Cognitive Alterations in Old Mice Are Associated with Intestinal Barrier Dysfunction and Induced Toll-like Receptor 2 and 4 Signaling in Different Brain Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Working Memory in a Spatial Novelty Preference Y-Maze Paradigm
2.3. Everted Gut Sac and Xylose Permeation Measurement
2.4. Histological Evaluation of Brain and Intestinal Sections
2.5. Measurement of Ligands of TLR2 and TLR4
2.6. RNA Isolation and Real-Time qPCR
2.7. ELISA
2.8. Western Blot
2.9. Immunohistochemical Staining
2.10. Microbiota Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of Aging on Markers of Cognitive Function and Senescence
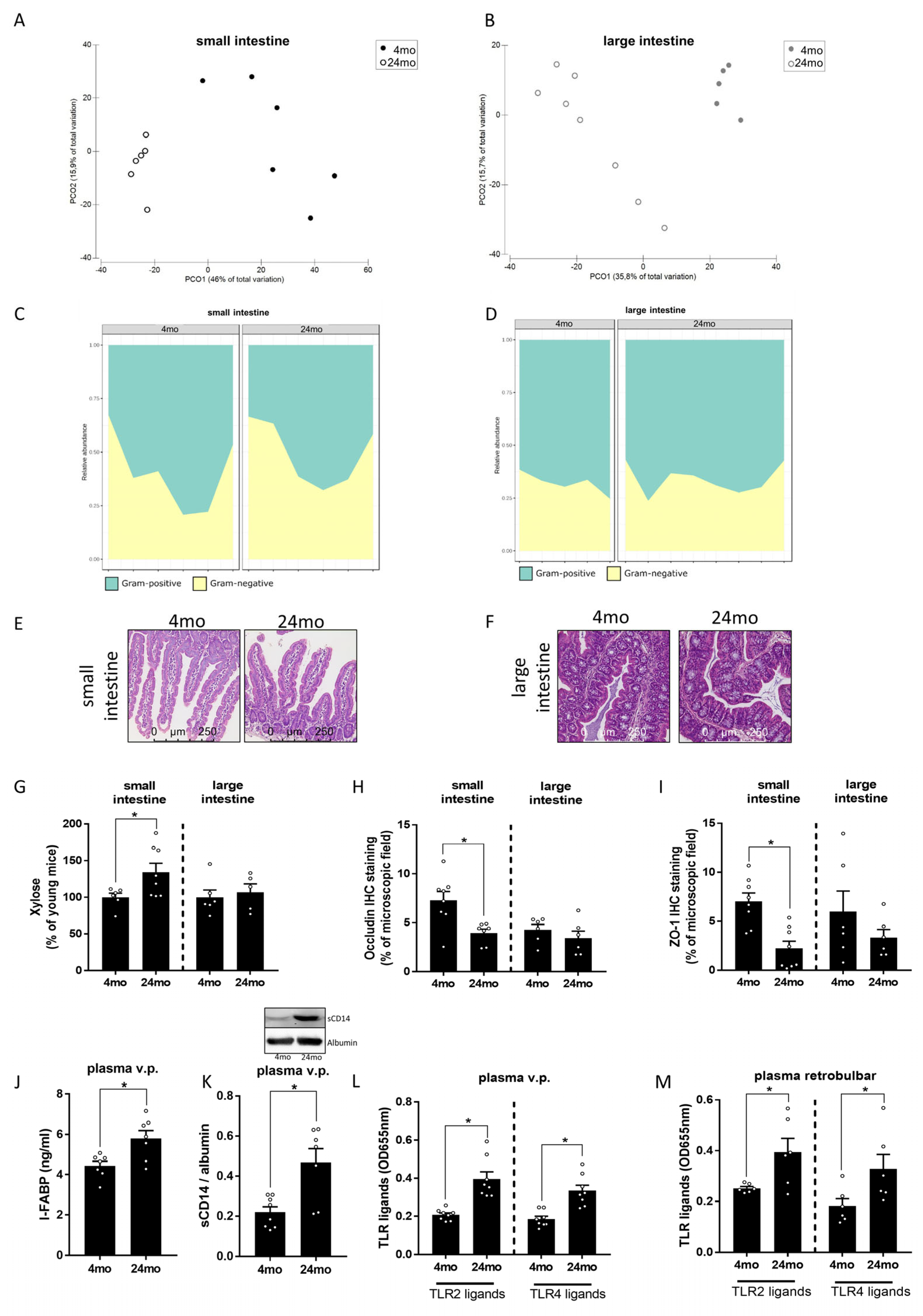
3.2. Effect of Aging on Intestinal Microbiota Composition and Markers of Intestinal Barrier Function
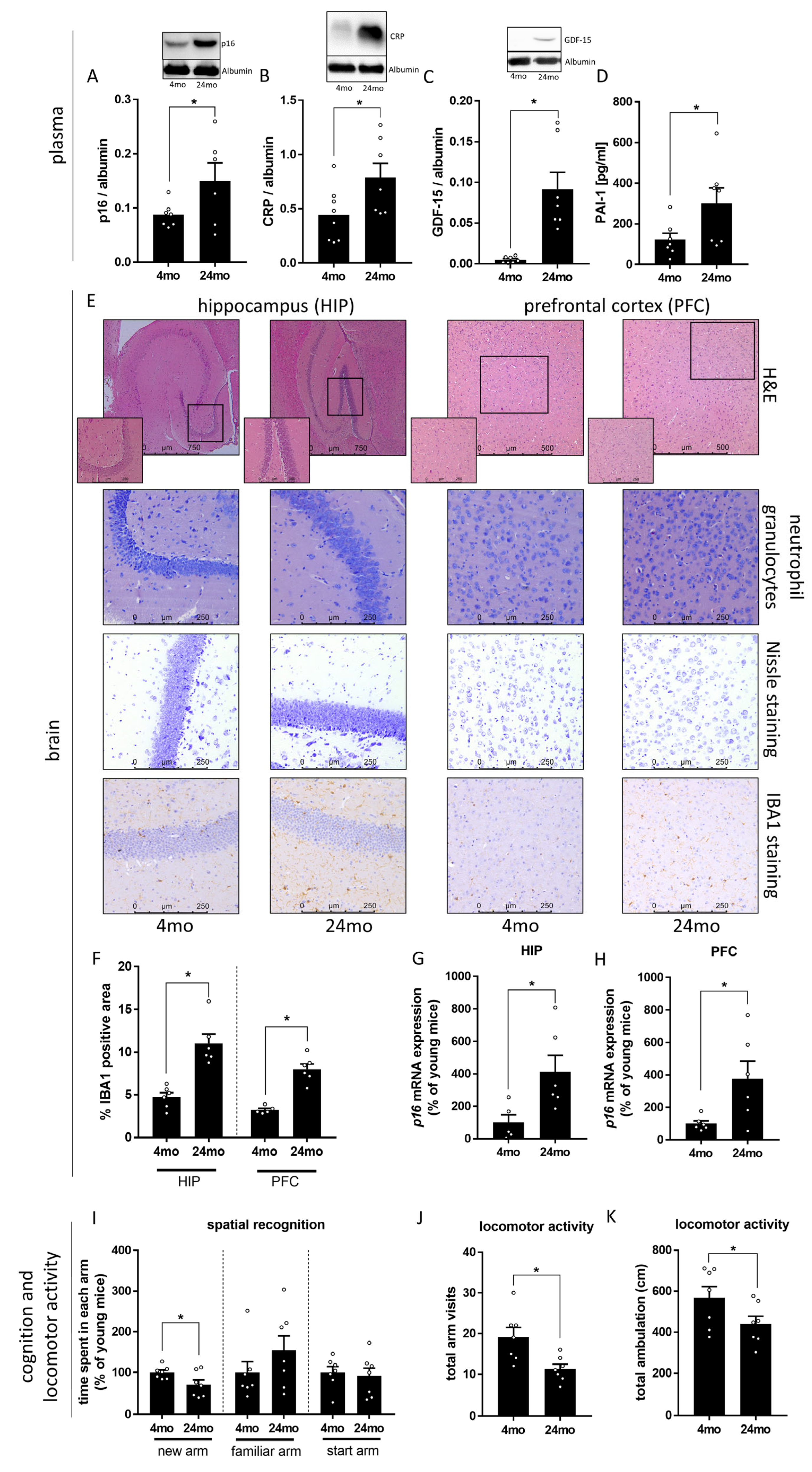
3.3. Effect of Aging on TLR2 and TLR4 Ligand Concentration as Well as on Markers of TLR2 and TLR4 Signaling in Prefrontal Cortex and Hippocampus
4. Discussion
4.1. Healthy Aging Related Cognitive Alterations Parallel Alterations of Intestinal Microbiota Composition and Barrier Function
4.2. Healthy Aging Associated Cognitive Alterations Parallels with Alterations of TLR2 and TLR4 Signaling in Brain
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.D. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1160–1203. [Google Scholar] [CrossRef]
- Eurostat. Healthy Life Years at Birth by Sex. Available online: https://ec.europa.eu/eurostat/databrowser/view/tps00150/default/table?lang=en (accessed on 16 December 2022).
- Eurostat. Life Expectancy at Birth by Sex. Available online: https://ec.europa.eu/eurostat/databrowser/view/tps00205/default/table?lang=en (accessed on 16 December 2022).
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Bilson, J.; Sethi, J.K.; Byrne, C.D. Non-alcoholic fatty liver disease: A multi-system disease influenced by ageing and sex, and affected by adipose tissue and intestinal function. Proc. Nutr. Soc. 2021, 81, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Meijer, E.; Casanova, M.; Kim, H.; Llena-Nozal, A.; Lee, J. Economic costs of dementia in 11 countries in Europe: Estimates from nationally representative cohorts of a panel study. Lancet Reg. Health-Eur. 2022, 20, 100445. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Kaiyrlykyzy, A.; Kozhakhmetov, S.; Babenko, D.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Kushugulova, A.R.; Askarova, S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022, 12, 15115. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Saji, N.; Saito, Y.; Yamashita, T.; Murotani, K.; Tsuduki, T.; Hisada, T.; Sugimoto, T.; Niida, S.; Toba, K.; Sakurai, T. Relationship Between Plasma Lipopolysaccharides, Gut Microbiota, and Dementia: A Cross-Sectional Study. J. Alzheimer’s Dis. 2022, 86, 1947–1957. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Contreras-Rodríguez, O.; Blasco, G.; Coll, C.; Biarnés, C.; Miranda-Olivos, R.; Latorre, J.; Moreno-Navarrete, J.M.; et al. Obesity Impairs Short-Term and Working Memory through Gut Microbial Metabolism of Aromatic Amino Acids. Cell Metab. 2020, 32, 548–560.e547. [Google Scholar] [CrossRef] [PubMed]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Contreras-Rodríguez, O.; Burokas, A.; Ortega-Sanchez, J.-A.; Blasco, G.; Coll, C.; Biarnés, C.; Castells-Nobau, A.; Puig, J.; et al. Obesity-associated deficits in inhibitory control are phenocopied to mice through gut microbiota changes in one-carbon and aromatic amino acids metabolic pathways. Front. Neurosci. 2021, 70, 2283. [Google Scholar] [CrossRef]
- Ganz, T.; Fainstein, N.; Elad, A.; Lachish, M.; Goldfarb, S.; Einstein, O.; Ben-Hur, T. Microbial pathogens induce neurodegeneration in Alzheimer’s disease mice: Protection by microglial regulation. J. Neuroinflammation 2022, 19, 5. [Google Scholar] [CrossRef]
- Ganz, T.; Fainstein, N.; Ben-Hur, T. When the infectious environment meets the AD brain. Mol. Neurodegener. 2022, 17, 53. [Google Scholar] [CrossRef]
- Lax, N.; Fainstein, N.; Nishri, Y.; Ben-Zvi, A.; Ben-Hur, T. Systemic microbial TLR2 agonists induce neurodegeneration in Alzheimer’s disease mice. J. Neuroinflammation 2020, 17, 55. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Xu, C.; Zhang, H.; Lin, C. TLR4 Targeting as a Promising Therapeutic Strategy for Alzheimer Disease Treatment. Front. Neurosci. 2020, 14, 602508. [Google Scholar] [CrossRef] [PubMed]
- Pourbadie, H.G.; Sayyah, M.; Khoshkholgh-Sima, B.; Choopani, S.; Nategh, M.; Motamedi, F.; Shokrgozar, M.A. Early minor stimulation of microglial TLR2 and TLR4 receptors attenuates Alzheimer’s disease-related cognitive deficit in rats: Behavioral, molecular, and electrophysiological evidence. Neurobiol. Aging 2018, 70, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ali, T.; Kim, M.O. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway. Sci. Rep. 2016, 6, 24493. [Google Scholar] [CrossRef]
- Fei, X.; Dou, Y.-N.; Lv, W.; Ding, B.; Wei, J.; Wu, X.; He, X.; Fei, Z.; Fei, F. TLR4 Deletion Improves Cognitive Brain Function and Structure in Aged Mice. Neuroscience 2022, 492, 1–17. [Google Scholar] [CrossRef]
