Glycolysis in Chronic Liver Diseases: Mechanistic Insights and Therapeutic Opportunities
Abstract
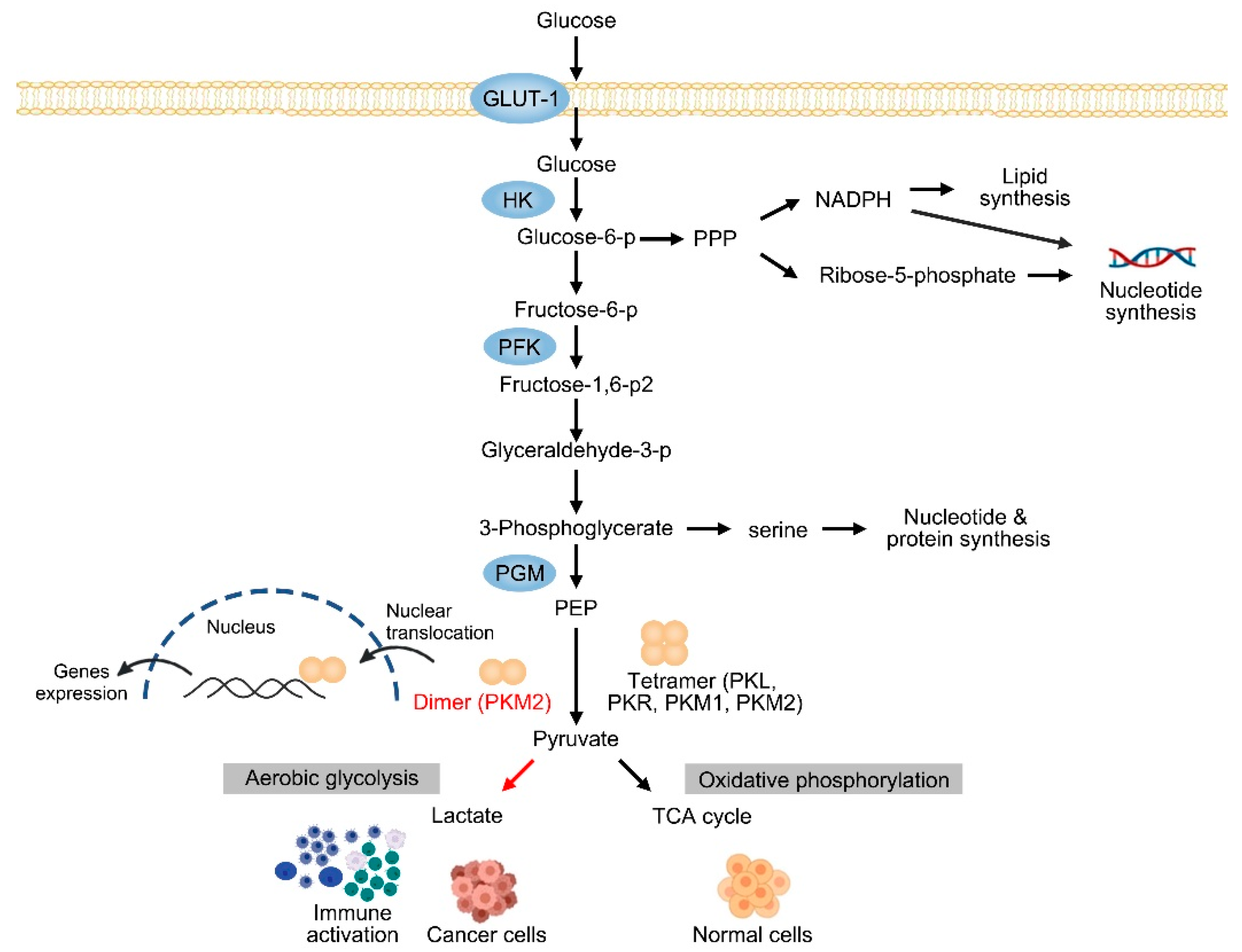
1. Introduction
2. The Expression of PKM2 in CLDs
3. Current Status and Challenge of Liver Cancer
3.1. PKM2 in HCC
3.2. PKM2 in CCA
4. Inflammatory Liver Diseases
4.1. PKM2 in Fatty Liver Diseases
4.2. PKM2 in Liver Fibrosis and Cirrhosis
5. Therapeutic Opportunities of PKM2-Targeted Therapy
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-DG | 2-deoxy-D-glucose |
| ALD | alcoholic liver disease |
| AMPK | AMP-activated protein kinase |
| BAG3 | BAG cochaperone 3 |
| CLD | chronic liver disease |
| CCA | cholangiocarcinoma |
| CCL20 C-C | motif chemokine ligand 20 |
| CNRIP1 | cannabinoid receptor interacting protein 1 |
| DRAM1 | DNA damage regulated autophagy modulator 1 |
| EMT | Epithelial–mesenchymal transition |
| FSTL1 | follistatin-like 1 |
| GLUT1 | glucose transporter protein type 1 |
| GTPBP4 | GTP binding protein 4 |
| HCC | hepatocellular carcinoma |
| HSC | hepatic stellate cell |
| HSP90 | heat shock protein 90 |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| HSPA12A | heat shock protein family A member 12A |
| HFD | high-fat diet |
| ICI | immune-checkpoint inhibitor |
| LSEC | liver sinusoidal endothelial cell |
| LF | liver fibrosis |
| MAT2B | methionine adenosyltransferase II beta |
| MyD88 | myeloid differentiation primary response 88 |
| MCD | methionine-choline deficient diet |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| OXPHOS | oxidative phosphorylation |
| PD-L1 | programmed death-ligand 1 |
| PRMT6 | protein arginine N-methyltransferase 6 |
| STAT3 | signal transducer and activator of transcription 3 |
| SMADS | suppressor of mothers against decapentaplegic |
| Th17 | T helper 17 |
| TACE | trans-arterial chemoembolization |
| TGF-β1 | transforming growth factor beta 1 |
References
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Francque, S.; Sanyal, A. Breakthroughs in therapies for NASH and remaining challenges. J. Hepatol. 2022, 76, 1263–1278. [Google Scholar] [CrossRef]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef]
- Kubes, P.; Mehal, W.Z. Sterile inflammation in the liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Stenmark, K.R. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 2015, 27, 267–275. [Google Scholar] [CrossRef]
- Shang, R.Z.; Qu, S.B.; Wang, D.S. Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects. World J. Gastroenterol. 2016, 22, 9933–9943. [Google Scholar] [CrossRef]
- Rui, L.; Lin, J.D. Reprogramming of Hepatic Metabolism and Microenvironment in Nonalcoholic Steatohepatitis. Annu. Rev. Nutr. 2022, 42, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wang, C.; Tian, Y.; Dai, C.; Zhu, Y.; Xu, F. Mitochondrial metabolic reprogramming: An important player in liver cancer progression. Cancer Lett. 2020, 470, 197–203. [Google Scholar] [CrossRef]
- Delgado, M.E.; Cárdenas, B.I.; Farran, N.; Fernandez, M. Metabolic Reprogramming of Liver Fibrosis. Cells 2021, 10, 3604. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Taddei, M.L.; Rae, C.; Braconi, C.; Marra, F. Metabolic reprogramming in cholangiocarcinoma. J. Hepatol. 2022, 77, 849–864. [Google Scholar] [CrossRef]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free. Radic. Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, X.; Liu, Y.; Liu, Y.; Sun, L.; Chen, F. PKM2, function and expression and regulation. Cell. Biosci. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Ojo, D.; Yan, J.; Tang, D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015, 356, 184–191. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Xia, Y.; Ji, H.; Chen, X.; Guo, F.; Lyssiotis, C.A.; Aldape, K.; Cantley, L.C.; Lu, Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell. Biol. 2012, 14, 1295–1304. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.; et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016, 17, 1721–1730. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Zhang, X.; Liu, H.; Yin, M.; Chen, X.; Peng, C. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021, 503, 240–248. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Fu, R. Research progress on the role of PKM2 in the immune response. Front. Immunol. 2022, 13, 936967. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Das, A.; Meshram, P.; Sharma, A.; Chowdhury, A.; Jariyal, H.; Datta, A.; Sarmah, D.; Nalla, L.V.; Sahu, B.; et al. Pyruvate kinase M2 in chronic inflammations: A potpourri of crucial protein-protein interactions. Cell. Biol. Toxicol. 2021, 37, 653–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Han, S.N.; Arumugam, S.; Yousaf, M.N.; Qin, Y.; Jiang, J.X.; Torok, N.J.; Chen, Y.; Mankash, M.S.; Liu, J.; et al. Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G387–G397. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Chen, H.; Gao, Z.; Li, Y.; Sun, Z.; Xiang, R.; Zhang, S. Phage display library selection of a hypoxia-binding scFv antibody for liver cancer metabolic marker discovery. Oncotarget 2016, 7, 38105–38121. [Google Scholar] [CrossRef] [PubMed]
- Bluemlein, K.; Grüning, N.M.; Feichtinger, R.G.; Lehrach, H.; Kofler, B.; Ralser, M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget 2011, 2, 393–400. [Google Scholar] [CrossRef]
- Hou, P.P.; Luo, L.J.; Chen, H.Z.; Chen, Q.T.; Bian, X.L.; Wu, S.F.; Zhou, J.X.; Zhao, W.X.; Liu, J.M.; Wang, X.M.; et al. Ectosomal PKM2 Promotes HCC by Inducing Macrophage Differentiation and Remodeling the Tumor Microenvironment. Mol. Cell 2020, 78, 1192–1206.e1110. [Google Scholar] [CrossRef]
- Li, T.E.; Wang, S.; Shen, X.T.; Zhang, Z.; Chen, M.; Wang, H.; Zhu, Y.; Xu, D.; Hu, B.Y.; Wei, R.; et al. PKM2 Drives Hepatocellular Carcinoma Progression by Inducing Immunosuppressive Microenvironment. Front. Immunol. 2020, 11, 589997. [Google Scholar] [CrossRef]
- Lv, W.W.; Liu, D.; Liu, X.C.; Feng, T.N.; Li, L.; Qian, B.Y.; Li, W.X. Effects of PKM2 on global metabolic changes and prognosis in hepatocellular carcinoma: From gene expression to drug discovery. BMC Cancer 2018, 18, 1150. [Google Scholar] [CrossRef]
- Zhao, R.; Li, L.; Yang, J.; Niu, Q.; Wang, H.; Qin, X.; Zhu, N.; Shi, A. Overexpression of Pyruvate Kinase M2 in Tumor Tissues Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Pathol. Oncol. Res. 2020, 26, 853–860. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Wang, Z.; Jin, G.; Wang, Q.; Chen, D.; Chen, T.; Li, J.; Fan, J.; Cong, W.; et al. Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget 2015, 6, 2538–2548. [Google Scholar] [CrossRef]
- Tai, W.T.; Hung, M.H.; Chu, P.Y.; Chen, Y.L.; Chen, L.J.; Tsai, M.H.; Chen, M.H.; Shiau, C.W.; Boo, Y.P.; Chen, K.F. SH2 domain-containing phosphatase 1 regulates pyruvate kinase M2 in hepatocellular carcinoma. Oncotarget 2016, 7, 22193–22205. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.S.; Hansen, R.M.; Gray, M.E.; Massoud, O.I.; McGuire, B.M.; Shoreibah, M.G. Hepatocellular carcinoma surveillance: An evidence-based approach. World J. Gastroenterol. 