Targeting Soluble Guanylyl Cyclase during Ischemia and Reperfusion
Abstract
1. Introduction
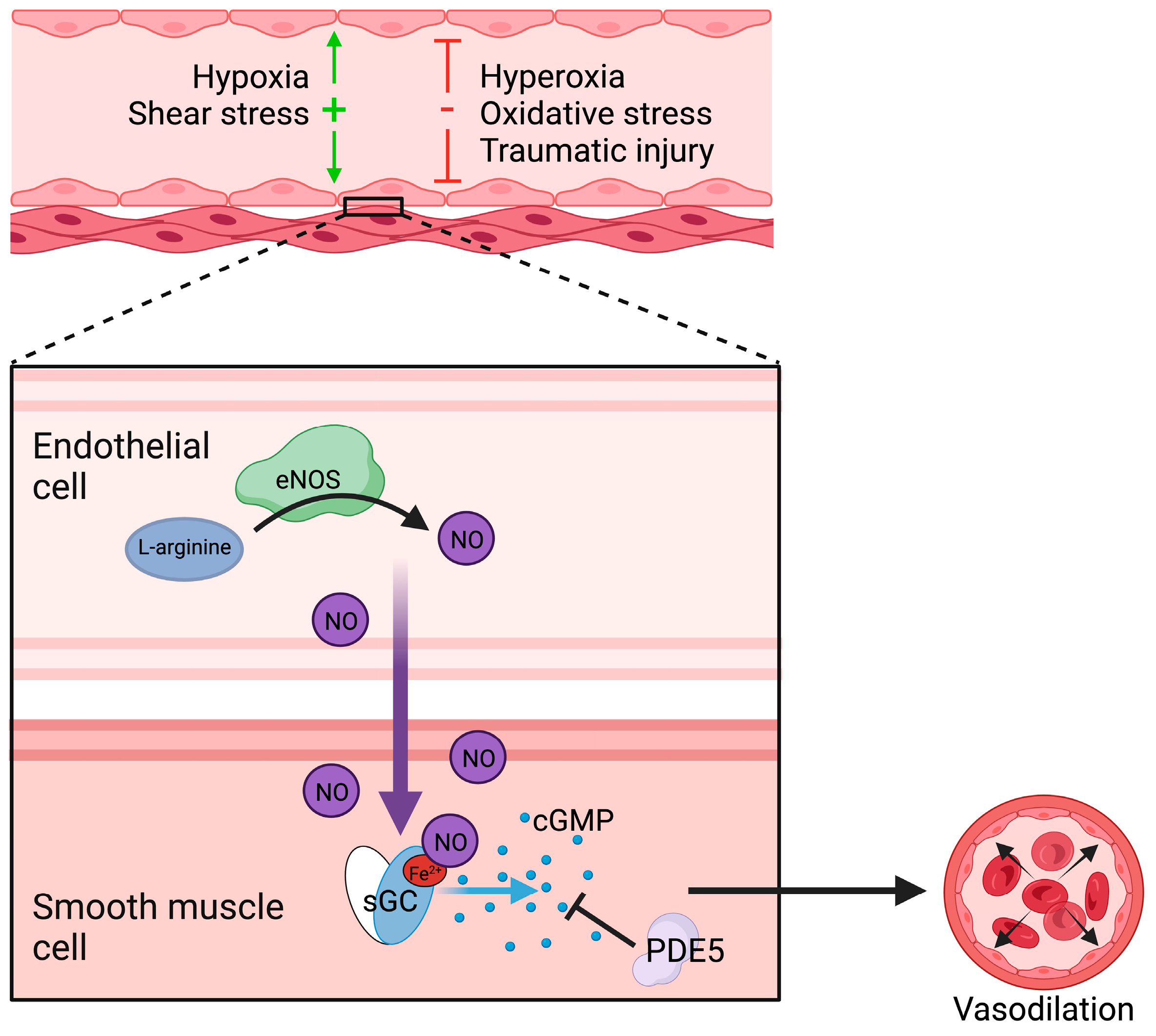
2. Regulation of Vasodilation
2.1. Nitric Oxide Synthesis and Function
2.2. Soluble Guanylyl Cyclase Regulation and Function
3. Vascular Dysfunction in Ischemia and Reperfusion
3.1. Endothelial Responses to Ischemia-Reperfusion
3.2. Impact of Endothelial Dysfunction on Organ Injury
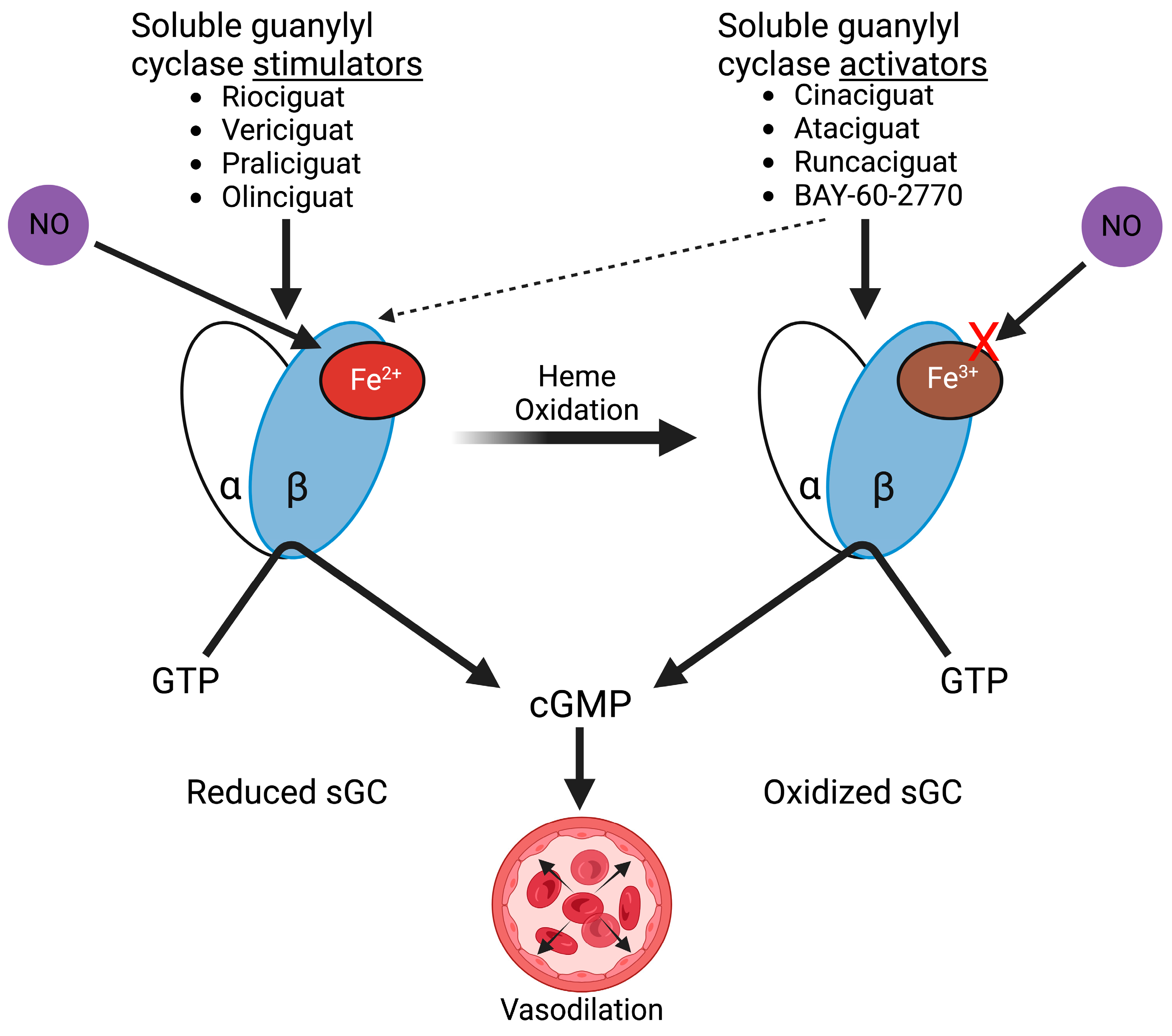
4. Targeting Soluble Guanylyl Cyclase in Ischemia-Reperfusion
Soluble Guanylyl Cyclase Stimulators and Activators
5. Protective Effects of sGC Stimulation and Activation
5.1. Cardiac Protection
5.2. Cerebral Protection
5.3. Renal, Pulmonary, and Vascular Protection
5.4. Soluble Guanylyl Cyclase Stimulation in Humans and Future Potential Uses
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, H.; Zuo, X.; Zhang, J.; Liu, X.; Liu, L.; Xu, Q.; Wu, Z.; Ji, A. A-lipoic Acid Protects against Cerebral Ischemia/Reperfusion-induced Injury in Rats. Mol. Med. Rep. 2015, 11, 3659–3665. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Ye, X.; Hao, Q.; Zhang, T.; Cui, G.; Yu, M. Nrf2/ARE Pathway Inhibits ROS-Induced NLRP3 Inflammasome Activation in BV2 Cells after Cerebral Ischemia Reperfusion. Inflamm. Res. 2018, 67, 57–65. [Google Scholar] [CrossRef]
- Pomerantz, B.J.; Reznikov, L.L.; Harken, A.H.; Dinarello, C.A. Inhibition of Caspase 1 Reduces Human Myocardial Ischemic Dysfunction via Inhibition of IL-18 and IL-1beta. Proc. Natl. Acad. Sci. USA 2001, 98, 2871–2876. [Google Scholar] [CrossRef]
- Webb, A.; Bond, R.; McLean, P.; Uppal, R.; Benjamin, N.; Ahluwalia, A. Reduction of Nitrite to Nitric Oxide during Ischemia Protects against Myocardial Ischemia–Reperfusion Damage. Proc. Natl. Acad. Sci. USA 2004, 101, 13683–13688. [Google Scholar] [CrossRef]
- Kanno, S.; Lee, P.C.; Zhang, Y.; Ho, C.; Griffith, B.P.; Shears, L.L.; Billiar, T.R. Attenuation of Myocardial Ischemia/Reperfusion Injury by Superinduction of Inducible Nitric Oxide Synthase. Circulation 2000, 101, 2742–2748. [Google Scholar] [CrossRef]