- Okun, E.; Barak, B.; Saada-Madar, R.; Rothman, S.M.; Griffioen, K.J.; Roberts, N.; Castro, K.; Mughal, M.R.; Pita, M.A.; Stranahan, A.M.; et al. Evidence for a Developmental Role for TLR4 in Learning and Memory. PLoS ONE 2012, 7, e47522. [Google Scholar] [CrossRef]
- Islam, R.; Vrionis, F.; Hanafy, K.A. Microglial TLR4 is Critical for Neuronal Injury and Cognitive Dysfunction in Subarachnoid Hemorrhage. Neurocritical Care 2022, 37, 761–769. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Li, D.; Zhu, W.; Duan, K.; Le, Y.; Liao, Y.; Ou, Y. The role of the TLR4 signaling pathway in cognitive deficits following surgery in aged rats. Mol. Med. Rep. 2013, 7, 1137–1142. [Google Scholar] [CrossRef]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Richetto, J.; Labouesse, M.A.; Poe, M.M.; Cook, J.M.; Grace, A.A.; Riva, M.A.; Meyer, U. Behavioral effects of the benzodiazepine-positive allosteric modulator SH-053-2’F-S-CH₃ in an immune-mediated neurodevelopmental disruption model. Int. J. Neuropsychopharmacol. 2015, 18, pyu055. [Google Scholar] [CrossRef]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Butt, A.G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 2013, 37, 415–426. [Google Scholar] [CrossRef]
- Eberts, T.J.; Sample, R.H.; Glick, M.R.; Ellis, G.H. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin. Chem. 1979, 25, 1440–1443. [Google Scholar] [CrossRef]
- Rajcic, D.; Baumann, A.; Hernández-Arriaga, A.; Brandt, A.; Nier, A.; Jin, C.J.; Sánchez, V.; Jung, F.; Camarinha-Silva, A.; Bergheim, I. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biol. 2021, 41, 101879. [Google Scholar] [CrossRef]
- Jung, F.; Burger, K.; Staltner, R.; Brandt, A.; Mueller, S.; Bergheim, I. Markers of Intestinal Permeability Are Rapidly Improved by Alcohol Withdrawal in Patients with Alcohol-Related Liver Disease. Nutrients 2021, 13, 1659. [Google Scholar] [CrossRef]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef]
- Wagnerberger, S.; Spruss, A.; Kanuri, G.; Volynets, V.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Toll-like receptors 1–9 are elevated in livers with fructose-induced hepatic steatosis. Br. J. Nutr. 2012, 107, 1727–1738. [Google Scholar] [CrossRef]
- Spruss, A.; Kanuri, G.; Wagnerberger, S.; Haub, S.; Bischoff, S.C.; Bergheim, I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009, 50, 1094–1104. [Google Scholar] [CrossRef]
- Baumann, A.; Hernández-Arriaga, A.; Brandt, A.; Sánchez, V.; Nier, A.; Jung, F.; Kehm, R.; Höhn, A.; Grune, T.; Frahm, C.; et al. Microbiota profiling in aging-associated inflammation and liver degeneration. Int. J. Med. Microbiol. 2021, 311, 151500. [Google Scholar] [CrossRef]
