Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Strains and Maintenance
2.2. DHA-TG and Oleic-TG Treatment: Triglyceride Nanoemulsions
2.3. Nile Red Staining
2.4. Lifespan Analysis
2.5. Motility Assay
2.6. Chemotaxis Assay
2.7. Preparation of Worm Lysates
2.8. Lipid Peroxidation
2.9. Superoxide Dismutase Activity
2.10. Glutathione Quantification
2.11. RNA Extraction, Retrotranscription, and (RT)-qPCR
2.12. Statistical Analysis
3. Results
3.1. Internalization of Triglyceride Nanoemulsions
3.2. DHA and Oleic-TG Effect on the Lifespan of Wild-Type Nematodes
3.3. Effect of DHA-TG on the Behavior of Wild-Type Nematodes
3.3.1. DHA-TG Improves Locomotive Function
3.3.2. Sensorial Response after DHA-TG Treatment
3.4. Antioxidant-Promoting Effect of DHA-TG in Wild-Type Nematodes
3.5. Upregulation of Antioxidant Genes in Wild-Type Nematodes after DHA-TG Treatment
3.6. Loss of Effect in DAF-16 Deficient Nematodes after DHA-TG Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haveman-Nies, A.; De Groot, L.C.; Van Staveren, W.A. Dietary quality, lifestyle factors and healthy ageing in Europe: The SENECA study. Age Ageing 2003, 32, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ruthsatz, M.; Candeias, V. Non-communicable disease prevention, nutrition and aging. Acta Biomed. 2020, 91, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Chowdhury, S.; Gatto, N.M.; Fraser, G.E.; Lee, G.J. Omega-3 fatty acids are associated with blood–brain barrier integrity in a healthy aging population. Brain Behav. 2021, 11, e2273. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Minhas, P.S.; Latif-Hernandez, A.; McReynolds, M.R.; Durairaj, A.S.; Wang, Q.; Rubin, A.; Joshi, A.U.; He, J.Q.; Gauba, E.; Liu, L.; et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021, 590, 122–128. [Google Scholar] [CrossRef]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins. Leukot. Essent. Fatty Acids 2018, 132, 23–29. [Google Scholar] [CrossRef]
- Wong, S.W.; Kwon, M.J.; Choi, A.M.K.; Kim, H.P.; Nakahira, K.; Hwang, D.H. Fatty Acids Modulate Toll-like Receptor 4 Activation through Regulation of Receptor Dimerization and Recruitment into Lipid Rafts in a Reactive Oxygen Species-dependent Manner. J. Biol. Chem. 2009, 284, 27384. [Google Scholar] [CrossRef]