- Meade, M.O.; Granton, J.T.; Matte-Martyn, A.; McRae, K.; Weaver, B.; Cripps, P.; Keshavjee, S.H. A Randomized Trial of Inhaled Nitric Oxide to Prevent Ischemia–Reperfusion Injury after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2003, 167, 1483–1489. [Google Scholar] [CrossRef]
- Follmann, M.; Ackerstaff, J.; Redlich, G.; Wunder, F.; Lang, D.; Kern, A.; Fey, P.; Griebenow, N.; Kroh, W.; Becker-Pelster, E.-M.; et al. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J. Med. Chem. 2017, 60, 5146–5161. [Google Scholar] [CrossRef]
- Mittendorf, J.; Weigand, S.; Alonso-Alija, C.; Bischoff, E.; Feurer, A.; Gerisch, M.; Kern, A.; Knorr, A.; Lang, D.; Muenter, K.; et al. Discovery of Riociguat (BAY 63-2521): A Potent, Oral Stimulator of Soluble Guanylate Cyclase for the Treatment of Pulmonary Hypertension. ChemMedChem 2009, 4, 853–865. [Google Scholar] [CrossRef]
- Ko, F.N.; Wu, C.C.; Kuo, S.C.; Lee, F.Y.; Teng, C.M. YC-1, a Novel Activator of Platelet Guanylate Cyclase. Blood 1994, 84, 4226–4233. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Carvalho, M.H.; Khan, M.T.; Matsunaga, K. Evidence for Endothelium-Dependent Vasodilation of Resistance Vessels by Acetylcholine. Blood Vessel. 2008, 24, 145–149. [Google Scholar] [CrossRef]
- Rapoport, R.M.; Murad, F. Agonist-Induced Endothelium-Dependent Relaxation in Rat Thoracic Aorta May Be Mediated through CGMP. Circ. Res. 1983, 52, 352–357. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Jackson, W.F.; Konig, A.; Dambacher, T.; Busse, R. Prostacyclin-Induced Vasodilation in Rabbit Heart is Mediated by ATP-Sensitive Potassium Channels. Am. J. Physiol. Heart Circ. Physiol. 1993, 264, H238–H243. [Google Scholar] [CrossRef]
- Wilson, J.R.; Kapoor, S.C. Contribution of Prostaglandins to Exercise-Induced Vasodilation in Humans. Am. J. Physiol. Heart Circ. Physiol. 1993, 265, H171–H175. [Google Scholar] [CrossRef]
- Bache, R.J.; Dai, X.Z.; Schwartz, J.S.; Homans, D.C. Role of Adenosine in Coronary Vasodilation during Exercise. Circ. Res. 1988, 62, 846–853. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-Derived Relaxing Factor Produced and Released from Artery and Vein Is Nitric Oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Rees, D.D.; Ashton, D.S.; Moncada, S. L-Arginine is the Physiological Precursor for the Formation of Nitric Oxide in Endothelium-Dependent Relaxation. Biochem. Biophys. Res. Commun. 