- Spruss, A.; Kanuri, G.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Metformin protects against the development of fructose-induced steatosis in mice: Role of the intestinal barrier function. Lab. Investig. 2012, 92, 1020–1032. [Google Scholar] [CrossRef]
- Hernández-Arriaga, A.; Baumann, A.; Witte, O.W.; Frahm, C.; Bergheim, I.; Camarinha-Silva, A. Changes in Oral Microbial Ecology of C57BL/6 Mice at Different Ages Associated with Sampling Methodology. Microorganisms 2019, 7, 283. [Google Scholar] [CrossRef]
- Baumann, A.; Nier, A.; Hernández-Arriaga, A.; Brandt, A.; Lorenzo Pisarello, M.J.; Jin, C.J.; Pilar, E.; Camarinha-Silva, A.; Schattenberg, J.M.; Bergheim, I. Toll-like receptor 1 as a possible target in non-alcoholic fatty liver disease. Sci. Rep. 2021, 11, 17815. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statisticl Methods; Primer-E Limited: Albany, New Zealand, 2008. [Google Scholar]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- di Candia, A.M.; de Avila, D.X.; Moreira, G.R.; Villacorta, H.; Maisel, A.S. Growth differentiation factor-15, a novel systemic biomarker of oxidative stress, inflammation, and cellular aging: Potential role in cardiovascular diseases. Am. Heart J. Plus 2021, 9, 100046. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef]
- Brandt, A.; Baumann, A.; Hernández-Arriaga, A.; Jung, F.; Nier, A.; Staltner, R.; Rajcic, D.; Schmeer, C.; Witte, O.W.; Wessner, B.; et al. Impairments of intestinal arginine and NO metabolisms trigger aging-associated intestinal barrier dysfunction and ‘inflammaging’. Redox Biol. 2022, 58, 102528. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef]
- Wu, M.L.; Yang, X.Q.; Xue, L.; Duan, W.; Du, J.R. Age-related cognitive decline is associated with microbiota-gut-brain axis disorders and neuroinflammation in mice. Behav. Brain Res. 2021, 402, 113125. [Google Scholar] [CrossRef]
- Adriaanse, M.P.; Tack, G.J.; Passos, V.L.; Damoiseaux, J.G.; Schreurs, M.W.; van Wijck, K.; Riedl, R.G.; Masclee, A.A.; Buurman, W.A.; Mulder, C.J.; et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment. Pharmacol. Ther. 2013, 37, 482–490. [Google Scholar] [CrossRef]