2019, 25, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.C.W.; Carella, M.A.; Papa, S.; Bubici, C. High Expression of Glycolytic Genes in Cirrhosis Correlates With the Risk of Developing Liver Cancer. Front. Cell. Dev. Biol. 2018, 6, 138. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Mei, Y.; Ding, X.; Yang, X.; Li, C.; Deng, M.; Gong, J. Clinicopathological and prognostic significance of PKM2 protein expression in cirrhotic hepatocellular carcinoma and non-cirrhotic hepatocellular carcinoma. Sci. Rep. 2017, 7, 15294. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, J.; Xu, D.; Gao, X.M.; Zhang, Z.; Hsu, J.L.; Li, C.W.; Lim, S.O.; Sheng, Y.Y.; Zhang, Y.; et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 2019, 68, 1653–1666. [Google Scholar] [CrossRef]
- Lu, L.G.; Zhou, Z.L.; Wang, X.Y.; Liu, B.Y.; Lu, J.Y.; Liu, S.; Zhang, G.B.; Zhan, M.X.; Chen, Y. PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma. Gut 2022, 71, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yu, W.; Jin, G.; Xu, D.; Chen, Y.; Xia, T.; Yu, A.; Fang, W.; Zhang, X.; Li, Z.; et al. PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol. Cancer 2015, 14, 193. [Google Scholar] [CrossRef]
- Cuenco, J.; Wehnert, N.; Blyuss, O.; Kazarian, A.; Whitwell, H.J.; Menon, U.; Dawnay, A.; Manns, M.P.; Pereira, S.P.; Timms, J.F. Identification of a serum biomarker panel for the differential diagnosis of cholangiocarcinoma and primary sclerosing cholangitis. Oncotarget 2018, 9, 17430–17442. [Google Scholar] [CrossRef]
- Inomata, Y.; Oh, J.W.; Taniguchi, K.; Sugito, N.; Kawaguchi, N.; Hirokawa, F.; Lee, S.W.; Akao, Y.; Takai, S.; Kim, K.P.; et al. Downregulation of miR-122-5p Activates Glycolysis via PKM2 in Kupffer Cells of Rat and Mouse Models of Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 5230. [Google Scholar] [CrossRef]
- Moreno-Fernandez, M.E.; Giles, D.A.; Oates, J.R.; Chan, C.C.; Damen, M.; Doll, J.R.; Stankiewicz, T.E.; Chen, X.; Chetal, K.; Karns, R.; et al. PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell. Metab. 2021, 33, 1187–1204.e1189. [Google Scholar] [CrossRef]
- Zheng, D.; Jiang, Y.; Qu, C.; Yuan, H.; Hu, K.; He, L.; Chen, P.; Li, J.; Tu, M.; Lin, L.; et al. Pyruvate Kinase M2 Tetramerization Protects against Hepatic Stellate Cell Activation and Liver Fibrosis. Am. J. Pathol. 2020, 190, 2267–2281. [Google Scholar] [CrossRef]
- Meoli, L.; Gupta, N.K.; Saeidi, N.; Panciotti, C.A.; Biddinger, S.B.; Corey, K.E.; Stylopoulos, N. Nonalcoholic fatty liver disease and gastric bypass surgery regulate serum and hepatic levels of pyruvate kinase isoenzyme M2. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E613–E621. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Lurie, Y.; Webb, M.; Cytter-Kuint, R.; Shteingart, S.; Lederkremer, G.Z. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J. Gastroenterol. 2015, 21, 11567–11583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Donne, R.; Lujambio, A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology 2023, 77, 1773–1796. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 1604–1626. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.; Liu, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef]
- Li, Q.; Pan, X.; Zhu, D.; Deng, Z.; Jiang, R.; Wang, X. Circular RNA MAT2B Promotes Glycolysis and Malignancy of Hepatocellular Carcinoma Through the miR-338-3p/PKM2 Axis Under Hypoxic Stress. Hepatology 2019, 70, 1298–1316. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kim, J.; Jiang, L.; Feng, B.; Ying, Y.; Ji, K.Y.; Tang, Q.; Chen, W.; Mai, T.; Dou, W.; et al. MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat. Commun. 2020, 11, 708. [Google Scholar] [CrossRef]
