 5, 853–856. [Google Scholar] [CrossRef]
- Xu, Z.M.; Ikuta, T.; Kawakami, K.; Kise, R.; Qian, Y.; Xia, R.X.; Sun, M.X.; Zhang, A.Q.; Guo, C.Y.; Cai, X.H.; et al. Structural basis of sphingosine-1-phosphate receptor 1 activation and biased agonism. Nat. Chem. Biol. 2022, 18, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.J.; Fan, T.J.; An, J.; Choi, W.; Duo, Y.H.; Ge, Y.Q.; Zhang, B.; Nie, G.H.; Xie, N.; Zheng, T.T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Zhou, F.F.; Yang, J.X.; Zhang, Y.Q.; Liu, M.Y.; Lang, M.L.; Li, M.; Chen, W.R. Local phototherapy synergizes with immunoadjuvant for treatment of pancreatic cancer through induced immunogenic tumor vaccine. Clin. Cancer Res. 2018, 24, 5335–5346. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B. Photocontrol of antibacterial activity: Shifting from UV to red Light activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef]
- Fan, W.P.; Huang, P.; Chen, X.Y. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Winsett, R.P.; Stratta, R.J.; Alloway, R.; Wicks, M.N.; Hathaway, D.K. Immunosuppressant side effect profile does not differ between organ transplant types. Clin. Transpl. 2001, 15 (Suppl. S6), 46–50. [Google Scholar] [CrossRef]
- Miyamoto, T.; Razavi, S.; DeRose, R.; Inoue, T. Synthesizing biomolecule-based Boolean logic gates. ACS Synth. Biol. 2013, 2, 72–82. [Google Scholar] [CrossRef]
- Chen, Z.B.; Kibler, R.D.; Hunt, A.; Busch, F.; Pearl, J.; Jia, M.X.; VanAernum, Z.L.; Wicky, B.I.M.; Dods, G.; Liao, H.; et al. De novo design of protein logic gates. Science 2020, 368, 78–84. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; Turchiello, R.D.F.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Thapa, P.; Li, M.J.; Bio, M.; Rajaputra, P.; Nkepang, G.; Sun, Y.J.; Woo, S.; You, Y. Far-red light-activatable prodrug of paclitaxel for the combined effects of photodynamic therapy and site-specific paclitaxel chemotherapy. J. Med. Chem. 2016, 59, 3204–3214. [Google Scholar] [CrossRef]
- Yang, Y.J.; Chen, F.M.; Xu, N.; Yao, Q.C.; Wang, R.; Xie, X.C.; Zhang, F.; He, Y.; Shao, D.; Dong, W.F.; et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials 2022, 281, 121368. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Dissanayake, K.C.; Gehrmann, E.J.; Wijesooriya, C.S.; Mukhopadhyay, A.; Smith, E.A.; Winter, A.H. Efficient far-red/near-IR absorbing BODIPY photocages by blocking unproductive conical intersections. J. Am. Chem. Soc. 2020, 142, 15505–15512. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Sharma, A.; Chang, M.J.; Lee, J.; Son, S.; Sessler, J.L.; Kang, C.; Kim, J.S. Fluorogenic reaction-based prodrug conjugates as targeted cancer theranostics. Chem. Soc. Rev. 2018, 47, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Min, X.; Xiao, S.H.; Johnstone, S.; Romanow, W.; Meininger, D.; Xu, H.D.; Liu, J.S.; Dai, J.; An, S.Z.; et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure 2013, 21, 798–809. [Google Scholar] [CrossRef]
- Kemp, J.A.; Keebaugh, A.; Edson, J.A.; Chow, D.; Kleinman, M.T.; Chew, Y.C.; McCracken, A.N.; Edinger, A.L.; Kwon, Y.J. Biocompatible chemotherapy for leukemia by acid-cleavable, PEGylated FTY720. Bioconjug. Chem. 2020, 31, 673–684. [Google Scholar] [CrossRef]
- Vicar, T.; Raudenska, M.; Gumulec, J.; Balvan, J. The quantitative-phase dynamics of apoptosis and lytic cell death. Sci. Rep. 2020, 10, 1566. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zheng, W.; Li, Y.Q.; Zhong, J.Y.; Ji, J.G.; Shen, P.P. Apoptosis induced by methylene-blue-mediated photodynamic therapy in melanomas and the involvement of mitochondrial dysfunction revealed by proteomics. Cancer Sci. 2008, 99, 2019–2027. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef]
- Tosato, M.G.; Schilardi, P.; de Mele, M.F.L.; Thomas, A.H.; Lorente, C.; Minan, A. Synergistic effect of carboxypterin and methylene blue applied to antimicrobial photodynamic therapy against mature biofilm of Klebsiella pneumoniae. Heliyon 2020, 6, e03522. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.D.; Ding, K.; Xu, J.G. FTY720 induces autophagy-related apoptosis and necroptosis in human glioblastoma cells. Toxicol. Lett. 2015, 236, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Yamamoto, D.; Sakurai, S.; Hasegawa, M.; Aizu-Yokota, E.; Momoi, T.; Kasahara, T. FTY720, a novel immunosuppressive agent, induces apoptosis in human glioma cells. Biochem. Biophys. Res. Commun. 2001, 281, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Tsuchida, M.; Isoyama, N.; Takai, K.; Matsuyama, H. FTY720 mediates cytochrome c release from mitochondria during rat thymocyte apoptosis. Transpl. Immunol. 2010, 23, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Chiu, C.F.; Chiu, S.J.; Chu, P.C.; Weng, J.R. FTY720 induces autophagy-associated apoptosis in human oral squamous carcinoma cells, in part, through a reactive oxygen species/Mcl-1-dependent mechanism. Sci. Rep. 2017, 7, 5600. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy-Current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhang, Y.; Xia, S.; Chen, X. Red-Light-Activatable AND-Gated Antitumor Immunosuppressant. Cells 2023, 12, 2351. https://doi.org/10.3390/cells12192351
Zhou Z, Zhang Y, Xia S, Chen X. Red-Light-Activatable AND-Gated Antitumor Immunosuppressant. Cells. 2023; 12(19):2351. https://doi.org/10.3390/cells12192351
Chicago/Turabian StyleZhou, Ziqi, Yan Zhang, Simin Xia, and Xi Chen. 2023. "Red-Light-Activatable AND-Gated Antitumor Immunosuppressant" Cells 12, no. 19: 2351. https://doi.org/10.3390/cells12192351
APA StyleZhou, Z., Zhang, Y., Xia, S., & Chen, X. (2023). Red-Light-Activatable AND-Gated Antitumor Immunosuppressant. Cells, 12(19), 2351. https://doi.org/10.3390/cells12192351