- Grimaldi, D.; Guivarch, E.; Neveux, N.; Fichet, J.; Pène, F.; Marx, J.S.; Chiche, J.D.; Cynober, L.; Mira, J.P.; Cariou, A. Markers of intestinal injury are associated with endotoxemia in successfully resuscitated patients. Resuscitation 2013, 84, 60–65. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Pilly, P.K.; Grossberg, S. How do spatial learning and memory occur in the brain? Coordinated learning of entorhinal grid cells and hippocampal place cells. J. Cogn. Neurosci. 2012, 24, 1031–1054. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.R.; Constantinidis, C. Role of Prefrontal Persistent Activity in Working Memory. Front. Syst. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Blazer, D.G.; Yaffe, K.; Karlawish, J. Cognitive aging: A report from the Institute of Medicine. JAMA 2015, 313, 2121–2122. [Google Scholar] [CrossRef]
- Boyle, P.A.; Wilson, R.S.; Aggarwal, N.T.; Tang, Y.; Bennett, D.A. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology 2006, 67, 441–445. [Google Scholar] [CrossRef]
- Campbell, N.L.; Unverzagt, F.; LaMantia, M.A.; Khan, B.A.; Boustani, M.A. Risk factors for the progression of mild cognitive impairment to dementia. Clin. Geriatr. Med. 2013, 29, 873–893. [Google Scholar] [CrossRef]
- Hallam, B.; Petersen, I.; Cooper, C.; Avgerinou, C.; Walters, K. Time Trends in Incidence of Reported Memory Concerns and Cognitive Decline: A Cohort Study in UK Primary Care. Clin. Epidemiol. 2022, 14, 395–408. [Google Scholar] [CrossRef]
- Li, M.; Su, S.; Cai, W.; Cao, J.; Miao, X.; Zang, W.; Gao, S.; Xu, Y.; Yang, J.; Tao, Y.X.; et al. Differentially Expressed Genes in the Brain of Aging Mice with Cognitive Alteration and Depression- and Anxiety-Like Behaviors. Front. Cell Dev. Biol. 2020, 8, 814. [Google Scholar] [CrossRef]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain 2016, 9, 11. [Google Scholar] [CrossRef]
- Shoji, H.; Miyakawa, T. Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacol. Rep. 2019, 39, 100–118. [Google Scholar] [CrossRef]
- Yanai, S.; Endo, S. Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Front. Aging Neurosci. 2021, 13, 697621. [Google Scholar] [CrossRef]
- Abdouh, M.; Chatoo, W.; El Hajjar, J.; David, J.; Ferreira, J.; Bernier, G. Bmi1 is down-regulated in the aging brain and displays antioxidant and protective activities in neurons. PLoS ONE 2012, 7, e31870. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Branen, L.; Sivasubramanian, M.K.; Monteiro, R.; Subramanian, M. Aging is associated with glial senescence in the brainstem—Implications for age-related sympathetic overactivity. Aging 2021, 13, 13460–13473. [Google Scholar] [CrossRef]