- Lafuente, M.; González-Herrero, M.E.R.; Villadóniga, S.R.; Domingo, J.C. Antioxidant Activity and Neuroprotective Role of Docosahexaenoic Acid (DHA) Supplementation in Eye Diseases That Can Lead to Blindness: A Narrative Review. Antioxidants 2021, 10, 386. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.C.; Villegas, J.A. Use of DHA for treating a pathology associated with cellular oxidative damage. World Intellect. Prop. Organ. 2007, 100. [Google Scholar]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.H.; Galano, J.-M.; Crauste, C.; Durand, T.; Lee, J.C.-Y. Combination of Lutein and Zeaxanthin, and DHA Regulated Polyunsaturated Fatty Acid Oxidation in H2O2-Stressed Retinal Cells. Neurochem. Res. 2020, 45, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Arab, K.; Rossary, A.; Flourié, F.; Tourneur, Y.; Steghens, J.-P. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating γ-glutamyl-cysteinyl ligase and glutathione reductase. Br. J. Nutr. 2006, 95, 18–26. [Google Scholar] [CrossRef]
- Gasso, F.; Bogdanov, P.; Domingo, J.C. Docosahexaenoic Acid Improves Endogen Antioxidant Defense in Arpe-19 Cells. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5932. [Google Scholar]
- Mora, I.; Arola, L.; Caimari, A.; Escoté, X.; Puiggròs, F. Structured Long-Chain Omega-3 Fatty Acids for Improvement of Cognitive Function during Aging. Int. J. Mol. Sci. 2022, 23, 3472. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef]
- Kitson, A.P.; Metherel, A.H.; Chen, C.T.; Domenichiello, A.F.; Trépanier, M.O.; Berger, A.; Bazinet, R.P. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J. Nutr. Biochem. 2016, 33, 91–102. [Google Scholar] [CrossRef]
- Peris-Martínez, C.; Piá-Ludeña, J.V.; Rog-Revert, M.J.; Fernández-López, E.; Domingo, J.C. Antioxidant and Anti-Inflammatory Effects of Oral Supplementation with a Highly-Concentrated Docosahexaenoic Acid (DHA) Triglyceride in Patients with Keratoconus: A Randomized Controlled Preliminary Study. Nutrients 2023, 15, 1300. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, J.; Kannan, S.; Govindasamy, A. Structured form of DHA prevents neurodegenerative disorders: A better insight into the pathophysiology and the mechanism of DHA transport to the brain. Nutr. Res. 2021, 85, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Sofela, S.; Sahloul, S.; Song, Y.A. Biophysical analysis of drug efficacy on C. elegans models for neurodegenerative and neuromuscular diseases. PLoS ONE 2021, 16, e0246496. [Google Scholar] [CrossRef]
