1. Introduction
In recent years, a number of preclinical reports have been published suggesting inhibition of the enzyme monoacylglycerol lipase (MAGL) as a promising concept to inhibit cancer progression (for review, see [
1]). Thus, MAGL inhibitors show antiproliferative [
2,
3], anti-invasive [
2,
4], and antimetastatic [
4,
5] properties in a variety of tumor cells. Furthermore, inhibition of MAGL exhibits beneficial effects on the quality of life of experimental animals, such as reduction of cachexia in a mouse model of bone cancer [
5], suppression of lithium chloride-induced vomiting in shrews [
6], and reversal of chemotherapy-induced neuropathy in mice [
7]. The overall rarely studied associations between the level of MAGL expression in different tumor types and patient outcomes have recently been reviewed [
1].
Depending on the experimental model, the mechanism of anticancer effects of MAGL inhibitors is either via inhibition of the degradation of 2-arachidonoylglycerol (2-AG), an endocannabinoid with anticancer properties, or via reduced concentrations of protumorigenic free fatty acids resulting from the inhibition of the degradation of other monoacylglycerides, or both processes [
2,
8]. With respect to the first mechanism, MAGL inhibitors, along with inhibitors of anandamide (AEA)-degrading fatty acid amidohydrolase (FAAH), are also classified as compounds that enhance endocannabinoid signaling and augment the therapeutic benefits of endocannabinoids (for review, see [
1,
9]).
One area that remains very poorly investigated concerns the effect of MAGL inhibitors on tumor neovascularization, which is essential for tumor growth and spread. Tumor angiogenesis allows tumors to grow beyond a diameter of 2–3 mm [
10]. A major proangiogenic factor in this process represents the vascular endothelial growth factor (VEGF), which is expressed and released under hypoxic conditions and promotes neovascularization via endothelial receptor tyrosine kinases (for review, see [
11]). In previously published work, MAGL inhibitor-induced reduction in colon carcinoma xenograft growth was accompanied by decreased VEGF and fibroblast growth factor (FGF)-2 expression [
12], suggesting the involvement of antiangiogenic mechanisms of action. In another recent study, parameters of endothelial angiogenesis were reduced by conditioned media (CM) of lung cancer cells previously treated with MAGL inhibitors, clarifying TIMP-1 (tissue inhibitor of metalloproteinases-1) induction in cancer cells as the underlying mechanism [
13]. However, mechanistic cell biology studies under hypoxic conditions have not yet been performed with MAGL inhibitors.
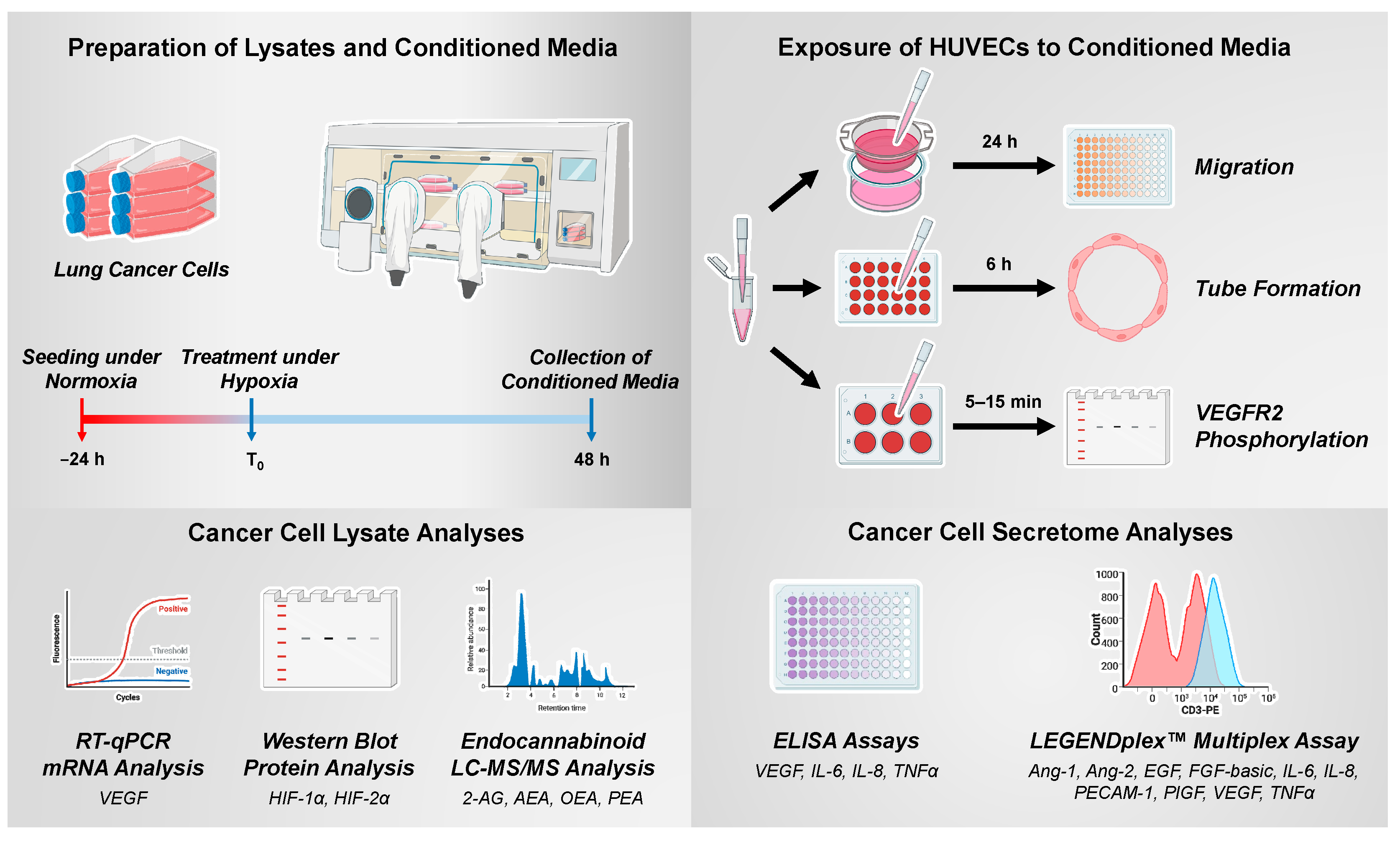
In the present study, the effect of the CM of A549 and H358 lung cancer cells incubated under hypoxic conditions with the MAGL inhibitor JZL184 on the angiogenic properties of human umbilical vein endothelial cells (HUVECs) was investigated. JZL184, a piperidine carbamate, is a highly potent and selective MAGL inhibitor that irreversibly inhibits the enzyme by carbamoylation of the active site catalytic serine nucleophile (Ser122), showing IC
50 values of 2 nM for murine and human MAGL and 25 nM for rat MAGL [
14]. In our previous studies, this MAGL inhibitor has already shown anti-invasive and antiangiogenic effects in normoxic lung cancer cells in vitro as well as antimetastatic and tumor-regressive effects in mice subjected to A549 lung cancer metastasis and xenograft models, respectively [
4,
13]. Here, we present cannabinoid receptor-dependent antiangiogenic properties of JZL184 due to decreased expression of VEGF in hypoxic lung cancer cells, which in turn leads to decreased activation of VEGF receptor 2 (VEGFR2) in HUVECs. Consequently, a novel mechanism of antiangiogenic action of MAGL inhibitors under conditions of hypoxic tumor–endothelial communication was elucidated.
