PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-Dependent Regulation of Small GTPase RhoA in Osteoclasts
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Cell Culture
2.2. Pull-Down Assay and Western Blotting
2.3. Measurement of PP2A Activity
2.4. Statistical Analysis
3. Results
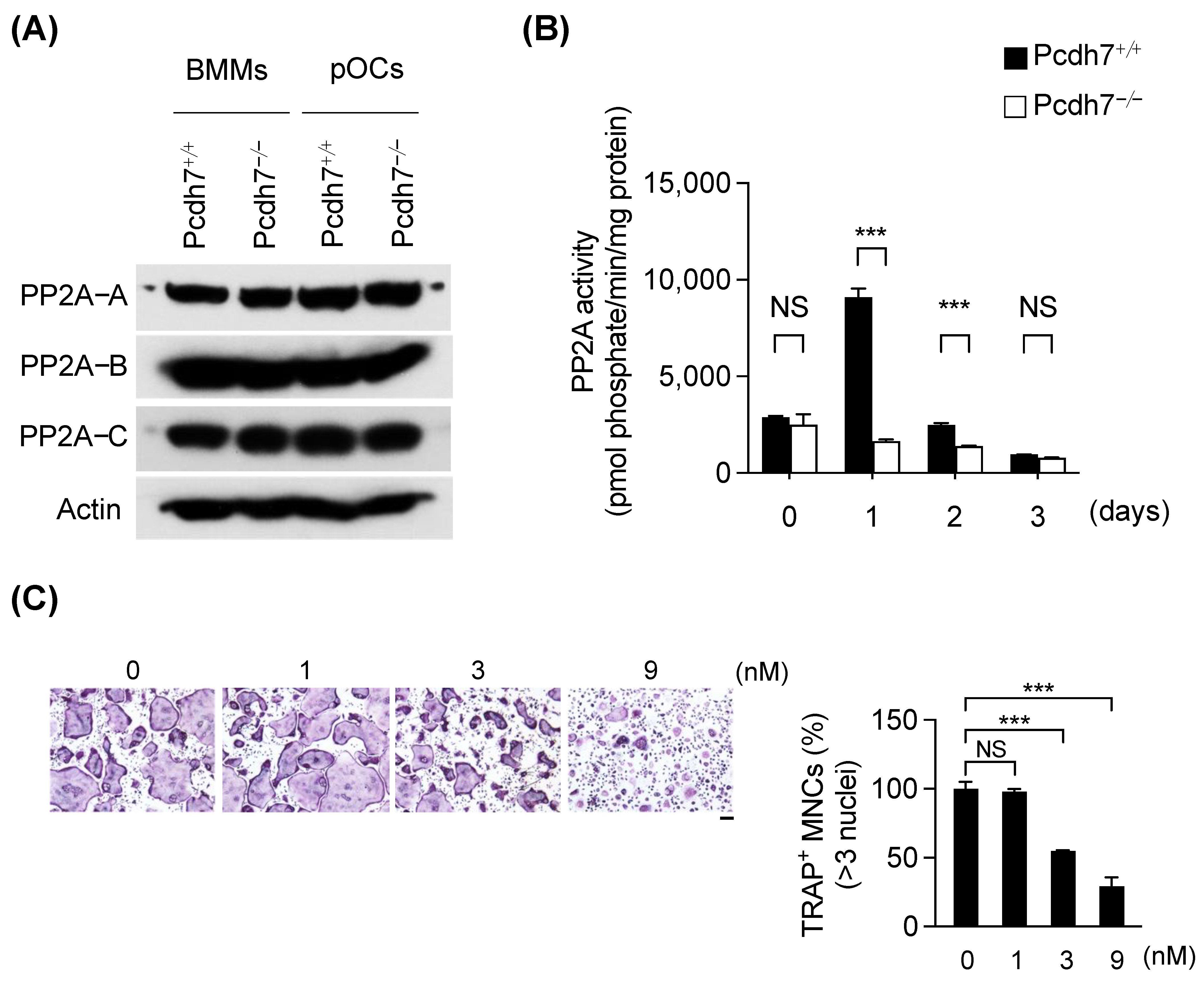
3.1. Pcdh7 Deficiency Results in Impaired PP2A Activity in Osteoclasts
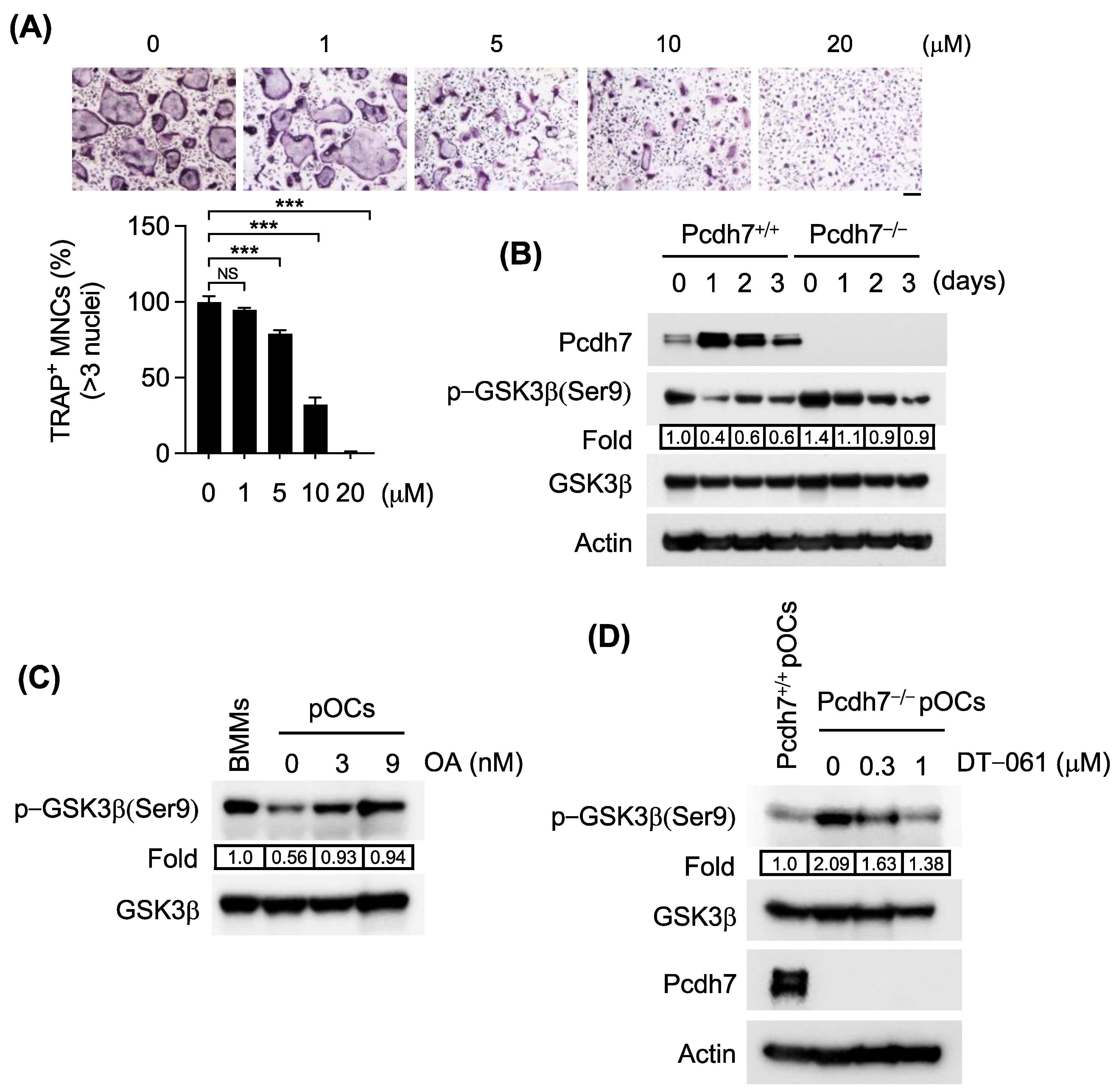
3.2. Pcdh7 Deficiency Results in Impaired PP2A−Mediated GSK3β Activation in Osteoclasts
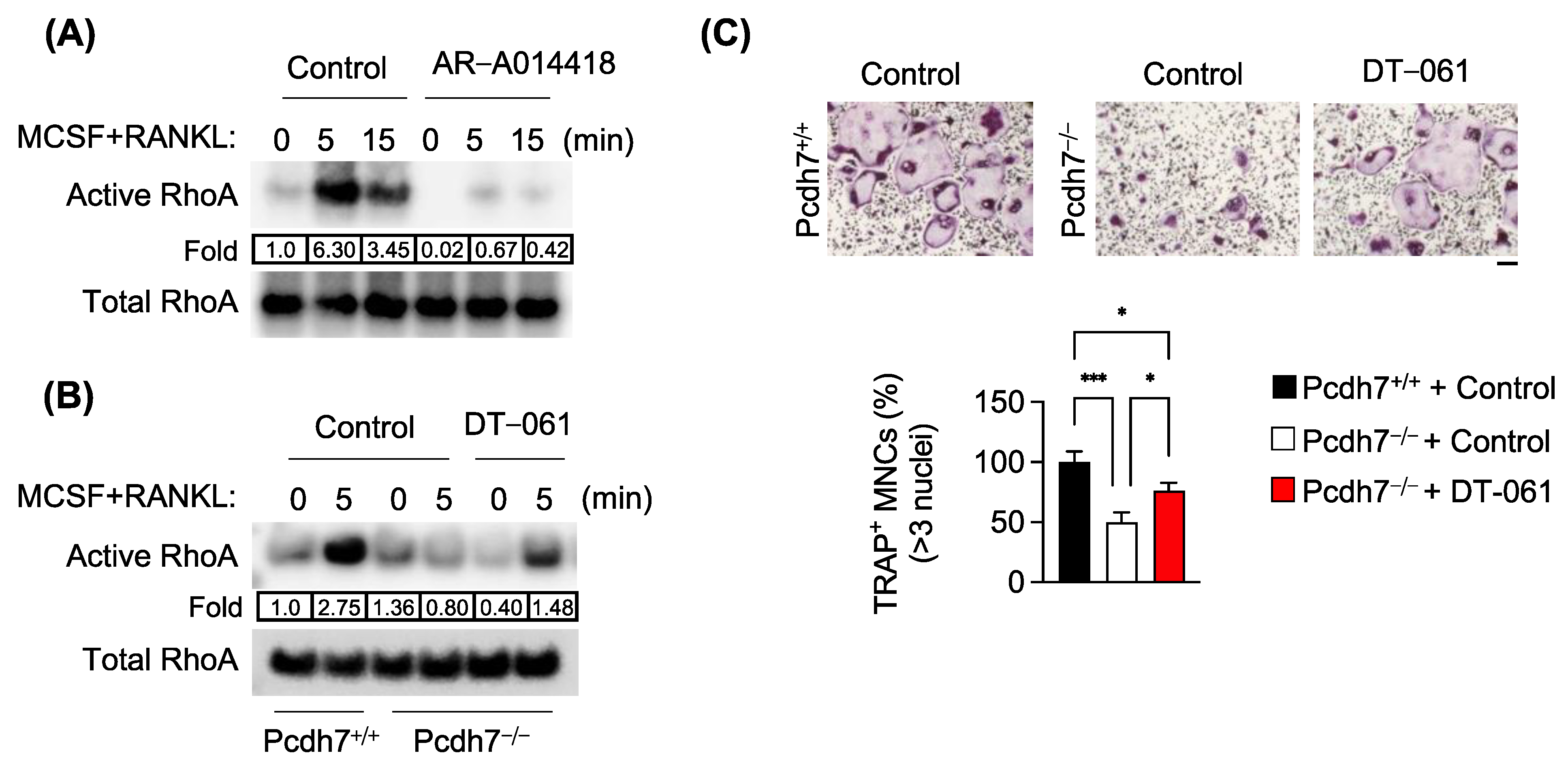
3.3. Pcdh7-Dependent RhoA Activation Requires PP2A and GSK3β
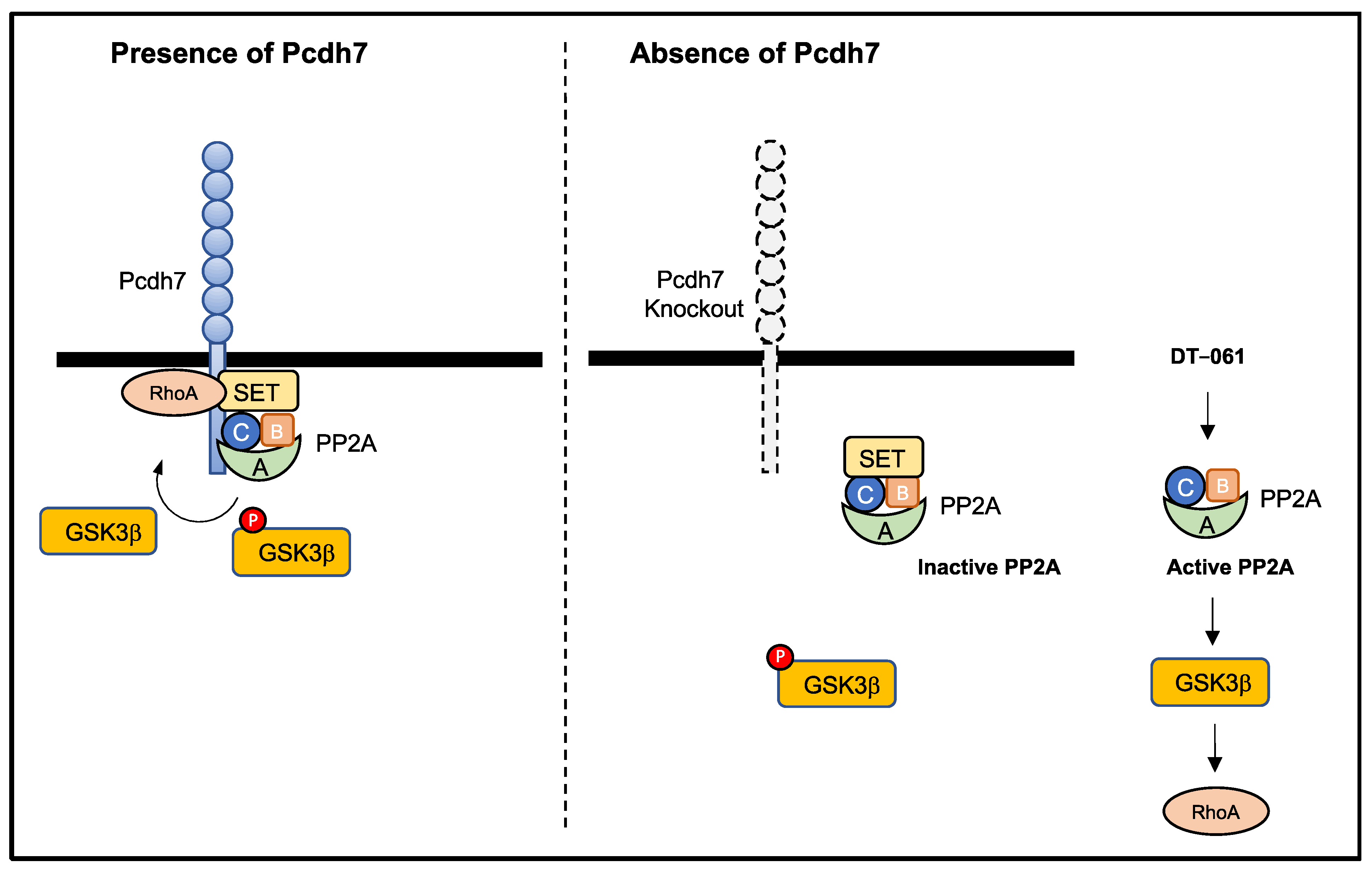
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Thostenson, J.D.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone 2014, 66, 146–154. [Google Scholar] [CrossRef]
- Takegahara, N.; Kim, H.; Choi, Y. RANKL biology. Bone 2022, 159, 116353. [Google Scholar] [CrossRef]
- Humphrey, M.B.; Nakamura, M.C. A Comprehensive Review of Immunoreceptor Regulation of Osteoclasts. Clin. Rev. Allergy Immunol. 2016, 51, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef]
- Volkmer, H.; Schreiber, J.; Rathjen, F.G. Regulation of adhesion by flexible ectodomains of IgCAMs. Neurochem. Res. 2013, 38, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Takegahara, N.; Choi, Y. IgSF11-mediated phosphorylation of pyruvate kinase M2 regulates osteoclast differentiation and prevents pathological bone loss. Bone Res. 2023, 11, 17. [Google Scholar] [CrossRef]