- Gottschling, D.-C.; Döring, F. Is C. elegans a suitable model for nutritional science? Genes. Nutr. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Olivares-Martín, M.; Bañuelos-Hortigüela, O.; Pallàs, M. Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model. Nutrients 2021, 13, 2411. [Google Scholar] [CrossRef] [PubMed]
- Marza, E.; Lesa, G.M. Polyunsaturated fatty acids and neurotransmission in Caenorhabditis elegans. Biochem. Soc. Trans. 2006, 34, 77–80. [Google Scholar] [CrossRef]
- Kosel, M.; Wild, W.; Bell, A.; Rothe, M.; Lindschau, C.; Steinberg, C.E.W.; Schunck, W.H.; Menzel, R. Eicosanoid formation by a cytochrome P450 isoform expressed in the pharynx of Caenorhabditis elegans. Biochem. J. 2011, 435, 689–700. [Google Scholar] [CrossRef]
- Hoang, H.D.; Prasain, J.K.; Dorand, D.; Miller, M.A. A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Caenorhabditis elegans Reproductive Tract. PLoS Genet. 2013, 9, e1003271. [Google Scholar] [CrossRef]
- Lesa, G.M.; Palfreyman, M.; Hall, D.H.; Clandinin, M.T.; Rudolph, C.; Jorgensen, E.M.; Schiavo, G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 2003, 116, 4965–4975. [Google Scholar] [CrossRef]
- Watts, J.L.; Brown, L.; Rauch, B.; Poudyal, H. Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids. J. Clin. Med. 2016, 5, 19. [Google Scholar] [CrossRef]
- Qi, W.; Gutierrez, G.E.; Gao, X.; Dixon, H.; McDonough, J.A.; Marini, A.M.; Fisher, A.L. The ω-3 fatty acid α-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARα and oxidation to oxylipins. Aging Cell 2017, 16, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Chabot, C.; Wang, L.; Smarun, A.V.; Vidović, D.; Shchepinov, M.S.; Thibault, G. Deuterated Polyunsaturated Fatty Acids Reduce Oxidative Stress and Extend the Lifespan of C. elegans. Front. Physiol. 2019, 10, 641. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, T.; Guo, Y.; Pei, W.; Liu, R.; Chang, M.; Wang, X. Linolenic acid ameliorates sarcopenia in C. elegans by promoting mitophagy and fighting oxidative stress. Food Funct. 2023, 14, 1498–1509. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Honma, T.; Ito, J.; Kijima, R.; Tsuduki, T. Fish oil changes the lifespan of Caenorhabditis elegans via lipid peroxidation. J. Clin. Biochem. Nutr. 2013, 52, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Saul, N.; Möller, S.; Cirulli, F.; Berry, A.; Luyten, W.; Fuellen, G. Health and longevity studies in C. elegans: The “healthy worm database” reveals strengths, weaknesses and gaps of test compound-based studies. Biogerontology 2021, 22, 215–236. [Google Scholar] [CrossRef]