2. Materials and Methods
2.1. Materials
JZL184 (#Cay13158), AM-251 (#Cay71670), AM-630 (#Cay10006974), 2-AG (#Cay62160), arachidonoyl ethanolamide (AEA; #Cay90050), palmitic acid (PA; #Cay10006627), palmitoyl ethanolamide (PEA; #Cay90350), oleoyl ethanolamide (OEA; #Cay90265), 2-AG-d5 (#Cay362162), and AEA-d8 (#Cay390050) were obtained from Cayman Chemical (Ann Arbour, MI, USA). Recombinant human VEGF-165 (rVEGF, #HZ-1038) and recombinant human serum albumin (rHSA, #HZ-3001) were purchased from Proteintech (Planegg-Martinsried, Germany). Dimethyl sulfoxide (DMSO), glycerin, glycine, sodium chloride (NaCl), sodium hydroxide (NaOH), hydrochloric acid 37% (HCl), Tris hydrocloride (Tris-HCl), and Tris ultrapure were from AppliChem (Darmstadt, Germany). Bortezomib (#sc-217785) was obtained from Santa Cruz Biotechnology (Heidelberg, Germany). β-Mercaptoethanol was purchased from Ferak (Berlin, Germany). Aqua ad iniectabilia was bought from Braun Melsungen (Melsungen, Germany). Ethyl acetate and water for chromatography (LC-MS grade) were obtained from Merck (Darmstadt, Germany). Formic acid was purchased from Honeywell Fluka (Seelze, Germany). MJN110 (#SML0872), capsazepine (#C191), aprotinin, hydrogen peroxide solution (H2O2), luminol, bromophenol blue, methylthiazolyldiphenyl-tetrazolium bromide (MTT), orthovanadate, p-coumaric acid, paraformaldehyde (PFA), and phenylmethylsulfonyl fluoride (PMSF) were from Sigma-Aldrich (Taufkirchen, Germany). Methanol and acetonitrile (ACN) were from J. T. Baker (Phillipsburg, NJ, USA). Leupeptin was bought from Biomol (Hamburg, Germany). Acrylamide (Rotiphorese® Gel 30), ammonium peroxydisulphate (APS), crystal violet, N,N,N′,N′-tetramethylethylenediamine (TEMED), and Tween® 20 were obtained from Carl Roth (Karlsruhe, Germany). Dulbecco’s Phosphate-Buffered Saline (DPBS) and fetal bovine serum (FBS) were purchased from PAN-Biotech (Aidenbach, Germany). Non-Fat Milk (NFM) powder was obtained from Bio-Rad Laboratories (Munich, Germany). GibcoTM Penicillin-Streptomycin (10,000 U/mL), GibcoTM Trypsin-EDTA, and GibcoTM Trypan Blue Solution were from Thermo Fisher Scientific (Schwerte, Germany).
2.2. Cell Culture
The human lung carcinoma cell line A549 was obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany; #ACC-107; RRID:CVCL_0023). NCI-H358 human lung carcinoma cells were purchased from ATCC (Manassas, Virginia, USA; #CRL-5807TM; RRID:CVCL_1559). Both cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/L glucose and with UltraGlutamineTM I from Lonza Cologne (Cologne, Germany) supplemented with 10% (v/v) heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Human umbilical vein endothelial cells (HUVECs) were obtained from PromoCell as single donor cryogenic vials (Heidelberg, Germany; #C-12200; RRID:CVCL_2959) and cultured with the Endothelial Cell Growth Medium Kit (#C-22110, designated HUVEC complete medium) from the same company. In addition, 100 U/mL penicillin and 100 μg/mL streptomycin were added to this medium. All three cell lines used were cultured in a humidified incubator at 37 °C and 5% CO2. In experiments with HUVECs, the latter were cultivated for a maximum of five weeks. A549 and H358 cells were frozen in large stock at early passages and used within three months following resuscitation.
With the exception of viability assays with HUVECs, all incubations with test compounds were performed in serum-free DMEM after cells were washed with DPBS. Test substances were dissolved in DMSO (JZL184, MJN110, AM-251, AM-630, capsazepine, palmitic acid) or DPBS (rVEGF, rHSA), after which stock solutions were diluted with DPBS. The final solvent concentrations per compound used in the cell incubates were 0.1% (v/v) DMSO or 0.000025% (w/v) rHSA. Even when multiple test substances were added to the cells, the final concentration of DMSO in the incubates did not exceed 0.3% (v/v) DMSO. In all experiments, the incubation media of vehicle- and substance-treated cells contained the same amount of solvent. When experiments were performed under hypoxia, cells were incubated in the humidified Baker Ruskinn InvivO2® 400 chamber (I&L Biosystems, Königswinter, Germany; hereafter referred to as hypoxia chamber) at 1% O2, 5% CO2, and 37 °C. For equilibration, cells were washed and incubated in serum-free medium under normoxic conditions and subsequently placed in the hypoxia chamber for 15 min. The time after equilibration was defined as the onset of hypoxia. Following equilibration, incubation with the respective test substance or vehicle was performed for the indicated time in the hypoxia chamber. In experiments with the receptor antagonists AM-251, AM-630, and capsazepine, A549 or H358 cells were pre-incubated for 30 min under normoxia followed by hypoxia-equilibration.
2.3. Generation and Further Use of Conditioned Media from A549 and H358 Lung Cancer Cells
To generate conditioned media (CM) from A549 and H358 cells, they were seeded at a density of 5 × 104 cells per well of a 48-well plate, grown for 16–24 h under normoxic conditions, washed, equilibrated to hypoxia, and treated for 48 h with the indicated test compounds in a hypoxia chamber with a final volume of 300 µL of serum-free DMEM. In addition, for the wells containing the respective vehicle control without cells, 300 µL of serum-free DMEM, referred to as unconditioned medium (UCM), was added. For experiments with AM-251, AM-630, and capsazepine, a 30 min pre-incubation with these compounds under normoxia was performed prior to hypoxia equilibration. After a 48 h incubation period, UCM/CM were collected and centrifuged at 1300× g and 4 °C for 5 min.
CM were then used for HUVEC viability, migration, and tube formation analysis (
Section 2.4,
Section 2.5,
Section 2.6), VEGFR2 Western blot analysis (
Section 2.8), LEGENDplex™ multiplex analysis (
Section 2.12), and ELISA determination of interleukin (IL)-6, IL-8, tumor necrosis factor (TNF) α, and VEGF (
Section 2.13). To obtain a higher total volume of UCM/CM, the corresponding media of a treatment group were pooled in some experiments.
To analyze the viability, migration, or tube formation of HUVECs in relation to the tumor cell secretome, reoxygenated UCM/CM of appropriately treated tumor cells were used to resuspend previously harvested, washed, and counted HUVECs therein.
The experimental procedures for generating and using CM of hypoxic cancer cells as well as the analyses of their lysates are shown schematically in
Figure 1.
2.4. Analysis of HUVEC Viability
Viability of HUVEC was determined by a colorimetric assay that detects the cleavage of WST-1 (Roche Diagnostics, Mannheim, Germany), a water-soluble tetrazolium salt, into a soluble formazan dye by metabolically active cells. Absorbance was measured at 450 nm (wavelength correction at 690 nm) using a microplate reader (Infinite F200 Pro Tecan, Tecan Group, Männedorf, Switzerland).
To assess viability after direct exposure to JZL184 and MJN110 (
Supplementary Figures S1C and S3C), HUVECs were seeded at a density of 5 × 10
3 cells per well in 96-well plates and incubated for 24 h with vehicle or the respective test compounds in DMEM supplemented with 2% (
v/
v) FBS. For testing the viability of HUVECs after costimulation with rVEGF and JZL184 (Figure 12C), an incubation time of 48 h was chosen. After the experiment-dependent incubation time, the WST-1 reagent was added.
In the case of viability analysis of HUVECs suspended in CM from hypoxic tumor cells (
Section 2.3), 100 μL UCM/CM containing 5 × 10
3 HUVECs were seeded per well of a 96-well plate. In addition, 2% (
v/
v) FBS was added to the medium. After an incubation period of 24 h, viability was measured.
2.5. Analysis of HUVEC Migration
The effect of test compounds or CM from hypoxic cancer cells on migration of HUVECs was determined by a modified Boyden chamber assay using collagen-coated Falcon
® cell culture inserts (#353097; Corning, Corning, NY, USA). In this assay, HUVECs seeded onto the inserts must migrate through a polyethylene terephthalate membrane with 8 µm pores to a chemoattractant in the 24-well companion plate (lower compartment) as previously described [
15] with slight modifications. To perform the assay, the lower side of the insert was coated with Corning™ Collagen I (Thermo Fisher Scientific) to improve adherence. DMEM containing 10% (
v/
v) FBS, which acts as a chemoattractant, was added to the lower companion plate. After incubation for 24 h in a humidified incubator at 37 °C and 5% CO
2, the non-migrating HUVECs on the upper surface of the inserts were removed with a cotton swab. Viability, which correlates with the cell number of migrated cells on the bottom side, was quantified using the WST-1 assay.
To examine the direct effects of JZL184 and MJN110 on migration of HUVECs (
Supplementary Figures S1A and S3A), 1 × 10
5 HUVECs were suspended in serum-free DMEM containing vehicle or the indicated concentrations of each test compound.
2.6. Analysis of HUVEC Tube Formation
To visualize and quantify the angiogenic potential of test compounds or CM derived from A549 or H358 cells on HUVECs, tube formation assays were performed on 48-well plates coated with Corning
® Matrigel
® Matrix (#356234; BD Biosciences, Heidelberg, Germany) as previously described [
15,
16] with slight modifications. Briefly, 48-well plates were coated with 30 µL of ice-cooled Matrigel per well and polymerized at 37 °C for 30 min.