- Hayashi, S.; Takeichi, M. Emerging roles of protocadherins: From self-avoidance to enhancement of motility. J. Cell Sci. 2015, 128, 1455–1464. [Google Scholar] [PubMed]
- Kim, S.Y.; Yasuda, S.; Tanaka, H.; Yamagata, K.; Kim, H. Non-clustered protocadherin. Cell Adh Migr. 2011, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Peek, S.L.; Mah, K.M.; Weiner, J.A. Regulation of neural circuit formation by protocadherins. Cell. Mol. Life Sci. CMLS 2017, 74, 4133–4157. [Google Scholar] [CrossRef] [PubMed]
- Heggem, M.; Bradley, R. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev. Cell 2003, 4, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Padanad, M.S.; Evers, B.M.; Smith, B.; Novaresi, N.; Suresh, S.; Richardson, J.A.; Stein, E.; Zhu, J.; Hammer, R.E.; et al. Modulation of Mutant Kras(G12D)-Driven Lung Tumorigenesis In Vivo by Gain or Loss of PCDH7 Function. Mol. Cancer Res. 2019, 17, 594–603. [Google Scholar] [CrossRef]
- Piper, M.; Dwivedy, A.; Leung, L.; Bradley, R.S.; Holt, C.E. NF-protocadherin and TAF1 regulate retinal axon initiation and elongation in vivo. J. Neurosci. 2008, 28, 100–105. [Google Scholar] [CrossRef]