- Vásquez, V.; Krieg, M.; Lockhead, D.; Goodman, M.B. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Rep. 2014, 6, 70–80. [Google Scholar] [CrossRef]
- Horikawa, M.; Sakamoto, K. Polyunsaturated fatty acids are involved in regulatory mechanism of fatty acid homeostasis via daf-2/insulin signaling in Caenorhabditis elegans. Mol. Cell. Endocrinol. 2010, 323, 183–192. [Google Scholar] [CrossRef]
- Di Rosa, G.; Brunetti, G.; Scuto, M.; Salinaro, A.T.; Calabrese, E.J.; Crea, R.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Enhancement by Olive Polyphenols in C. elegans Wild Type and Parkinson’s Models. Int. J. Mol. Sci. 2020, 21, 3893. [Google Scholar] [CrossRef]
- Lionaki, E.; Tavernarakis, N. Assessing aging and senescent decline in Caenorhabditis elegans: Cohort survival analysis. Methods Mol. Biol. 2013, 965, 473–484. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, e4019. [Google Scholar] [CrossRef]
- Mitchell, D.H.; Stiles, J.W.; Santelli, J.; Rao Sanadi, D. Synchronous Growth and Aging of Caenorhabditis elegans in the Presence of Fluorodeoxyuridine. J. Gerontol. 1979, 34, 28–36. [Google Scholar] [CrossRef]
- Chang, M.; Yang, J.; Guo, X.; Zhang, T.; Liu, R.; Jin, Q.; Wang, X. Medium / long-chain structured triglycerides are superior to physical mixtures triglycerides in Caenorhabditis elegans lifespan through an AMPK modified pathway. Food Biosci. 2021, 39, 100815. [Google Scholar] [CrossRef]
- Colmenares, D.; Sun, Q.; Shen, P.; Yue, Y.; McClements, D.J.; Park, Y. Delivery of dietary triglycerides to Caenorhabditis elegans using lipid nanoparticles: Nanoemulsion-based delivery systems. Food Chem. 2016, 202, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Zhu, L.J.; Yen, K.; Tissenbaum, H.A. Uncoupling lifespan and healthspan in Caenorhabditis Elegans longevity mutants. Proc. Natl. Acad. Sci. USA 2015, 112, E277–E286. [Google Scholar] [CrossRef]
- Gómez-Escribano, A.P.; Bono-Yagüe, J.; García-Gimeno, M.A.; Sequedo, M.D.; Hervás, D.; Fornés-Ferrer, V.; Torres-Sánchez, S.C.; Millán, J.M.; Sanz, P.; Vázquez-Manrique, R.P. Synergistic activation of AMPK prevents from polyglutamine-induced toxicity in Caenorhabditis elegans. Pharmacol. Res. 2020, 161, 105105. [Google Scholar] [CrossRef] [PubMed]
- Margie, O.; Palmer, C.; Chin-Sang, I.C. elegans Chemotaxis Assay. J. Vis. Exp. 2013, 50069. [Google Scholar] [CrossRef]