To examine the direct effects of JZL184 and MJN110 (
Supplementary Figures S1B and S3B) on tube formation of HUVECs, cells were suspended in serum-free DMEM containing vehicle or the indicated concentrations of each test compound. Then, the corresponding HUVEC suspensions were seeded at a density of 5 × 10
4 cells per well in a prepared 48-well plate. For analysis of the effects of CM from A549 or H358 cells on HUVEC tube formation (
Figure 2B,E;
Supplementary Figure S3E,H; Figure 4B,D,F,H;
Supplementary Figure S4B,D), 5 × 10
4 HUVECs were seeded in 200 μL UCM/CM (
Section 2.3) per well of a prepared 48-well plate. The subsequent incubation period was 6 h in each case.
Thereafter, the intersections were fixed with 2% (v/v) PFA, and microscopic images were obtained using the Primovert inverted microscope (Carl Zeiss, Jena, Germany) at 50× magnification for evaluation. Tube formation was quantitatively analyzed in four microscopic fields by counting the number of tube-like structures that formed closed intersections.
2.7. Analysis of A549 and H358 Lung Cancer Cell Viability
To investigate the influence of test compounds and siRNAs on tumor cell viability (
Supplementary Table S3), A549 and H358 cells were seeded and treated as described in
Section 2.3 or
Section 2.14. To measure viability, an MTT assay was performed here, which, in contrast to the WST-1 assay, is not sensitive to changes in oxygenation [
17]. For this purpose, after an incubation period of 48 h, 60 µL of MTT solution (3 mg/mL in DPBS, 0.5 mg/mL final concentration in incubates) was added and the cells were incubated for another 2 h in the hypoxia chamber. Subsequently, the formed crystals were resolved with DMSO under normoxic conditions and the absorbance was measured at 570 nm (wavelength correction at 690 nm) using a microplate reader.
In the case of palmitic acid, an additional viability assay was performed to indirectly quantify adherent cells and cell death (
Supplementary Table S4). For this purpose, cells seeded and treated as described in
Section 2.3 were fixed for 30 min with ice-cold absolute ethanol before incubation with crystal violet staining solution (0.1% [
w/
v] crystal violet in 10% ethanol) for 30 min. After thoroughly washing off the excess dye, the stained cells were dissolved with 10% acetic acid and the dye intensity was measured at 570 nm with a microplate reader.
2.8. Isolation of Total Cellular Protein in Lung Cancer Cells and HUVECs
A549 and H358 cells were seeded in 6-well plates at a density of 2 × 105 per well, except for Figures 9A and 11A, where 2.5 × 106 cells were seeded per Petri dish. Cells were cultured in DMEM containing 10% (v/v) FBS in a humidified incubator at 37 °C and 5% CO2 for 16–24 h. Subsequently, lung cancer cells were washed and the medium was replaced with serum-free medium under normoxic conditions. After hypoxia equilibration for 15 min, cells were treated under hypoxia for fixed incubation periods. If a normoxia control was included, another plate was added with the appropriate incubation times. Cells were then washed with ice-cold DPBS, and after scraping the cells in lysis buffer (2% [w/v] SDS, 40% [v/v] H2O, 10% [v/v] glycerol, 50% [v/v] 125 mM Tris-HCl [pH 6.8]), proteins were immediately denatured at 95 °C under continuous shaking for 10 min. Thereafter, the lysates were centrifuged (20,817× g, 4 °C) for 5 min, and the resulting supernatant was collected for further protein analysis.
In the case of p-VEGFR2 and VEGFR2 analysis, HUVECs were seeded in 6-well plates at a density of 3 × 10
5 per well and cultured for 16–24 h in HUVEC complete medium. HUVECs were then washed and starved for 6 h in serum-free DMEM with or without JZL184 before treatment under normoxic conditions with rVEGF or UCM/CM from A549 or H358 cells (
Section 2.3). For neutralization studies, 1 µg/mL VEGF-A antibody (#MAB293; RRID:AB_358222) or 1 µg/mL IgG2B isotype control (#MAB004; RRID:AB_357346) was added to the CM 1 h before at room temperature. Both antibodies were from R&D Systems (Wiesbaden, Germany). After the incubation period, protein isolation was performed as described before.
Protein concentrations were measured using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). Before samples were stored at −20 °C, 4% (v/v) β-mercaptoethanol was added.
2.9. Isolation of Cytoplasmic and Nuclear Proteins in Lung Cancer Cells
Nuclear and cytoplasmic extraction was performed using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (#78833; Thermo Fisher Scientific). Briefly, 2.5 × 10
6 A549 cells were seeded per Petri dish, grown for 16–24 h, and treated as described in
Section 2.8. After the incubation period, cells were washed with DPBS supplemented with 1 µM bortezomib and detached with trypsin-EDTA also containing 1 µM bortezomib. Trypsinization was stopped with DMEM supplemented with 10% (
v/
v) FBS and 1 µM bortezomib. The cytoplasmic and nuclear pellet was isolated as described in the manufacturer’s instructions. To the CER I and NER solubilization buffers, 10 µg/mL aprotinin, 1 µg/mL leupeptin, 1 mM orthovanadate, 1 mM PMSF, and 10 µM bortezomib were additionally added. Protein concentrations of both fractions were measured using the Pierce™ BCA Protein Assay Kit and samples were stored at −80 °C for further analysis.
2.10. Western Blot Analysis of Proteins in Lung Cancer Cells and HUVECs
Equal amounts of protein were separated on an 8% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane, which was then blocked for 1 h in 5% (w/v) NFM in Tris-buffered saline containing 0.1% (v/v) Tween® 20 (TBS-T buffer). After washing with TBS-T buffer, membranes were incubated with primary antibodies in 5% (w/v) NFM overnight at 4 °C. Specific antibodies against VEGFR2 (#2479; RRID:AB_2212507) and p-VEGFR2 (Tyr1175) (#3770; RRID:AB_1642326) were purchased from Cell Signaling Technology (Frankfurt/Main, Germany). HIF-1α (#MA1-516; RRID:AB_325431) was obtained from Thermo Fisher Scientific, HIF-2α (#NB100-122; RRID:AB_10000872) from Novus Biologicals (Wiesbaden-Nordenstadt, Germany), β-actin (#A5441; RRID:AB_476744) from Sigma-Aldrich, and Lamin B1 (#ab16048; RRID:AB_443298) from Abcam (Berlin, Germany). Following washing with TBS-T buffer, membranes were incubated with secondary antibodies coupled to horseradish peroxidase (anti-rabbit antibody: #7074, RRID:AB_2099233 or anti-mouse antibody: #7076; RRID:AB_330924) from Cell Signaling Technology in 5% (w/v) NFM in TBS-T buffer for 1 h at room temperature. To visualize antibody binding, a chemiluminescent substrate solution (100 mM Tris-HCl [pH 8.5], 1.25 mM luminol; 200 µM p-coumaric acid; 0.09% [v/v] H2O2) was added, and signal detection was performed using the ChemiDoc XRS gel documentation system from Bio-Rad Laboratories (Munich, Germany). The Precision Plus ProteinTM Dual Colour Standard from Bio-Rad Laboratories was used to determine the molecular weight of the bands. Quantification of signal intensity was performed using Quantity One 1-D analysis software (Bio-Rad Laboratories). The signal of a specific protein band was normalized to the signal of the loading control (β-actin for whole-cell lysate and cytoplasmic fraction, lamin B1 for nuclear fraction, and VEGFR2 for p-VEGFR2 analysis).
2.11. Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR)
A549 and H358 cells were seeded at a density of 2.0 × 105 cells per well in a 6-well plate, cultured for 16–24 h in DMEM containing 10% (v/v) FBS, washed, and the medium was replaced with serum-free medium under normoxic conditions. After 15 min of hypoxia equilibration, cells were treated under hypoxia for the respective incubation times. Cells were then lysed under hypoxic conditions and total RNA was isolated using the RNeasy Mini Kit from Qiagen (Hilden, Germany). Total RNA concentrations were measured using the NanoDrop™ OneC Microvolume UV-Vis spectrophotometer from Thermo Fisher Scientific to allow the use of equal amounts of RNA for further RT-qPCR. For quantification of VEGF-A, the Applied Biosystems® TaqMan® Gene Expression Assay (Assay ID: Hs00900055_m1; FAM-MGB) and the Applied Biosystems® TaqMan® RNA-to-CT™ 1-Step Kit from Thermo Fisher Scientific were used according to the manufacturer’s instructions. Peptidylprolyl isomerase A (PPIA; Assay ID: Hs999904_m1; VIC-MGB) was used as a housekeeping gene to normalize VEGF-A mRNA levels before comparison with the corresponding vehicle or zero time control.