- Liu, B.; Qu, X.; Wang, J.; Xu, L.; Zhang, L.; Xu, B.; Su, J.; Bian, X. LINC00365 functions as a tumor suppressor by inhibiting HIF-1α-mediated glucose metabolism reprogramming in breast cancer. Exp. Cell. Res. 2023, 425, 113514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Zhu, W.; Han, J.; Yang, X.; Zhou, R.; Lu, H.C.; Yu, H.; Yuan, W.B.; Li, P.C.; Tao, J.; et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021, 41, 560–575. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Y.; Yang, L.; Wang, T.; Meng, X.; Zong, Z.; Sun, X.; Hua, X.; Li, H. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. 2018, 37, 216. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hu, H.; Chang, R.; Zhong, J.; Knabel, M.; O’Meally, R.; Cole, R.N.; Pandey, A.; Semenza, G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011, 145, 732–744. [Google Scholar] [CrossRef]
- Pan, R.Y.; He, L.; Zhang, J.; Liu, X.; Liao, Y.; Gao, J.; Liao, Y.; Yan, Y.; Li, Q.; Zhou, X.; et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell. Metab. 2022, 34, 634–648.e636. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Hou, L.; Chen, Q.; Liu, Y. Shikonin differentially regulates glucose metabolism via PKM2 and HIF1α to overcome apoptosis in a refractory HCC cell line. Life Sci. 2021, 265, 118796. [Google Scholar] [CrossRef]
- Feng, J.; Dai, W.; Mao, Y.; Wu, L.; Li, J.; Chen, K.; Yu, Q.; Kong, R.; Li, S.; Zhang, J.; et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J. Exp. Clin. Cancer Res. 2020, 39, 24. [Google Scholar] [CrossRef]
- Wong, T.L.; Ng, K.Y.; Tan, K.V.; Chan, L.H.; Zhou, L.; Che, N.; Hoo, R.L.C.; Lee, T.K.; Richard, S.; Lo, C.M.; et al. CRAF Methylation by PRMT6 Regulates Aerobic Glycolysis-Driven Hepatocarcinogenesis via ERK-Dependent PKM2 Nuclear Relocalization and Activation. Hepatology 2020, 71, 1279–1296. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, Y.; Yu, M.; Gao, D.; Sun, J.; Yang, Z.; Weng, J.; Chen, W.; Atyah, M.; Shen, Y.; et al. GTPBP4 promotes hepatocellular carcinoma progression and metastasis via the PKM2 dependent glucose metabolism. Redox Biol. 2022, 56, 102458. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, J.; Liu, Y.; Chen, H.; Liu, H.; Wang, J.; Niu, M.; Hou, L.; Wu, Z.; Chen, Z.; et al. MyD88 in myofibroblasts regulates aerobic glycolysis-driven hepatocarcinogenesis via ERK-dependent PKM2 nuclear relocalization and activation. J. Pathol. 2022, 256, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, D.; Tang, Y. PKM2 promotes cell metastasis and inhibits autophagy via the JAK/STAT3 pathway in hepatocellular carcinoma. Mol. Cell. Biochem. 2021, 476, 2001–2010. [Google Scholar] [CrossRef]
- Liu, W.R.; Tian, M.X.; Yang, L.X.; Lin, Y.L.; Jin, L.; Ding, Z.B.; Shen, Y.H.; Peng, Y.F.; Gao, D.M.; Zhou, J.; et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget 2015, 6, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, L.; Wu, M.; Cao, K.; Jiang, F.; Chen, D.; Li, N.; Li, W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol. Cancer 2020, 19, 1. [Google Scholar] [CrossRef]
- Dayton, T.L.; Gocheva, V.; Miller, K.M.; Israelsen, W.J.; Bhutkar, A.; Clish, C.B.; Davidson, S.M.; Luengo, A.; Bronson, R.T.; Jacks, T.; et al. Germline loss of PKM2 promotes metabolic distress and hepatocellular carcinoma. Genes. Dev. 2016, 30, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Y.; Tang, D.; Dorfman, R.G.; Xu, L.; Zhou, Q.; Zhou, L.; Wang, Y.; Li, Y.; Yin, Y.; et al. Low levels of pyruvate induced by a positive feedback loop protects cholangiocarcinoma cells from apoptosis. Cell. Commun. Signal. 2019, 17, 23. [Google Scholar] [CrossRef]