- Saul, N.; Dhondt, I.; Kuokkanen, M.; Perola, M.; Verschuuren, C.; Wouters, B.; von Chrzanowski, H.; De Vos, W.H.; Temmerman, L.; Luyten, W.; et al. Identification of healthspan-promoting genes in Caenorhabditis elegans based on a human GWAS study. Biogerontology 2022, 23, 431–452. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.-S.; Lee, S.-J.V.; Kim, S.; Han, S.K.; Lee, D.; et al. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef]
- Beaudoin-Chabot, C.; Wang, L.; Celik, C.; Abdul Khalid, A.T.F.; Thalappilly, S.; Xu, S.; Koh, J.H.; Lim, V.W.X.; Low, A.D.; Thibault, G. The unfolded protein response reverses the effects of glucose on lifespan in chemically-sterilized C. elegans. Nat. Commun. 2022, 13, 5889. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.D.; Cavataio, J.P.; Berry, Y.; Vang, B.; Maddali, A.; Sukpraphrute, R.J.; Schnell, S.; Truttmann, M.C. Explaining inter-lab variance in C. elegans N2 lifespan: Making a case for standardized reporting to enhance reproducibility. Exp. Gerontol. 2021, 156, 111622. [Google Scholar] [CrossRef]
- Gómez-Escribano, A.P.; Mora-Martínez, C.; Roca, M.; Walker, D.S.; Panadero, J.; Sequedo, M.D.; Saini, R.; Knölker, H.-J.; Blanca, J.; Burguera, J.; et al. Changes in lipid metabolism driven by steroid signalling modulate proteostasis in C. elegans. EMBO Rep. 2023, 24, e55556. [Google Scholar] [CrossRef]
- Back, P.; De Vos, W.H.; Depuydt, G.G.; Matthijssens, F.; Vanfleteren, J.R.; Braeckman, B.P. Exploring real-time in vivo redox biology of developing and aging Caenorhabditis elegans. Free Radic. Biol. Med. 2012, 52, 850–859. [Google Scholar] [CrossRef]
- Jenkins, N.L.; James, S.A.; Salim, A.; Sumardy, F.; Speed, T.P.; Conrad, M.; Richardson, D.R.; Bush, A.I.; McColl, G. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis Elegans. Elife 2020, 9, e56580. [Google Scholar] [CrossRef]
- Van Voorhies, W.A. The influence of metabolic rate on longevity in the nematode Caenorhabditis elegans. Aging Cell 2002, 1, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Shmookler Reis, R.J.; Xu, L.; Lee, H.; Chae, M.; Thaden, J.J.; Bharill, P.; Tazearslan, C.; Siegel, E.; Alla, R.; Zimniak, P.; et al. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging 2011, 3, 125–147. [Google Scholar] [CrossRef]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef]