2.12. LEGENDplex™ Multiplex Assay
CM of A549 were obtained as described in
Section 2.3 and analyzed for 10 major targets involved in angiogenesis using the LEGENDplex™ multiplex assay (BioLegend, Amsterdam, The Netherlands). The specific kit is the Human Angiogenesis Panel 1 (10-plex) with V-bottom plate (#740698), which allows simultaneous quantification of angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), epidermal growth factor (EGF), fibroblast growth factor-basic (FGF-basic), IL-6, IL-8, platelet endothelial cell adhesion molecule-1 (PECAM-1), placental growth factor (PlGF), VEGF, and TNFα. For the experiments, in brief, samples were incubated with capture beads with fluorescent barcodes and coated with monoclonal antibodies against 10 different analytes. After washing, the beads were incubated with phycoerythrin (PE)-conjugated monoclonal antibodies specific for a different epitope of each analyte tested. Following further washing, samples were detected by flow cytometry (CytoFLEX LX; Beckman Coulter, Krefeld, Germany). PE intensities informed absolute analyte amounts by interpolation from 5-log analyte standards measured in parallel.
2.13. Determination of VEGF-A, IL-6, IL-8, and TNFα Protein Levels
CM of A549 and H358 cells were obtained as described in
Section 2.3. Subsequently, Quantikine
® ELISAs from R&D Systems were used for quantification of VEGF-A (VEGF Quantikine ELISA Kit, #DVE00), IL-6 (IL-6 Quantikine ELISA Kit, #D6050), IL-8 (IL-8/CXCL8 Quantikine ELISA Kit, #D8000C) and TNFα (TNF-alpha Quantikine ELISA Kit, #DTA00D). To detect TNFα, 2 mL of CM from a treatment group was pooled and concentrated using the Amicon
® Ultra-0.5 Centrifugal Filter Unit (Merck; NMWL: 3 kDa).
2.14. Transfection of siRNA in Lung Cancer Cells
Transfection of HIF-1α, HIF-2α, or negative control siRNA was performed using Lipofectamine™ RNAiMAX Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions provided under “Reverse Transfection” with slight modifications. RNAi-Lipofectamine™ RNAiMAX complexes were prepared in Opti-MEM
® I Reduced-Serum Medium (Thermo Fisher Scientific) and contained Lipofectamine™ RNAiMAX reagent and HIF-1α siRNA (Qiagen, #1027418, GeneGlobe ID: SI02664053), HIF-2α siRNA (Qiagen, #1027418, GeneGlobe ID: SI02663038) or negative control siRNA (Qiagen, #1027310). Gently mixed transfection mixtures were added to each well, and after 20 min, A549 or H358 cells in DMEM containing 10% (
v/
v) FBS were added (2 × 10
5 cells per well of a 6-well plate for protein analyses or 5 × 10
4 cells per well of a 48-well plate for VEGF and viability analyses), yielding a final concentration of 10 nM siRNA or negative control siRNA, respectively. For the use of 0.03 nmol siRNA (single knockdown) as well as 0.06 nmol (double knockdown), 1 µL Lipofectamine™ RNAiMAX reagent was used in each case. After a 24 h incubation period, cells were washed and incubated in serum-free DMEM for the indicated times. Finally, cells were harvested for Western blot analyses or the supernatants were used for VEGF ELISA measurements as described in
Section 2.13.
2.15. LC-MS/MS Analysis of Endocannabinoids and Endocannabinoid-like Substances in A549 and H358 Lung Cancer Cells
A total of 2.5 × 10
6 A549 or H358 cells were seeded per Petri dish, grown for 16–24 h, and processed as described in
Section 2.8. After each incubation period, cells were washed with DPBS and detached with trypsin-EDTA, and the detached cells from three Petri dishes were combined in a Falcon tube. Cells were harvested as pellets after 5 min centrifugation (173×
g, 4 °C). The cell pellet was washed with DPBS, recollected after 5 min centrifugation (173×
g, 4 °C), and stored at −80 °C for at least one day. For the analysis of endocannabinoids (2-AG, AEA) and endocannabinoid-like substances (OEA, PEA), the thawed pellet containing approximately 7.5 × 10
6 cells was used. The purification and mass spectrometry method was adapted according to previous work of our laboratory [
4,
18]. The pellets were resuspended in 1 mL of 100 mM Tris-HCl buffer pH 7.4. For subsequent normalization to the appropriate protein levels, 5 µL was taken for protein determination. Extraction was then performed twice with ethyl acetate. Supernatants were concentrated to dryness in a vacuum concentrator (SpeedVac SPD130DLX, Thermo Fisher Scientific, Asheville, NC, USA) and thereafter resuspended in 100 µL of 60% ACN/H
2O containing 0.2% [
v/
v] formic acid. AEA-d8 was added to the samples as an internal standard for AEA, OEA, and PEA and 2-AG-d5 for 2-AG, each at a final concentration of 10 ng/mL. Several standard solutions (for AEA, OEA, and PEA: 0.025, 0.05, 0.1, 0.2, 0.5, 0.75, 1, 2, and 10 ng/mL; for 2-AG: 0.125, 0.25, 0.5, 1, 2.5, 3.75, 5, 10, and 20 ng/mL) were used for calibration. 2-AG was supplied by the producer as a mixture of 2-AG and 1-AG (9:1). Therefore
, the stated concentrations refer to nine parts 2-AG and one part 1-AG. A sample volume of 50 µL was injected and separated using a Prominence LC-20AD system (Shimadzu, Duisburg, Germany) equipped with a Multospher 120 C18 AQ column 125 × 2 mm, 5 µm particle size and a guard column (20 mm × 3 mm, 5 µm particle size), both from CS-Chromatographie Service (Langerwehe, Germany). H
2O was chosen as mobile phase A and ACN as mobile phase B, both containing 0.2% (
v/
v) formic acid. The flow rate was 0.3 mL/min. After holding the starting gradient for 3 min, the gradient was increased from 60% B to 100% B in 10 min. 100% B was held for 7 min and then immediately decreased to 60% B to equilibrate the column for 14 min. The oven temperature was set at 40 °C. An LCMS-8050 triple quadrupole mass spectrometer (Shimadzu) was used for the quantification of 2-AG, AEA, OEA, and PEA in cell samples. All mass spectrometric data for positive electrospray ionization and precursor ions with corresponding fragment ions are presented in
Supplementary Tables S1 and S2. Argon was used as the gas for collision-induced dissociation (CID). The values shown correspond to the measured concentrations of the analytes normalized to the cellular protein concentrations determined using the Pierce™ BCA Protein Assay Kit.
2.16. Statistics
All statistical analyses were performed using GraphPad Prism 9.4.1 (GraphPad Software, San Diego, CA, USA). Comparisons between two groups were carried out using Student’s unpaired two-tailed t-test. Comparisons between more than two groups were conducted using one-way ANOVA with Dunnett’s post hoc test when all conditions were compared with vehicle control or using Bonferroni’s post hoc test for selected group comparisons. In some cases (Figures 5, 6A,B and 9), statistical analysis was omitted for content or experimental reasons.
4. Discussion
The present study is the first to demonstrate antiangiogenic properties of a MAGL inhibitor, and thus degradation inhibitor of the endocannabinoid 2-AG, under hypoxic conditions due to decreased expression of VEGF in lung cancer cells and subsequently diminished activation of VEGFR2 in human endothelial cells.