- Lei, G.L.; Li, Z.; Li, Y.Y.; Hong, Z.X.; Wang, S.; Bai, Z.F.; Sun, F.; Yan, J.; Yu, L.X.; Yang, P.H.; et al. Long noncoding RNA FAM66C promotes tumor progression and glycolysis in intrahepatic cholangiocarcinoma by regulating hsa-miR-23b-3p/KCND2 axis. Environ. Toxicol. 2021, 36, 2322–2332. [Google Scholar] [CrossRef]
- Peng, C.; Sun, Z.; Li, O.; Guo, C.; Yi, W.; Tan, Z.; Jiang, B. Leptin stimulates the epithelial-mesenchymal transition and pro-angiogenic capability of cholangiocarcinoma cells through the miR-122/PKM2 axis. Int. J. Oncol. 2019, 55, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, H.; Feng, X.; Chen, Y.; Lv, Z.; Kota, V.G.; Chen, J.; Wu, W.; Lu, Y.; Liu, H.; et al. DNA Methylation of Cannabinoid Receptor Interacting Protein 1 Promotes Pathogenesis of Intrahepatic Cholangiocarcinoma Through Suppressing Parkin-Dependent Pyruvate Kinase M2 Ubiquitination. Hepatology 2021, 73, 1816–1835. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, H.; Bu, Q.; Wei, S.; Li, L.; Zhou, J.; Zhou, S.; Su, W.; Liu, M.; Liu, Z.; et al. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J. Hepatol. 2022, 77, 312–325. [Google Scholar] [CrossRef]
- Kong, Q.; Li, N.; Cheng, H.; Zhang, X.; Cao, X.; Qi, T.; Dai, L.; Zhang, Z.; Chen, X.; Li, C.; et al. HSPA12A Is a Novel Player in Nonalcoholic Steatohepatitis via Promoting Nuclear PKM2-Mediated M1 Macrophage Polarization. Diabetes 2019, 68, 361–376. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, J.; Wang, M.; Wang, Y.; Dong, M.; Ma, X.; Sun, B.; Liu, S.; Zhao, Z.; Chen, L.; et al. DRAM1 increases the secretion of PKM2-enriched EVs from hepatocytes to promote macrophage activation and disease progression in ALD. Mol. Ther. Nucleic Acids 2022, 27, 375–389. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Rao, J.; Wang, H.; Ni, M.; Wang, Z.; Wang, Z.; Wei, S.; Liu, M.; Wang, P.; Qiu, J.; Zhang, L.; et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 2022, 71, 2539–2550. [Google Scholar] [CrossRef]
- Wan, L.; Xia, T.; Du, Y.; Liu, J.; Xie, Y.; Zhang, Y.; Guan, F.; Wu, J.; Wang, X.; Shi, C. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: A role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019, 33, 8530–8542. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, J.; Jiang, Z.; Wang, B.; Wang, Y.; Hu, X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011, 30, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, D.; Yu, Y.; Israelsen, W.J.; Jiang, J.K.; Boxer, M.B.; Hong, B.S.; Tempel, W.; Dimov, S.; Shen, M.; Jha, A.; et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012, 8, 839–847. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Q.; Jiang, L.; Lu, S.; Li, C.; Xu, C.; Wang, C.; Zhang, E.; Zhang, X. Shikonin ameliorated mice colitis by inhibiting dimerization and tetramerization of PKM2 in macrophages. Front. Pharmacol. 2022, 13, 926945. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, S.Y.; Zhang, J.; Yang, G.M.; Gao, G.B.; Yu, N.N.; Li, Y.P.; Li, Y.X.; Ma, Z.Q.; Wang, Y.; et al. Inhibition of BAG3 enhances the anticancer effect of shikonin in hepatocellular carcinoma. Am. J. Cancer Res. 2021, 11, 3575–3593. [Google Scholar]
- Angiari, S.; Runtsch, M.C.; Sutton, C.E.; Palsson-McDermott, E.M.; Kelly, B.; Rana, N.; Kane, H.; Papadopoulou, G.; Pearce, E.L.; Mills, K.H.G.; et al. Pharmacological Activation of Pyruvate Kinase M2 Inhibits CD4(+) T Cell Pathogenicity and Suppresses Autoimmunity. Cell. Metab. 2020, 31, 391–405.e398. [Google Scholar] [CrossRef]