- Leung, L.C.; Urbancic, V.; Baudet, M.L.; Dwivedy, A.; Bayley, T.G.; Lee, A.C.; Harris, W.A.; Holt, C.E. Coupling of NF-protocadherin signaling to axon guidance by cue-induced translation. Nat. Neurosci. 2013, 16, 166–173. [Google Scholar] [CrossRef]
- Yoshida, K. Fibroblast cell shape and adhesion in vitro is altered by overexpression of the 7a and 7b isoforms of protocadherin 7, but not the 7c isoform. Cell. Mol. Biol. Lett. 2003, 8, 735–741. [Google Scholar]
- Rashid, D.; Newell, K.; Shama, L.; Bradley, R. A requirement for NF-protocadherin and TAF1/set in cell adhesion and neural tube formation. Dev. Biol. 2006, 291, 170–181. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Takegahara, N.; Choi, Y. Protocadherin-7 Regulates Osteoclast Differentiation through Intracellular SET-Binding Domain-Mediated RhoA and Rac1 Activation. Int. J. Mol. Sci. 2021, 22, 13117. [Google Scholar] [CrossRef]
- Kim, H.; Takegahara, N.; Walsh, M.C.; Ueda, J.; Fujihara, Y.; Ikawa, M.; Choi, Y. Protocadherin-7 contributes to maintenance of bone homeostasis through regulation of osteoclast multinucleation. Bmb Rep. 2020, 53, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, A.; Ruan, B.; Niu, Z.; Su, Y.; Qin, H.; Zheng, Y.; Zhang, B.; Gao, L.; Chen, Z.; et al. PCDH7 Inhibits the Formation of Homotypic Cell-in-Cell Structure. Front. Cell Dev. Biol. 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Updegraff, B.L.; Guo, Y.; Peyton, M.; Girard, L.; Larsen, J.E.; Xie, X.J.; Zhou, Y.; Hwang, T.H.; Xie, Y.; et al. PROTOCADHERIN 7 Acts through SET and PP2A to Potentiate MAPK Signaling by EGFR and KRAS during Lung Tumorigenesis. Cancer Res. 2017, 77, 187–197. [Google Scholar] [CrossRef]