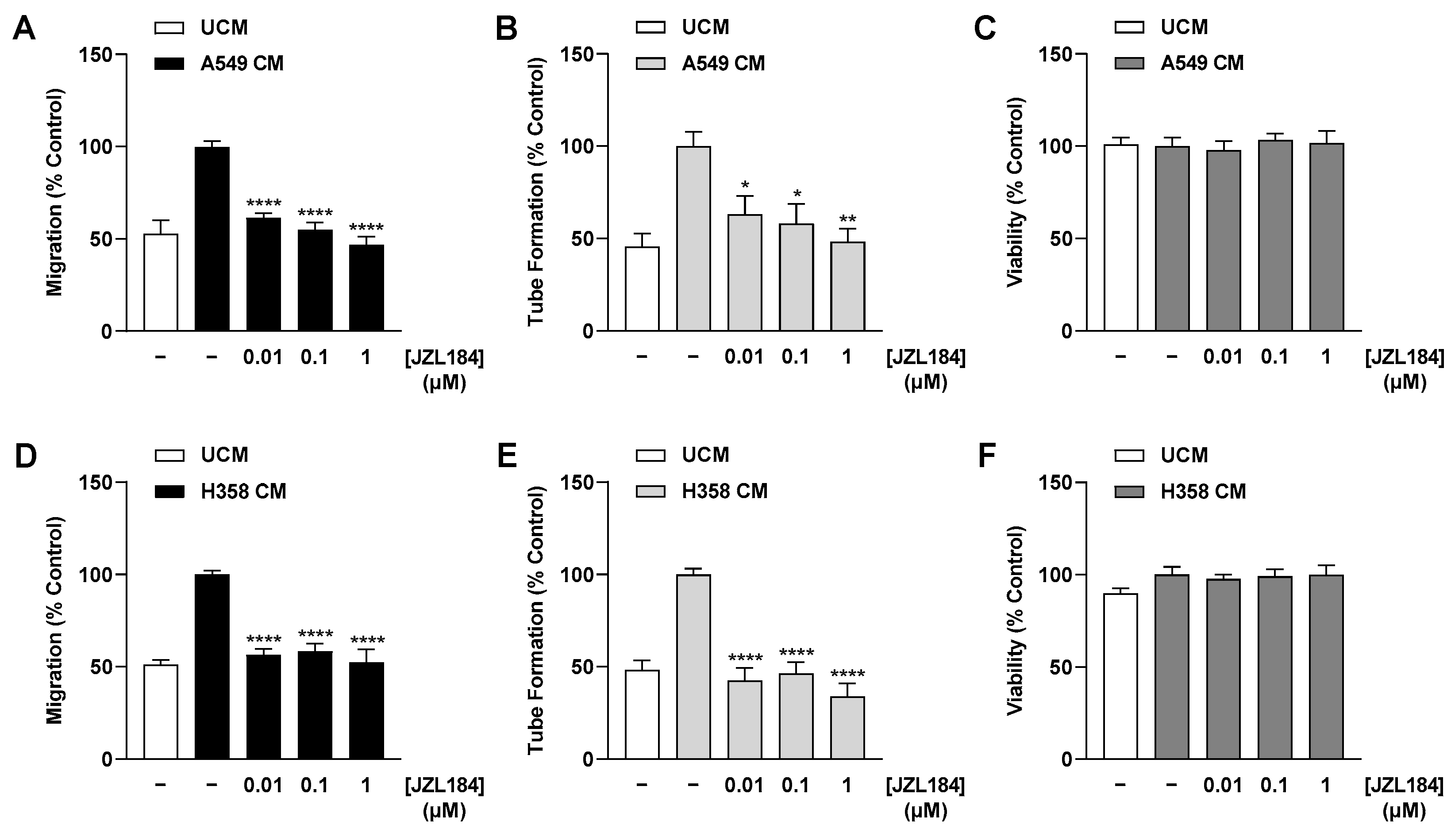
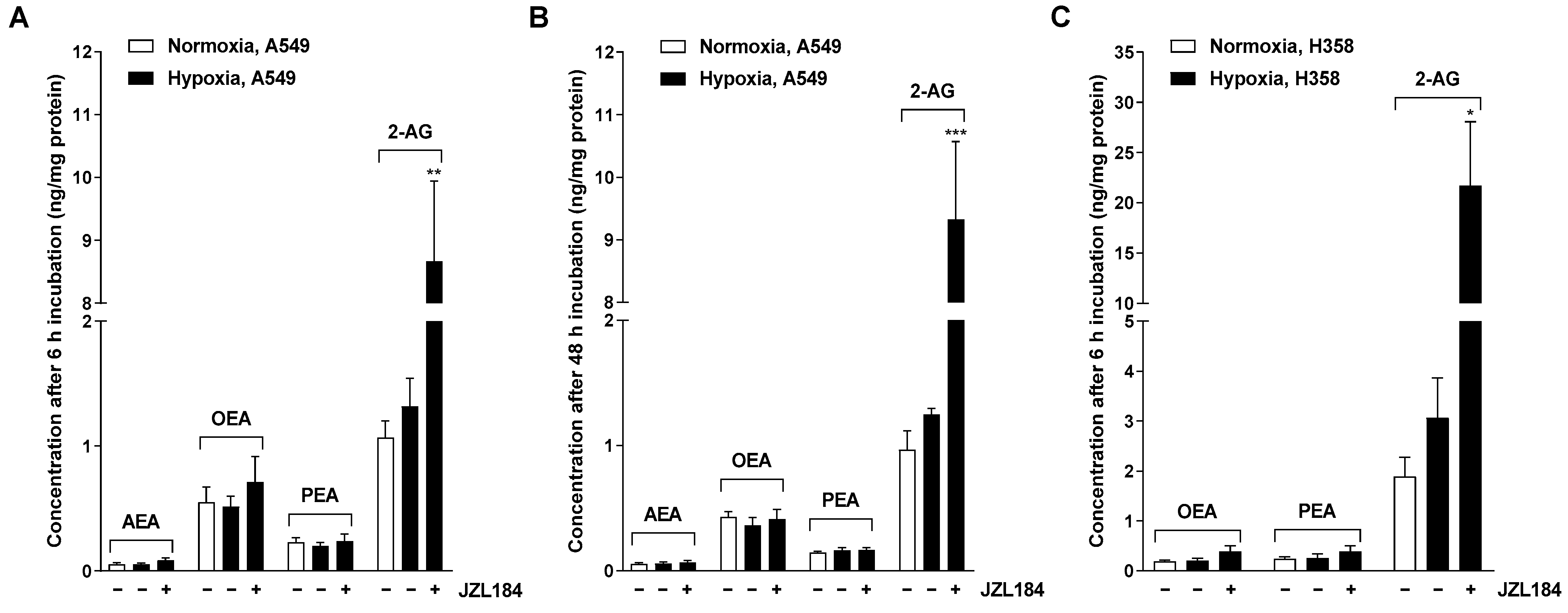
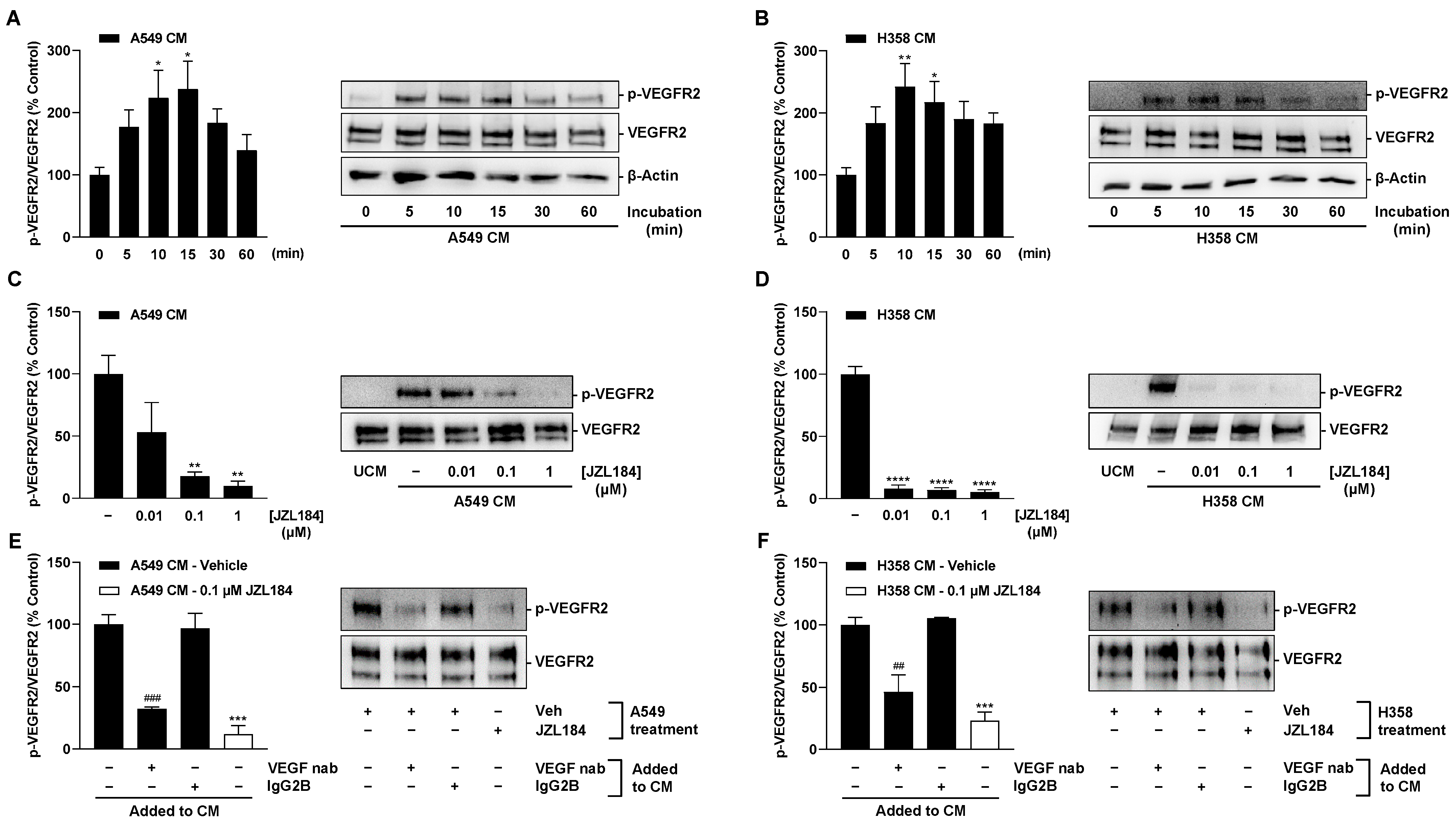
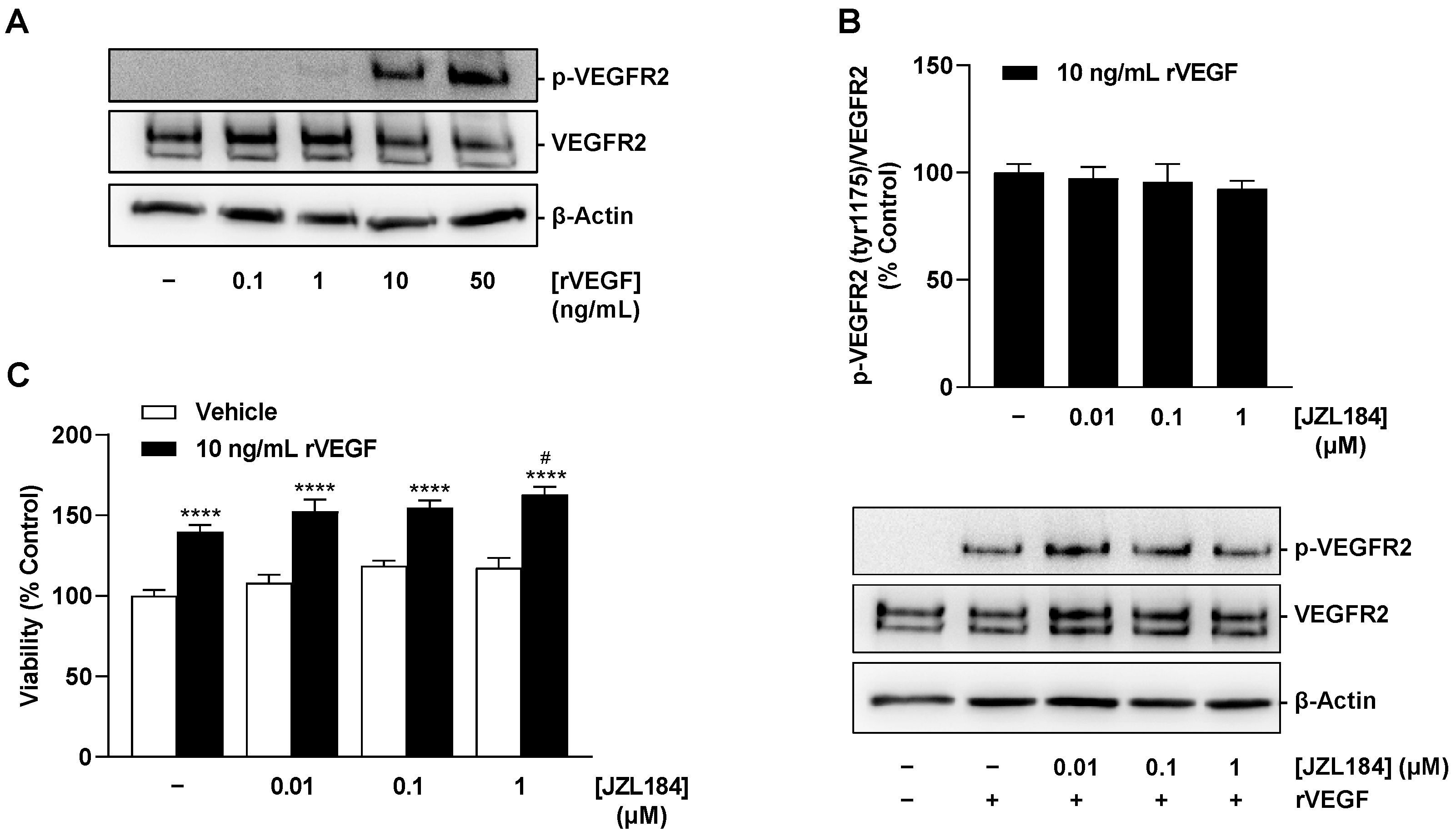
There are several results that support this assumption. Thus, CM derived from hypoxic A549 and H358 lung cancer cells treated with the MAGL inhibitors JZL184 and MJN110 inhibited HUVEC migration and tube formation, two crucial steps in angiogenesis. Involvement of the endocannabinoid system in the effects shown is supported by a selectively significant increase in MAGL substrate 2-AG in lung cancer cells upon incubation with MAGL inhibitors. In line with this finding, the inhibitory effect of CM from hypoxic A549 and H358 cancer cells treated with JZL184 on endothelial cell tube formation and migration potential was partially abolished by antagonists at the CB1 and CB2 receptors. Inhibition of VEGF secretion was confirmed in the presence of two structurally distinct MAGL inhibitors and, in the case of JZL184, was partially abolished by CB1 and CB2 antagonists, consistent with the data on angiogenesis. Finally, altered endothelial VEGF signaling resulting from VEGF downregulation triggered by JZL184 in hypoxic lung cancer cells was confirmed by analyzing the effect of CM of cancer cells on VEGFR2 phosphorylation of HUVECs. Here, VEGFR2 phosphorylation was significantly inhibited by CM of A549 or H358 cells previously treated with JZL184 compared with CM of vehicle-treated cells. On the other hand, JZL184 showed no effect on VEGFR2 activation induced by exogenous rVEGF, ruling out a direct intervention of JZL184 in VEGFR signaling in HUVECs.