- Hamieh, A.M.; Camperos, E.; Hernier, A.M.; Castagné, V. C57BL/6 mice as a preclinical model to study age-related cognitive deficits: Executive functions impairment and inter-individual differences. Brain Res. 2021, 1751, 147173. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.T.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut microbiota, cognitive frailty and dementia in older individuals: A systematic review. Clin. Interv. Aging 2018, 13, 1497–1511. [Google Scholar] [CrossRef]
- Lee, J.; Venna, V.R.; Durgan, D.J.; Shi, H.; Hudobenko, J.; Putluri, N.; Petrosino, J.; McCullough, L.D.; Bryan, R.M. Young versus aged microbiota transplants to germ-free mice: Increased short-chain fatty acids and improved cognitive performance. Gut Microbes 2020, 12, 1814107. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 2021, 1, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Sorbara, M.T.; Kim, S.G.; Littmann, E.L.; Dong, Q.; Walsh, G.; Wright, R.; Amoretti, L.; Fontana, E.; Hohl, T.M.; et al. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe 2021, 29, 378–393.e375. [Google Scholar] [CrossRef] [PubMed]
- Kühn, F.; Adiliaghdam, F.; Cavallaro, P.M.; Hamarneh, S.R.; Tsurumi, A.; Hoda, R.S.; Munoz, A.R.; Dhole, Y.; Ramirez, J.M.; Liu, E.; et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 2020, 5, e134049. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Greenwood-Van Meerveld, B. Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 1045–1056. [Google Scholar] [CrossRef]
- Lin, T.; Liu, G.A.; Perez, E.; Rainer, R.D.; Febo, M.; Cruz-Almeida, Y.; Ebner, N.C. Systemic Inflammation Mediates Age-Related Cognitive Deficits. Front. Aging Neurosci. 2018, 10, 236. [Google Scholar] [CrossRef]
- Rundek, T.; Roy, S.; Hornig, M.; Cheung, Y.K.; Gardener, H.; DeRosa, J.; Levin, B.; Wright, C.B.; Del Brutto, V.J.; Elkind, M.S.; et al. Gut permeability and cognitive decline: A pilot investigation in the Northern Manhattan Study. Brain Behav. Immun.-Health 2021, 12, 100214. [Google Scholar] [CrossRef]
- Zhao, Y.; Cong, L.; Jaber, V.; Lukiw, W.J. Microbiome-Derived Lipopolysaccharide Enriched in the Perinuclear Region of Alzheimer’s Disease Brain. Front. Immunol. 2017, 8, 1064. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Miron, J.; Picard, C.; Frappier, J.; Dea, D.; Théroux, L.; Poirier, J. TLR4 Gene Expression and Pro-Inflammatory Cytokines in Alzheimer’s Disease and in Response to Hippocampal Deafferentation in Rodents. J. Alzheimer’s Dis. 2018, 63, 1547–1556. [Google Scholar] [CrossRef]