1988, 153, 1251–1256. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Heymes, C.; Benessiano, J.; Geske, R.S.; Lévy, B.I.; Vanhoutte, P.M. Neuronal Nitric Oxide Synthase is Expressed in Rat Vascular Smooth Muscle Cells: Activation by Angiotensin II in Hypertension. Circ. Res. 1998, 83, 1271–1278. [Google Scholar] [CrossRef]
- Balligand, J.L.; Ungureanu-Longrois, D.; Simmons, W.W.; Kobzik, L.; Lowenstein, C.J.; Lamas, S.; Kelly, R.A.; Smith, T.W.; Michel, T. Induction of NO Synthase in Rat Cardiac Microvascular Endothelial Cells by IL-1 Beta and IFN-Gamma. Am. J. Physiol. Heart Circ. Physiol. 1995, 268, H1293–H1303. [Google Scholar] [CrossRef]
- Balligand, J.L.; Ungureanu-Longrois, D.; Simmons, W.W.; Pimental, D.; Malinski, T.A.; Kapturczak, M.; Taha, Z.; Lowenstein, C.J.; Davidoff, A.J.; Kelly, R.A. Cytokine-Inducible Nitric Oxide Synthase (INOS) Expression in Cardiac Myocytes. Characterization and Regulation of INOS Expression and Detection of INOS Activity in Single Cardiac Myocytes in Vitro. J. Biol. Chem. 1994, 269, 27580–27588. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Schmidt, H.H.; Pollock, J.S.; Sheng, H.; Mitchell, J.A.; Warner, T.D.; Nakane, M.; Murad, F. Isoforms of Nitric Oxide Synthase. Characterization and Purification from Different Cell Types. Biochem. Pharmacol. 1991, 42, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [PubMed]
- Andrew, P. Enzymatic Function of Nitric Oxide Synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Searles, C.D. Transcriptional and Posttranscriptional Regulation of Endothelial Nitric Oxide Synthase Expression. Am. J. Physiol. Cell Physiol. 2006, 291, C803–C816. [Google Scholar] [CrossRef]
- Aoyagi, M.; Arvai, A.S.; Tainer, J.A.; Getzoff, E.D. Structural Basis for Endothelial Nitric Oxide Synthase Binding to Calmodulin. EMBO J. 2003, 22, 766–775. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide Anions and Hyperoxia Inactivate Endothelium-Derived Relaxing Factor. Am. J. Physiol. Heart Circ. Physiol. 1986, 250, H822–H827. [Google Scholar] [CrossRef]