Antiangiogenic properties of CB
1 and CB
2 receptor agonists have been reported before in vitro and in vivo [
27,
28,
29,
30,
31,
32]. However, corresponding evidence on MAGL inhibitors is limited. In one investigation, the MAGL inhibitor URB602 reduced the number of microvessels in a mouse colonic xenograft, suggesting an involvement of antiangiogenic effects in the tumor regression induced here by this compound [
12]. In another recent study performed on normoxic lung cancer cells, angiogenic parameters of HUVECs were reduced by CM of lung cancer cells previously incubated with MAGL inhibitors, with the release of antiangiogenic TIMP-1 playing a critical role in this process [
13].
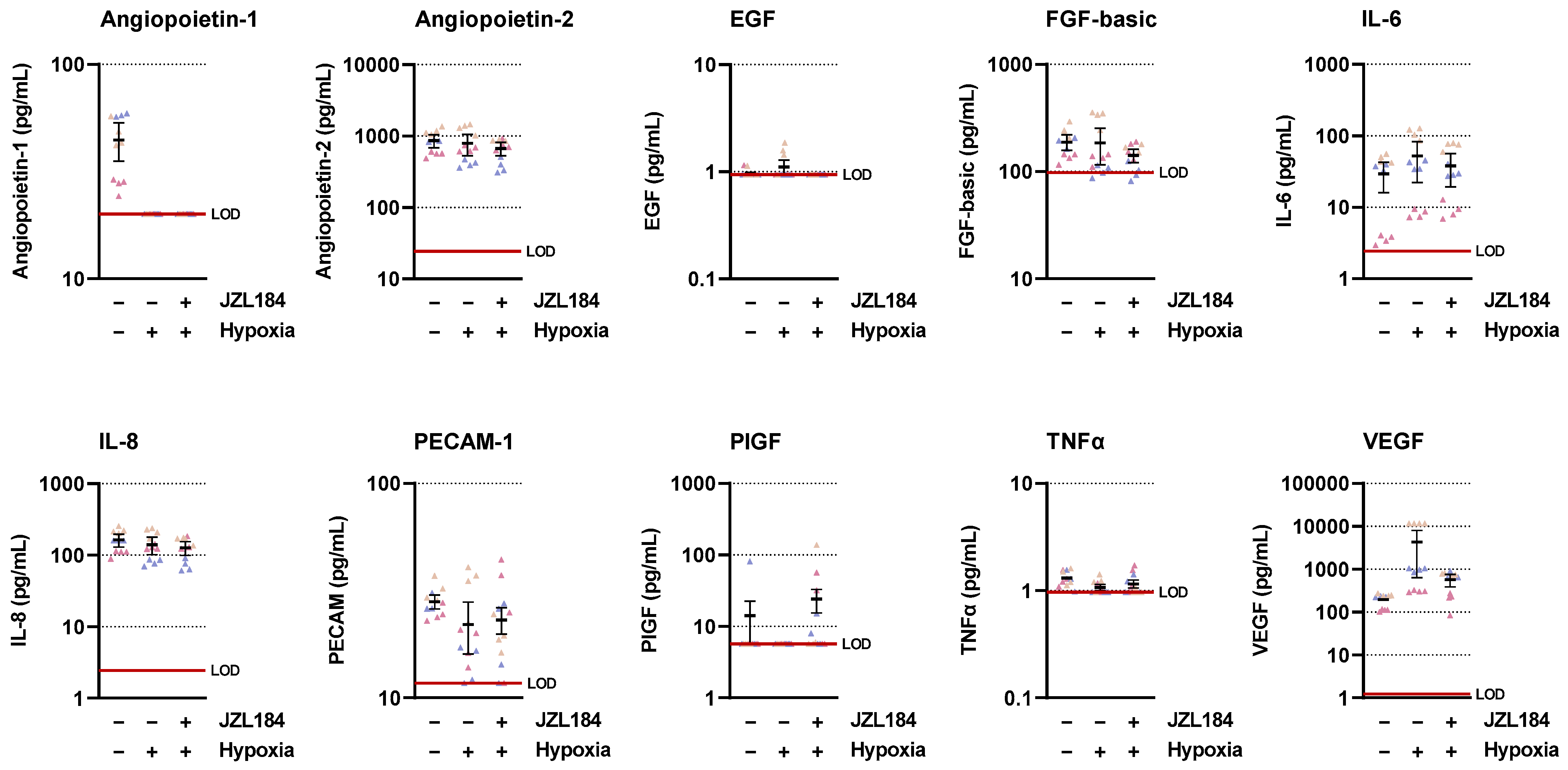
In the present work, multiplex technology was used to analyze 10 different factors involved in angiogenesis. This revealed a selective inhibition of VEGF formation by JZL184, which was confirmed by ELISA and also detected at the mRNA level by RT-qPCR. As shown by our data, inhibition of VEGF expression seems to be an important factor for the VEGF-lowering effect of JZL184, which completely inhibited hypoxia-induced VEGF mRNA and protein formation at a concentration as low as 0.01 µM. In agreement with our results, reduced VEGF expression was also demonstrated after in vivo administration of a MAGL inhibitor in mouse colon carcinoma xenografts [
12]. Moreover, activation of CB
1 [
29] and CB
2 receptors [
30] has been shown to reduce the expression of VEGF in tumors, accompanied by an antiangiogenic effect of the corresponding agonists.