- Gurung, P.; Fan, G.; Lukens, J.R.; Vogel, P.; Tonks, N.K.; Kanneganti, T.D. Tyrosine Kinase SYK Licenses MyD88 Adaptor Protein to Instigate IL-1α-Mediated Inflammatory Disease. Immunity 2017, 46, 635–648. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Letiembre, M.; Liu, Y.; Walter, S.; Hao, W.; Pfander, T.; Wrede, A.; Schulz-Schaeffer, W.; Fassbender, K. Screening of innate immune receptors in neurodegenerative diseases: A similar pattern. Neurobiol. Aging 2009, 30, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, I.E.; Lewen, A.; Galow, L.V.; Cesetti, T.; Scheffel, J.; Regen, T.; Hanisch, U.K.; Kann, O. TLR4-activated microglia require IFN-γ to induce severe neuronal dysfunction and death in situ. Proc. Natl. Acad. Sci. USA 2016, 113, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Chausse, B.; Dikmen, H.O.; Almouhanna, F.; Hollnagel, J.O.; Lewen, A.; Kann, O. TLR2- and TLR3-activated microglia induce different levels of neuronal network dysfunction in a context-dependent manner. Brain Behav. Immun. 2021, 96, 80–91. [Google Scholar] [CrossRef]
- Potter, O.V.; Giedraitis, M.E.; Johnson, C.D.; Cox, M.N.; Kohman, R.A. Young and aged TLR4 deficient mice show sex-dependent enhancements in spatial memory and alterations in interleukin-1 related genes. Brain Behav. Immun. 2019, 76, 37–47. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, X.; Wang, S.; Zhou, C.; Lin, L.; Ding, X.; Han, J.; Zhou, Y.; Jin, G.; Wang, Y.; et al. Toll-like receptor-2 gene knockout results in neurobehavioral dysfunctions and multiple brain structural and functional abnormalities in mice. Brain Behav. Immun. 2021, 91, 257–266. [Google Scholar] [CrossRef]
- Senatorov, V.V., Jr.; Friedman, A.R.; Milikovsky, D.Z.; Ofer, J.; Saar-Ashkenazy, R.; Charbash, A.; Jahan, N.; Chin, G.; Mihaly, E.; Lin, J.M.; et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 2019, 11, eaaw8283. [Google Scholar] [CrossRef]
- Adelöf, J.; Ross, J.M.; Lazic, S.E.; Zetterberg, M.; Wiseman, J.; Hernebring, M. Conclusions from a behavioral aging study on male and female F2 hybrid mice on age-related behavior, buoyancy in water-based tests, and an ethical method to assess lifespan. Aging 2019, 11, 7150–7168. [Google Scholar] [CrossRef]
- Benice, T.S.; Rizk, A.; Kohama, S.; Pfankuch, T.; Raber, J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 2006, 137, 413–423. [Google Scholar] [CrossRef]
- Tran, T.; Mach, J.; Gemikonakli, G.; Wu, H.; Allore, H.; Howlett, S.E.; Little, C.B.; Hilmer, S.N. Male–Female Differences in the Effects of Age on Performance Measures Recorded for 23 Hours in Mice. J. Gerontol. Ser. A 2021, 76, 2141–2146. [Google Scholar] [CrossRef]