- Pang, Y.; Lin, Y.; Wang, X.; Wang, J.; Liu, Q.; Ding, N.; Huang, L.; Xiang, Q.; Fang, J.; Tan, G.; et al. Inhibition of abnormally activated HIF-1α-GLUT1/3-glycolysis pathway enhances the sensitivity of hepatocellular carcinoma to 5-caffeoylquinic acid and its derivatives. Eur. J. Pharmacol. 2022, 920, 174844. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, J.; Lei, S.; Yang, Y.; He, Z.; Xue, Y.; Chen, T. Simultaneous Inhibition of Ornithine Decarboxylase 1 and Pyruvate Kinase M2 Exerts Synergistic Effects Against Hepatocellular Carcinoma Cells. Onco Targets Ther. 2020, 13, 11697–11709. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, L.; Jiang, W.; Wu, W. Shikonin induces apoptosis and autophagy via downregulation of pyrroline-5-carboxylate reductase1 in hepatocellular carcinoma cells. Bioengineered 2022, 13, 7904–7918. [Google Scholar] [CrossRef]
- Thonsri, U.; Seubwai, W.; Waraasawapati, S.; Wongkham, S.; Boonmars, T.; Cha’on, U.; Wongkham, C. Antitumor Effect of Shikonin, a PKM2 Inhibitor, in Cholangiocarcinoma Cell Lines. Anticancer Res. 2020, 40, 5115–5124. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, Z.; Wang, X.; Tao, R.; Zhou, Y. TRAIL Enhances Shikonin Induced Apoptosis through ROS/JNK Signaling in Cholangiocarcinoma Cells. Cell. Physiol. Biochem. 2017, 42, 1073–1086. [Google Scholar] [CrossRef]
- Gwon, S.Y.; Ahn, J.; Jung, C.H.; Moon, B.; Ha, T.Y. Shikonin Attenuates Hepatic Steatosis by Enhancing Beta Oxidation and Energy Expenditure via AMPK Activation. Nutrients 2020, 12, 1133. [Google Scholar] [CrossRef]
- Liu, T.; Xu, L.; Wang, C.; Chen, K.; Xia, Y.; Li, J.; Li, S.; Wu, L.; Feng, J.; Xu, S.; et al. Alleviation of hepatic fibrosis and autophagy via inhibition of transforming growth factor-β1/Smads pathway through shikonin. J. Gastroenterol. Hepatol. 2019, 34, 263–276. [Google Scholar] [CrossRef]
- Satyanarayana, G.; Turaga, R.C.; Sharma, M.; Wang, S.; Mishra, F.; Peng, G.; Deng, X.; Yang, J.; Liu, Z.R. Pyruvate kinase M2 regulates fibrosis development and progression by controlling glycine auxotrophy in myofibroblasts. Theranostics 2021, 11, 9331–9341. [Google Scholar] [CrossRef]
- Yang, S.H.; Wu, H.; Yi, Z.J.; Lai, X. The PKM2 activator TEPP-46 attenuates MCD feeding-induced nonalcoholic steatohepatitis by inhibiting the activation of Kupffer cells. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4017–4026. [Google Scholar] [CrossRef]
- Ouyang, X.; Han, S.N.; Zhang, J.Y.; Dioletis, E.; Nemeth, B.T.; Pacher, P.; Feng, D.; Bataller, R.; Cabezas, J.; Stärkel, P.; et al. Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1α Transactivation in Steatohepatitis. Cell. Metab. 2018, 27, 339–350.e333. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Sheng, L.; Yuan, M.; Wu, Y.; Chen, L.; Zheng, G.; Qiu, Z. Metformin delays AKT/c-Met-driven hepatocarcinogenesis by regulating signaling pathways for de novo lipogenesis and ATP generation. Toxicol. Appl. Pharmacol. 2019, 365, 51–60. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Wu, L.; Yu, Q.; Li, J.; Feng, J.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Experimental Study of Hepatocellular Carcinoma Treatment by Shikonin Through Regulating PKM2. J. Hepatocell. Carcinoma 2020, 7, 19–31. [Google Scholar] [CrossRef]