In our hands, VEGF inhibition was not due to cytotoxic effects of JZL184, which even under hypoxic conditions did not induce a decrease in viability of the lung cancer cells used. Consistent with this, JZL184 at a concentration range of 0.01 to 10 µM also did not significantly reduce viability or proliferation in a study we performed on normoxic A549 cells under serum-containing and serum-free conditions [
4]. Thereby, JZL184 likewise elicited no significant change in colony-forming properties of A549 cells [
4]. The effect of MAGL inhibitors on tumor cell viability was recently reviewed [
1] and appears to depend on the particular experimental conditions, the cell line used, the duration of the effect, and the concentration used.
According to a number of reports, VEGF and its membrane receptor VEGFR2 play an important role in regulating physiological and pathophysiological angiogenesis (for review, see [
11]). Bevacizumab, a monoclonal antibody that binds soluble VEGF, thereby preventing its binding to receptors, was therefore developed as the first pharmacotherapy to combat tumor angiogenesis. A small molecule strategy subsequently launched with sunitinib and sorafinib directly targets VEGFR2 activation by tyrosine kinase inhibition (for review, see [
33]). Remarkably, in the HUVECs we also used, MIL60, a VEGF-neutralizing antibody, inhibited VEGF-triggered proliferation, migration, and tube formation via the VEGFR2 pathway [
26]. In our control experiments, VEGFR2 activation induced by CM from vehicle-treated hypoxic lung cancer cells was inhibited by a neutralizing antibody against VEGF as well as when CM from hypoxic lung cancer cells treated with VEGF-lowering JZL184 were used. Surprisingly, the VEGF-neutralizing antibody did not completely inhibit the phosphorylation of VEGFR2 in HUVECs, which may be due to the fact that other factors, such as IL-8, which was also detectable in the CM, can lead to transactivation of VEGFR2 [
34] but are not inhibited by the neutralizing antibody.
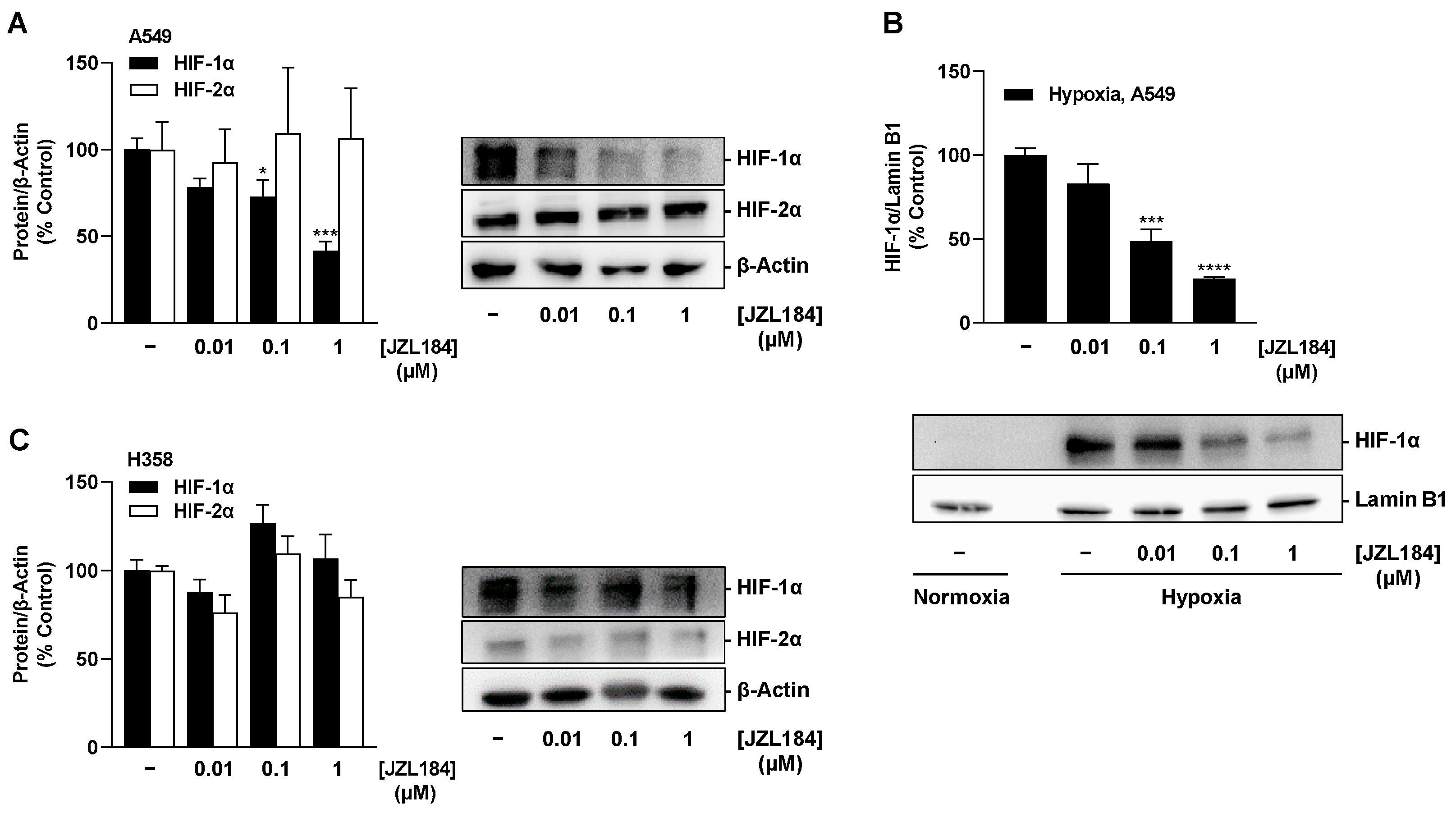
As expected, hypoxic treatment of A549 and H358 cells resulted in a time-dependent increase in HIF-1α and HIF-2α protein levels. These results confirm the known stabilization of both HIF-α subunits under hypoxia, which prevents the modification by HIF-specific prolyl hydroxylases and subsequent ubiquitination and proteasomal degradation that otherwise occurs under normoxic conditions (for review, see [
35]). After accumulation in the cytoplasm, HIF-α enters the nucleus and dimerizes with HIF-β to form the HIF complex, which acts as a transcription factor for hundreds of genes, including those involved in angiogenesis such as VEGF, platelet-derived growth factor (PDGF) and angiopoietin-1 (for review, see [
36]). The efficient accumulation of HIF-1 in the nucleus required for potential transcriptional modulation was convincingly demonstrated in this work with the hypoxia-induced HIF-1α increase in nuclear fractions of A549 cells as well as with the concentration-dependent inhibition by JZL184 also detected there. Downregulation of HIF-1α under hypoxic conditions has also been previously described in glioma cells exposed to cannabidiol [
37] and in colon cancer xenografts from mice treated with the hexahydrocannabinol analog LYR-8 [
38].