- Vanhalst, K.; Kools, P.; Staes, K.; van Roy, F.; Redies, C. delta-Protocadherins: A gene family expressed differentially in the mouse brain. Cell. Mol. Life Sci. CMLS 2005, 62, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Pippa, R.; Dominguez, A.; Christensen, D.J.; Moreno-Miralles, I.; Blanco-Prieto, M.J.; Vitek, M.P.; Odero, M.D. Effect of FTY720 on the SET-PP2A complex in acute myeloid leukemia; SET binding drugs have antagonistic activity. Leukemia 2014, 28, 1915–1918. [Google Scholar] [CrossRef]
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3beta-MCL-1 Axis. Cancer Cell 2019, 35, 798–815. [Google Scholar] [CrossRef]
- Wang, X.; Blanchard, J.; Kohlbrenner, E.; Clement, N.; Linden, R.M.; Radu, A.; Grundke-Iqbal, I.; Iqbal, K. The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment. FASEB J. 2010, 24, 4420–4432. [Google Scholar] [CrossRef]
- Kitano, A.; Shimasaki, T.; Chikano, Y.; Nakada, M.; Hirose, M.; Higashi, T.; Ishigaki, Y.; Endo, Y.; Takino, T.; Sato, H.; et al. Aberrant Glycogen Synthase Kinase 3β Is Involved in Pancreatic Cancer Cell Invasion and Resistance to Therapy. PLoS ONE 2013, 8, e55289. [Google Scholar]