- Liu, B.; Jin, J.; Zhang, Z.; Zuo, L.; Jiang, M.; Xie, C. Shikonin exerts antitumor activity by causing mitochondrial dysfunction in hepatocellular carcinoma through PKM2-AMPK-PGC1α signaling pathway. Biochem. Cell Biol. 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Sieghart, W.; Hucke, F.; Peck-Radosavljevic, M. Transarterial chemoembolization: Modalities, indication, and patient selection. J. Hepatol. 2015, 62, 1187–1195. [Google Scholar] [CrossRef]
- Martin, S.P.; Fako, V.; Dang, H.; Dominguez, D.A.; Khatib, S.; Ma, L.; Wang, H.; Zheng, W.; Wang, X.W. PKM2 inhibition may reverse therapeutic resistance to transarterial chemoembolization in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 99. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Li, H.; Li, C.; Gong, Z. Synergistic Induction of Potential Warburg Effect in Zebrafish Hepatocellular Carcinoma by Co-Transgenic Expression of Myc and xmrk Oncogenes. PLoS ONE 2015, 10, e0132319. [Google Scholar] [CrossRef]
- Pathi, S.; Peterson, P.; Mangelson, R.; Tyagi, E.; Foulks, J.M.; Whatcott, C.J.; Bearss, D.J.; Warner, S.L. Abstract B080: PKM2 activation modulates metabolism and enhances immune response in solid tumor models. Mol. Cancer Ther. 2019, 18, B080. [Google Scholar] [CrossRef]
- Xu, Q.; Dou, C.; Liu, X.; Yang, L.; Ni, C.; Wang, J.; Guo, Y.; Yang, W.; Tong, X.; Huang, D. Oviductus ranae protein hydrolysate (ORPH) inhibits the growth, metastasis and glycolysis of HCC by targeting miR-491-5p/PKM2 axis. Biomed. Pharmacother. 2018, 107, 1692–1704. [Google Scholar] [CrossRef]
- Ma, W.K.; Voss, D.M.; Scharner, J.; Costa, A.S.H.; Lin, K.T.; Jeon, H.Y.; Wilkinson, J.E.; Jackson, M.; Rigo, F.; Bennett, C.F.; et al. ASO-Based PKM Splice-Switching Therapy Inhibits Hepatocellular Carcinoma Growth. Cancer Res. 2022, 82, 900–915. [Google Scholar] [CrossRef]
- Wang, Z.; Jeon, H.Y.; Rigo, F.; Bennett, C.F.; Krainer, A.R. Manipulation of PK-M mutually exclusive alternative splicing by antisense oligonucleotides. Open. Biol. 2012, 2, 120133. [Google Scholar] [CrossRef]
- Liang, J.; Cao, R.; Wang, X.; Zhang, Y.; Wang, P.; Gao, H.; Li, C.; Yang, F.; Zeng, R.; Wei, P.; et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017, 27, 329–351. [Google Scholar] [CrossRef]
- Xu, F.; Guo, M.; Huang, W.; Feng, L.; Zhu, J.; Luo, K.; Gao, J.; Zheng, B.; Kong, L.D.; Pang, T.; et al. Annexin A5 regulates hepatic macrophage polarization via directly targeting PKM2 and ameliorates NASH. Redox Biol. 2020, 36, 101634. [Google Scholar] [CrossRef]
- Fan, N.; Zhang, X.; Zhao, W.; Zhao, J.; Luo, D.; Sun, Y.; Li, D.; Zhao, C.; Wang, Y.; Zhang, H.; et al. Covalent Inhibition of Pyruvate Kinase M2 Reprograms Metabolic and Inflammatory Pathways in Hepatic Macrophages against Non-alcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2022, 18, 5260–5275. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, J.; Sheng, Y.; Wang, J.; Zhou, X.; Li, W.; Kong, Y. Lapachol treats non-alcoholic fatty liver disease by modulating the M1 polarization of Kupffer cells via PKM2. Int. Immunopharmacol. 2023, 120, 110380. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, F.; Wan, T.; Chen, A.; Wang, H.; Jiang, B.; Zhao, W.; Liao, S.; Wang, S.; Li, G.; et al. ZEB1 enhances Warburg effect to facilitate tumorigenesis and metastasis of HCC by transcriptionally activating PFKM. Theranostics 2021, 11, 5926–5938. [Google Scholar] [CrossRef]
- Tarasenko, T.N.; Jestin, M.; Matsumoto, S.; Saito, K.; Hwang, S.; Gavrilova, O.; Trivedi, N.; Zerfas, P.M.; Barca, E.; DiMauro, S.; et al. Macrophage derived TNFα promotes hepatic reprogramming to Warburg-like metabolism. J. Mol. Med. 2019, 97, 1231–1243. [Google Scholar] [CrossRef]
- Beyoğlu, D.; Idle, J.R. The metabolomic window into hepatobiliary disease. J. Hepatol. 2013, 59, 842–858. [Google Scholar] [CrossRef]