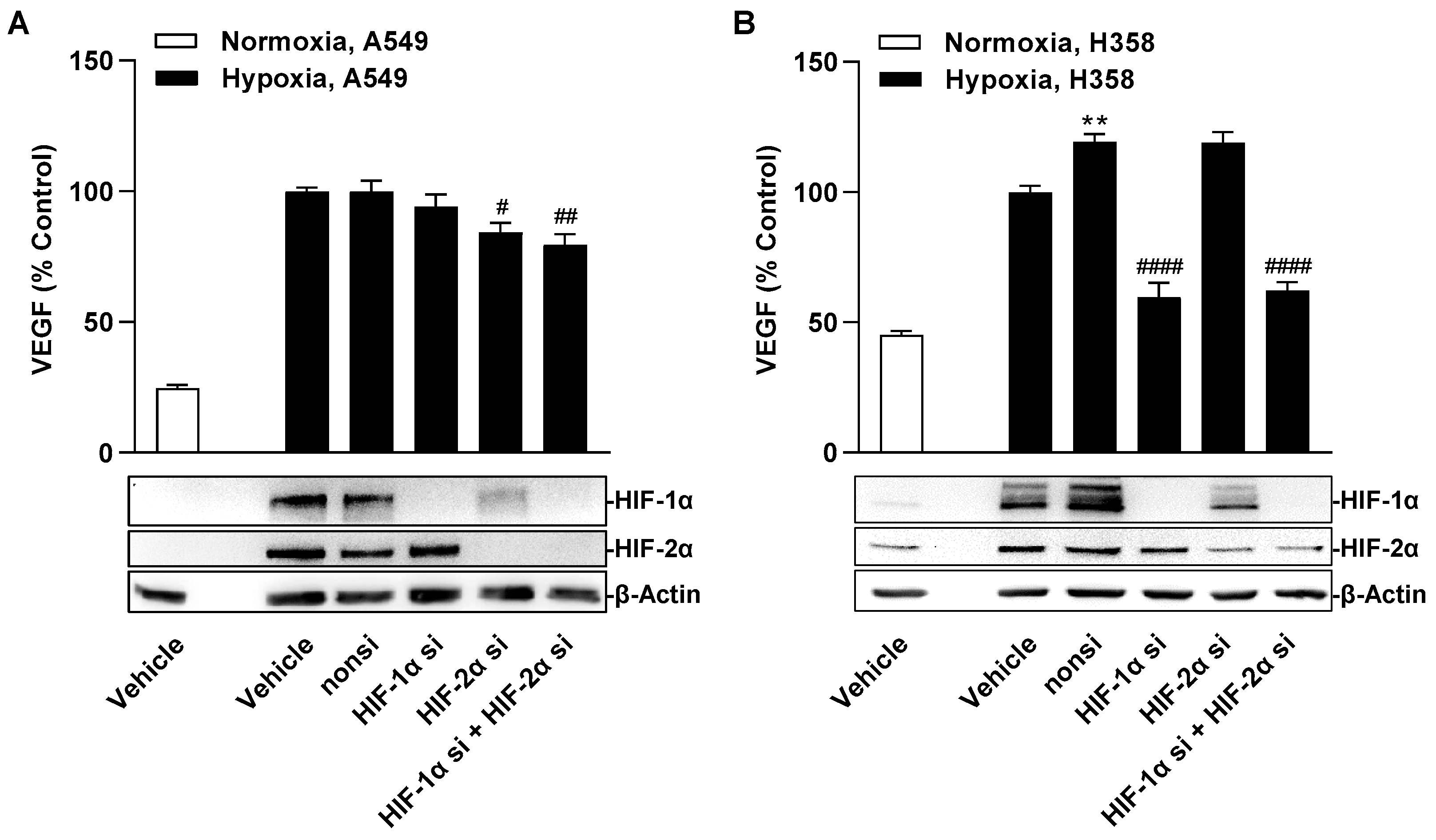
Regulation of VEGF under hypoxic conditions has been described for HIF-1α [
39,
40,
41] and HIF-2α [
42,
43,
44]. However, the siRNA experiments we performed do not indicate a major role for both HIF-1α and HIF-2α in hypoxia-induced VEGF secretion in A549 cells. On the other hand, HIF-1α knockdown was associated with a strong suppression of VEGF release from hypoxic H358 cells. These divergent cell type-dependent findings are consistent with the literature, which repeatedly reported HIF-1-independent VEGF inductions under hypoxia conditions in addition to HIF-1-dependent ones (for review, see [
45]). For example, in hypoxic colon cancer cells, activation of PI3K/Rho/ROCK and c-Myc have been revealed to be alternative pathways of appropriate VEGF induction [
46]. In another work, the flavonoids fisetin, luteolin, galangin, and quercetin were shown to inhibit hypoxia-induced VEGF formation in the lung cancer squamous cell carcinoma cell line NCI-H157, but concomitantly caused induction (rather than inhibition) of HIF-1α expression [
47].
Independent of the finding of HIF-1α-independent VEGF regulation in hypoxic A549 cells, the concentration-dependent decrease in HIF-1α protein in A549 whole cell lysates as well as in nuclear fractions triggered by JZL184 opens new perspectives and research approaches for this substance. Indeed, increased protein levels of HIF-1α and HIF-2α are associated with poor prognosis and metastasis in a number of cancers, including lung cancer (for review, see [
48]). In particular, HIF-1α activates the transcription of genes involved in virtually all aspects of tumor biology, inducing, for example, invasion and metastasis as well as resistance to radiation and chemotherapy, in addition to angiogenesis. Therefore, HIF inhibition represents a potential target that has been and is being investigated in preclinical and clinical studies (for review, see [
49,
50,
51,
52]) with mechanisms of action such as inhibition of HIF-1α protein synthesis and transcriptional activity or induction of HIF-1α hydroxylation and subsequent ubiquitination and proteasomal degradation. In light of this, the corresponding mechanism of JZL184 in terms of the HIF-1α decrease shown, as well as its functional consequence, should be addressed in future studies.
Another issue to be clarified in the present work was the possible effect of the MAGL inhibitor JZL184 via the reduction in free fatty acids. Thus, the experiments we performed with receptor antagonists suggested that only part of the antiangiogenic and VEGF-lowering effect of JZL184 proceeds via cannabinoid receptors. To address a corresponding mechanism, add-back experiments with palmitic acid have been repeatedly described in the literature. These found an involvement of fatty acid reduction by JZL184 in its antimigrative effect on prostate cancer cells [
2] as well as on MAGL-overexpressing melanoma and ovarian cancer cells [
8], but not in its anti-invasive effect on lung cancer cells [
4] or its antiangiogenic impact on the microenvironment of normoxic lung cancer cells [
13]. Consistent with the latter report, in the present study, the addition of palmitic acid also failed to restore the JZL184-induced decreased VEGF formation in hypoxic A549 and H358 cells as well as the decreased angiogenic properties of HUVECs upon contact with CM from JZL184-treated cancer cells. However, it is difficult to make a conclusive statement because many aspects of lipid metabolism are modulated under hypoxia (for review, see [
53,
54]). Thus, hypoxia-associated HIF-1 activation in various cancer cells leads to the downregulation of lipid droplet degradation mediated by adipose triglyceride lipase (ATGL), the key enzyme for intracellular lipolysis [
55]. Accordingly, a 24 h hypoxic treatment of cancer cells here resulted in a 2.5- to 3-fold lower release of free fatty acids compared to normoxic conditions [
55]. Under these circumstances, pharmacological suppression of glyceride degradation (e.g., by MAGL inhibitors) is likely to be superimposed by the hypoxia effect, making it difficult to draw a clear conclusion about the drug mechanism. In addition, the regulation of MAGL by hypoxia or HIF-1 is currently unclear [
54].
It should also be added that in the present study, we were unable to register clear concentration dependencies in the effect of JZL184 in many cases, with maximum effects already occurring at the lowest concentration of 0.01 µM used. In this context, however, it should be noted that JZL184 inhibits the MAGL enzyme highly potently with IC
50 values in the nanomolar range [
14]. Accordingly, in a previous study by our group, JZL184 already elicited a highly significant induction of 2-AG synthesis in normoxic A549 cells at a concentration as low as 0.01 µM [
4].
Collectively, the present study is the first to elucidate an antiangiogenic mechanism of MAGL inhibitors under hypoxia essential for neoangiogenesis in malignant tumors. Therefore, another necessary step was taken in the preclinical characterization of the anticancer activity of MAGL inhibitors.