- Fernando, L.M.; Adeel, S.; Basar, M.A.; Allen, A.K.; Duttaroy, A. The SOD-2 protein is the single active SOD enzyme in C. elegans. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cabreiro, F.; Ackerman, D.; Doonan, R.; Araiz, C.; Back, P.; Papp, D.; Braeckman, B.P.; Gems, D. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic. Biol. Med. 2011, 51, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 8905. [Google Scholar] [CrossRef]
- Scharf, A.; Pohl, F.; Egan, B.M.; Kocsisova, Z.; Kornfeld, K. Reproductive Aging in Caenorhabditis elegans: From Molecules to Ecology. Front. Cell Dev. Biol. 2021, 9, 2343. [Google Scholar] [CrossRef]
- Sugawara, T.; Sakamoto, K. Quercetin enhances motility in aged and heat-stressed Caenorhabditis elegans nematodes by modulating both HSF-1 activity, and insulin-like and p38-MAPK signalling. PLoS ONE 2020, 15, e0238528. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Molin, L.; Alcolei, A.; Solyga, M.; Bonneau, B.; Vachon, C.; Bessereau, J.L.; Solari, F. DAF-2/insulin IGF-1 receptor regulates motility during aging by integrating opposite signaling from muscle and neuronal tissues. Aging Cell 2022, 21, e13660. [Google Scholar] [CrossRef]
- Rollins, J.A.; Howard, A.C.; Dobbins, S.K.; Washburn, E.H.; Rogers, A.N. Assessing Health Span in Caenorhabditis elegans: Lessons From Short-Lived Mutants. J. Gerontol. A. Biol. Sci. Med. Sci. 2017, 72, 473–480. [Google Scholar] [CrossRef]
- Chávez, V.; Mohri-Shiomi, A.; Maadani, A.; Vega, L.A.; Garsin, D.A. Oxidative Stress Enzymes Are Required for DAF-16-Mediated Immunity Due to Generation of Reactive Oxygen Species by Caenorhabditis elegans. Genetics 2007, 176, 1567. [Google Scholar] [CrossRef]
- Park, S.; Park, S.K. Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis elegans. Antioxidants 2022, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Soo, S.K.; Rudich, Z.D.; Ko, B.; Moldakozhayev, A.; AlOkda, A.; Van Raamsdonk, J.M. Biological resilience and aging: Activation of stress response pathways contributes to lifespan extension. Ageing Res. Rev. 2023, 88, 101941. [Google Scholar] [CrossRef]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. DHA and Its Elaborated Modulation of Antioxidant Defenses of the Brain: Implications in Aging and AD Neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Yang, A.Y.; Guo, Y.; Kong, A.N.T. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2–ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.J.; Liu, J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J. Agric. Food Chem. 2021, 69, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wu, H.; Cong, M.; Zhan, J.; Li, F. Meta-analytic evidence for the anti-aging effect of hormesis on Caenorhabditis elegans. Aging 2020, 12, 2723. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Hsu, W.L.; Wu, C.Y.; Lai, Y.R.; Chao, H.R.; Chen, C.H.; Tsai, M.H. Effect of omega-6 linoleic acid on neurobehavioral development in Caenorhabditis elegans. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102557. [Google Scholar] [CrossRef] [PubMed]

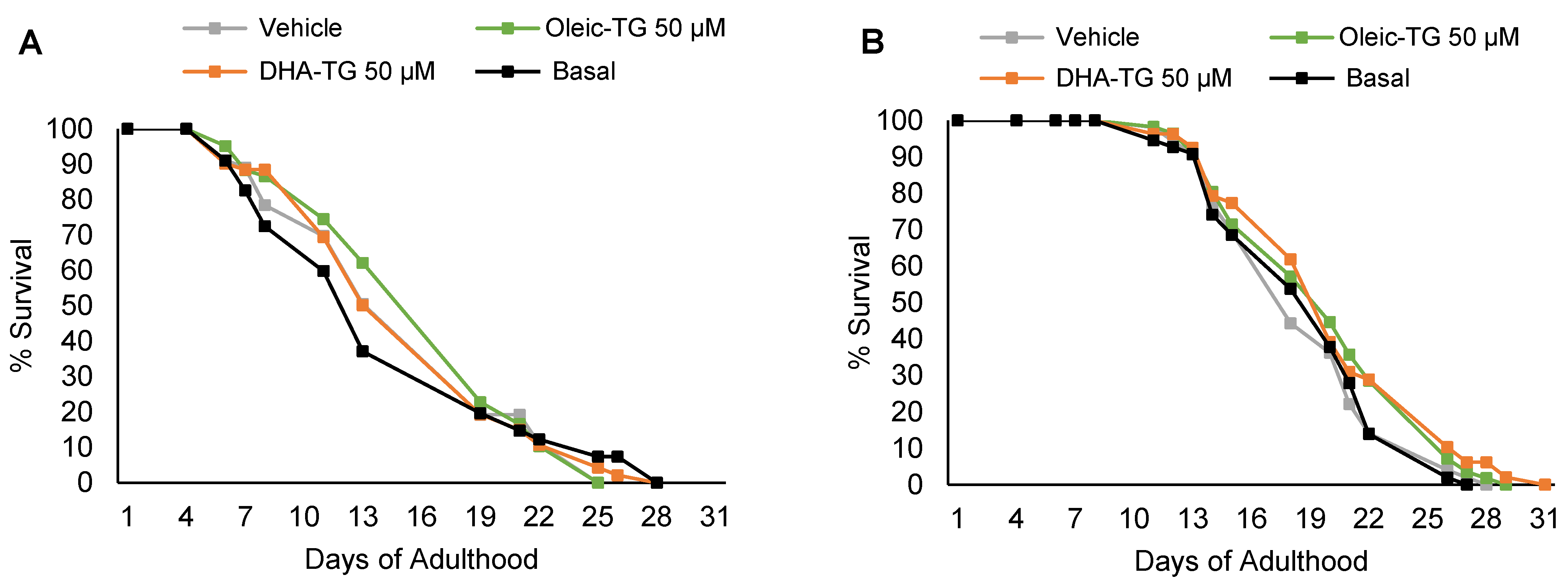

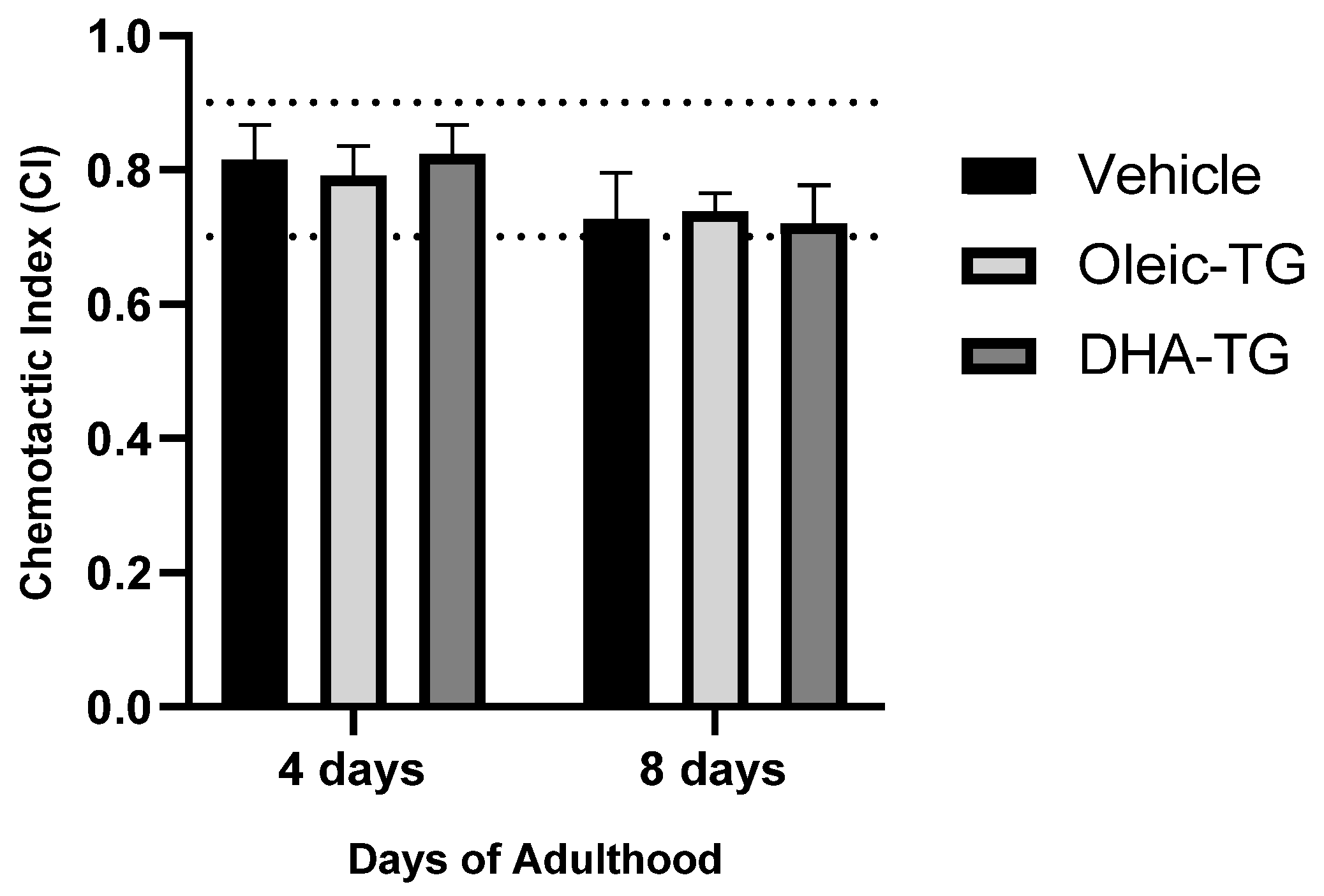

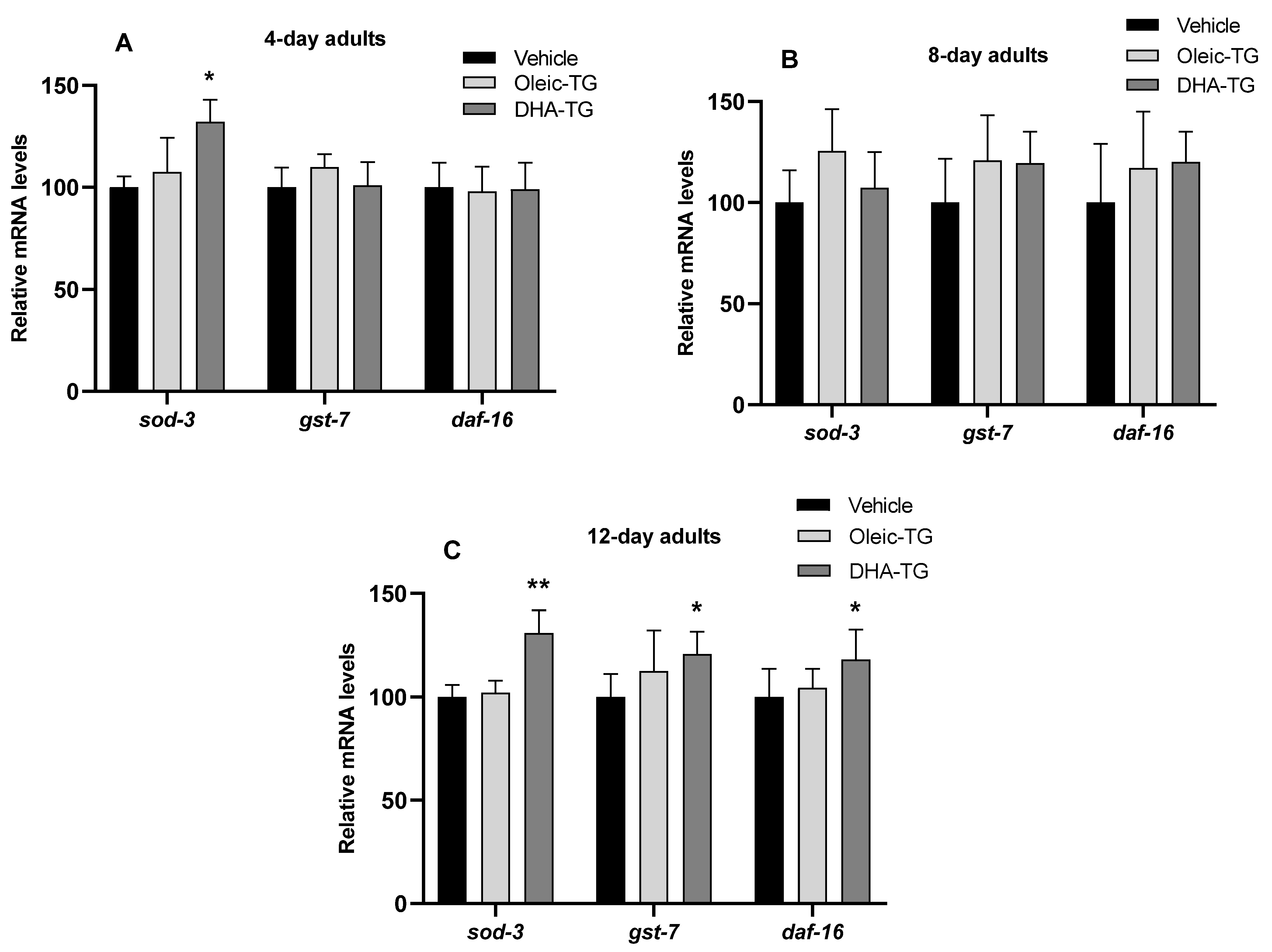

| FUdR | Treatment | Mean Lifespan | SEM | p-Value vs. Basal |
|---|---|---|---|---|
| No | Basal | 14.47 | 0.89 | - |
| Vehicle | 15.49 | 0.86 | 0.636 | |
| Oleic-TG | 16.58 | 0.75 | 0.262 | |
| DHA-TG | 15.78 | 0.76 | 0.524 | |
| Yes | Basal | 18.89 | 0.60 | - |
| Vehicle | 18.78 | 0.60 | 0.919 | |
| Oleic-TG | 20.02 | 0.66 | 0.126 | |
| DHA-TG | 20.33 | 0.70 | 0.067 |
| Data | Age (Days) | mu86 sod-3 | mu86 DHA-TG sod-3 | WT sod-3 | mu86 gst-7 | mu86 DHA-TG gst-7 | WT gst-7 |
|---|---|---|---|---|---|---|---|
| Mean | 4 | 1.0 | 0.9 | 36.4 *** | 1.0 | 1.1 | 3.9 *** |
| 8 | 1.0 | 1.2 | 336.0 *** | 1.0 | 1.2 | 10.4 *** | |
| 12 | 1.0 | 1.0 | 1342.8 *** | 1.0 | 0.8 | 6.4 *** | |
| SEM | 4 | 0.2 | 0.1 | 5.3 | 0.3 | 0.2 | 0.6 |
| 8 | 0.2 | 0.1 | 53.5 | 0.2 | 0.5 | 2.2 | |
| 12 | 0.1 | 0.2 | 50.4 | 0.2 | 0.1 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, I.; Pérez-Santamaria, A.; Tortajada-Pérez, J.; Vázquez-Manrique, R.P.; Arola, L.; Puiggròs, F. Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. elegans. Cells 2023, 12, 1932. https://doi.org/10.3390/cells12151932
Mora I, Pérez-Santamaria A, Tortajada-Pérez J, Vázquez-Manrique RP, Arola L, Puiggròs F. Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. elegans. Cells. 2023; 12(15):1932. https://doi.org/10.3390/cells12151932
Chicago/Turabian StyleMora, Ignasi, Alejandra Pérez-Santamaria, Julia Tortajada-Pérez, Rafael P. Vázquez-Manrique, Lluís Arola, and Francesc Puiggròs. 2023. "Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. elegans" Cells 12, no. 15: 1932. https://doi.org/10.3390/cells12151932
APA StyleMora, I., Pérez-Santamaria, A., Tortajada-Pérez, J., Vázquez-Manrique, R. P., Arola, L., & Puiggròs, F. (2023). Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. elegans. Cells, 12(15), 1932. https://doi.org/10.3390/cells12151932