- Dolin, C.E.; Arteel, G.E. The Matrisome, Inflammation, and Liver Disease. Semin. Liver Dis. 2020, 40, 180–188. [Google Scholar] [CrossRef]
- Seen, S. Chronic liver disease and oxidative stress—A narrative review. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 1021–1035. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Sové, R.J.; Verma, B.K.; Wang, H.; Ho, W.J.; Yarchoan, M.; Popel, A.S. Virtual clinical trials of anti-PD-1 and anti-CTLA-4 immunotherapy in advanced hepatocellular carcinoma using a quantitative systems pharmacology model. J. Immunother. Cancer 2022, 10, e005414. [Google Scholar] [CrossRef]
- Nijsen, M.; Wu, F.; Bansal, L.; Bradshaw-Pierce, E.; Chan, J.R.; Liederer, B.M.; Mettetal, J.T.; Schroeder, P.; Schuck, E.; Tsai, A.; et al. Preclinical QSP Modeling in the Pharmaceutical Industry: An IQ Consortium Survey Examining the Current Landscape. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 135–146. [Google Scholar] [CrossRef]
- Jeon, M.; Kang, H.W.; An, S. A Mathematical Model for Enzyme Clustering in Glucose Metabolism. Sci. Rep. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Mulukutla, B.C.; Yongky, A.; Daoutidis, P.; Hu, W.S. Bistability in glycolysis pathway as a physiological switch in energy metabolism. PLoS ONE 2014, 9, e98756. [Google Scholar] [CrossRef]
- Kapuy, O.; Makk-Merczel, K.; Szarka, A. Therapeutic Approach of KRAS Mutant Tumours by the Combination of Pharmacologic Ascorbate and Chloroquine. Biomolecules 2021, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.K.; Subramaniam, P.; Vadigepalli, R. Model-based virtual patient analysis of human liver regeneration predicts critical perioperative factors controlling the dynamic mode of response to resection. BMC Syst. Biol. 2019, 13, 9. [Google Scholar] [CrossRef] [PubMed]

| Name | Structure | MW | Disease Types | Pharmacological Properties | Refs. |
|---|---|---|---|---|---|
| C3k | 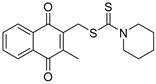 | 345.5 | HCC | Inhibitor | [87,88] |
| Shikonin | 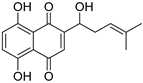 | 288.3 | HCC | Inhibitor | [59,89] |
| CCA | [90,91] | ||||
| NAFLD | [92] | ||||
| LF | [93] | ||||
| ML265 | 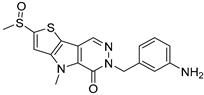 | 372.5 | LF | Agonist | [42,94] |
| NASH | [95] | ||||
| DASA-58 | 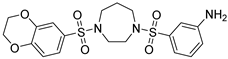 | 453.5 | LF | Agonist | [80] |
| Digoxin | 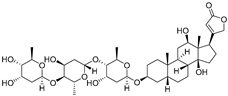 | 780.9 | NASH | Inhibiting PKM2 trans-activation | [24,96] |
| Meformin | 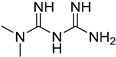 | 315.8 | HCC | Suppressing PKM2 activity | [97] |
| CCA | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, H.; Liu, J.; Zhang, D.; Xie, R.; Wang, L.; Hong, J. Glycolysis in Chronic Liver Diseases: Mechanistic Insights and Therapeutic Opportunities. Cells 2023, 12, 1930. https://doi.org/10.3390/cells12151930
Qu H, Liu J, Zhang D, Xie R, Wang L, Hong J. Glycolysis in Chronic Liver Diseases: Mechanistic Insights and Therapeutic Opportunities. Cells. 2023; 12(15):1930. https://doi.org/10.3390/cells12151930
Chicago/Turabian StyleQu, Hengdong, Junli Liu, Di Zhang, Ruoyan Xie, Lijuan Wang, and Jian Hong. 2023. "Glycolysis in Chronic Liver Diseases: Mechanistic Insights and Therapeutic Opportunities" Cells 12, no. 15: 1930. https://doi.org/10.3390/cells12151930
APA StyleQu, H., Liu, J., Zhang, D., Xie, R., Wang, L., & Hong, J. (2023). Glycolysis in Chronic Liver Diseases: Mechanistic Insights and Therapeutic Opportunities. Cells, 12(15), 1930. https://doi.org/10.3390/cells12151930