- Perrotti, D.; Neviani, P. Protein phosphatase 2A: A target for anticancer therapy. Lancet Oncol. 2013, 14, e229–e238. [Google Scholar]
- Takai, A.; Mieskes, G. Inhibitory effect of okadaic acid on the p-nitrophenyl phosphate phosphatase activity of protein phosphatases. Biochem. J. 1991, 275, 233–239. [Google Scholar] [CrossRef]
- Bennecib, M.; Gong, C.X.; Grundke-Iqbal, I.; Iqbal, K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5, and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000, 485, 87–93. [Google Scholar] [CrossRef]
- Mitra, A.; Menezes, M.E.; Pannell, L.K.; Mulekar, M.S.; Honkanen, R.E.; Shevde, L.A.; Samant, R.S. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3beta to downregulate beta-catenin transcription target, osteopontin. Oncogene 2012, 31, 4472–4483. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Espeseth, A.; Kintner, C. NF-protocadherin, a novel member of the cadherin superfamily, is required for Xenopus ectodermal differentiation. Curr. Biol. 1998, 8, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, H.; Damuni, Z. Purification and characterization of 2 potent heat-stable protein inhibitors of protein phosphatase 2a from bovine kidney. Biochemistry 1995, 34, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Makkinje, A.; Damuni, Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 1996, 271, 11059–11062. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.D.; Shin, J.H.; Park, D.R.; Hong, J.H.; Yoon, K.; Ko, R.; Ko, C.Y.; Kim, H.S.; Jeong, D.; Kim, N.; et al. Inactivation of glycogen synthase kinase-3beta is required for osteoclast differentiation. J. Biol. Chem. 2011, 286, 39043–39050. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.B.; Kim, J.H.; Kim, K.; Youn, B.U.; Ko, A.; Lee, S.Y.; Kim, N. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J. Immunol. 2012, 188, 163–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Takegahara, N.; Choi, Y. PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-Dependent Regulation of Small GTPase RhoA in Osteoclasts. Cells 2023, 12, 1967. https://doi.org/10.3390/cells12151967
Kim H, Takegahara N, Choi Y. PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-Dependent Regulation of Small GTPase RhoA in Osteoclasts. Cells. 2023; 12(15):1967. https://doi.org/10.3390/cells12151967
Chicago/Turabian StyleKim, Hyunsoo, Noriko Takegahara, and Yongwon Choi. 2023. "PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-Dependent Regulation of Small GTPase RhoA in Osteoclasts" Cells 12, no. 15: 1967. https://doi.org/10.3390/cells12151967
APA StyleKim, H., Takegahara, N., & Choi, Y. (2023). PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-Dependent Regulation of Small GTPase RhoA in Osteoclasts. Cells, 12(15), 1967. https://doi.org/10.3390/cells12151967