- Ma, J.; Hong, Y.; Zheng, N.; Xie, G.; Lyu, Y.; Gu, Y.; Xi, C.; Chen, L.; Wu, G.; Li, Y.; et al. Gut microbiota remodeling reverses aging-associated inflammation and dysregulation of systemic bile acid homeostasis in mice sex-specifically. Gut Microbes 2020, 11, 1450–1474. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.; Liu, A.T.; Rege, S.V.; Adams, J.M.; Akrapongpisak, L.; Le, D.; Alcantara-Lee, R.; Estrada, R.A.; Ray, R.; Ahadi, S.; et al. Molecular and histological correlates of cognitive decline across age in male C57BL/6J mice. Brain Behav. 2022, 12, e2736. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, X.; Ma, T. Memory Decline and Behavioral Inflexibility in Aged Mice Are Correlated With Dysregulation of Protein Synthesis Capacity. Front. Aging Neurosci. 2019, 11, 246. [Google Scholar] [CrossRef] [PubMed]

| 4 Months | 24 Months | ||
|---|---|---|---|
| small intestine | villus height (µm) | 239 ± 14 | 241 ± 9.0 |
| crypt depth (µm) | 94.4 ± 4.7 | 98.4 ± 7.3 | |
| large intestine | crypt depth (µm) | 91.9 ± 1.8 | 89.0 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, A.; Kromm, F.; Hernández-Arriaga, A.; Martínez Sánchez, I.; Bozkir, H.Ö.; Staltner, R.; Baumann, A.; Camarinha-Silva, A.; Heijtz, R.D.; Bergheim, I. Cognitive Alterations in Old Mice Are Associated with Intestinal Barrier Dysfunction and Induced Toll-like Receptor 2 and 4 Signaling in Different Brain Regions. Cells 2023, 12, 2153. https://doi.org/10.3390/cells12172153
Brandt A, Kromm F, Hernández-Arriaga A, Martínez Sánchez I, Bozkir HÖ, Staltner R, Baumann A, Camarinha-Silva A, Heijtz RD, Bergheim I. Cognitive Alterations in Old Mice Are Associated with Intestinal Barrier Dysfunction and Induced Toll-like Receptor 2 and 4 Signaling in Different Brain Regions. Cells. 2023; 12(17):2153. https://doi.org/10.3390/cells12172153
Chicago/Turabian StyleBrandt, Annette, Franziska Kromm, Angélica Hernández-Arriaga, Inés Martínez Sánchez, Haktan Övül Bozkir, Raphaela Staltner, Anja Baumann, Amélia Camarinha-Silva, Rochellys Diaz Heijtz, and Ina Bergheim. 2023. "Cognitive Alterations in Old Mice Are Associated with Intestinal Barrier Dysfunction and Induced Toll-like Receptor 2 and 4 Signaling in Different Brain Regions" Cells 12, no. 17: 2153. https://doi.org/10.3390/cells12172153
APA StyleBrandt, A., Kromm, F., Hernández-Arriaga, A., Martínez Sánchez, I., Bozkir, H. Ö., Staltner, R., Baumann, A., Camarinha-Silva, A., Heijtz, R. D., & Bergheim, I. (2023). Cognitive Alterations in Old Mice Are Associated with Intestinal Barrier Dysfunction and Induced Toll-like Receptor 2 and 4 Signaling in Different Brain Regions. Cells, 12(17), 2153. https://doi.org/10.3390/cells12172153