- Wennmalm, A.; Benthin, G.; Edlund, A.; Jungersten, L.; Kieler-Jensen, N.; Lundin, S.; Westfelt, U.N.; Petersson, A.S.; Waagstein, F. Metabolism and Excretion of Nitric Oxide in Humans. An Experimental and Clinical Study. Circ. Res. 1993, 73, 1121–1127. [Google Scholar] [CrossRef]
- Espey, M.G.; Miranda, K.M.; Thomas, D.D.; Xavier, S.; Citrin, D.; Vitek, M.P.; Wink, D.A. A Chemical Perspective on the Interplay Between NO, Reactive Oxygen Species, and Reactive Nitrogen Oxide Species. Ann. N. Y. Acad. Sci. 2002, 962, 195–206. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, R.; Wu, J.-X.; Chen, L. Structural Insights into the Mechanism of Human Soluble Guanylate Cyclase. Nature 2019, 574, 206–210. [Google Scholar] [CrossRef]
- Liu, R.; Kang, Y.; Chen, L. Activation Mechanism of Human Soluble Guanylate Cyclase by Stimulators and Activators. Nat. Commun. 2021, 12, 5492. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Heme-Dependent Activation of Cytosolic Guanylate Cyclase by L-Arginine-Derived Nitric Oxide Represents a Novel Transduction Mechanism for Transcellular Signaling. Eur. J. Pharmacol. 1990, 183, 1624–1625. [Google Scholar] [CrossRef]
- Cornwell, T.L.; Lincoln, T.M. Regulation of Intracellular Ca2+ Levels in Cultured Vascular Smooth Muscle Cells. Reduction of Ca2+ by Atriopeptin and 8-Bromo-Cyclic GMP Is Mediated by Cyclic GMP-Dependent Protein Kinase. J. Biol. Chem. 1989, 264, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Li, L.; Kitazawa, T. Cyclic GMP Causes Ca2+ Desensitization in Vascular Smooth Muscle by Activating the Myosin Light Chain Phosphatase. J. Biol. Chem. 1997, 272, 5063–5068. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Flierl, U.; Kobsar, A.; Eigenthaler, M.; Ertl, G.; Bauersachs, J. Soluble Guanylyl Cyclase Activation With HMR1766 Attenuates Platelet Activation in Diabetic Rats. ATVB 2006, 26, 2813–2818. [Google Scholar] [CrossRef]
- Tiefenbacher, C.P.; Chilian, W.M.; Mitchell, M.; DeFily, D.V. Restoration of Endothelium-Dependent Vasodilation after Reperfusion Injury by Tetrahydrobiopterin. Circulation 1996, 94, 1423–1429. [Google Scholar] [CrossRef]
- Yassin, M.M.; Harkin, D.W.; D’Sa, A.A.B.B.; Halliday, M.I.; Rowlands, B.J. Lower Limb Ischemia-Reperfusion Injury Triggers a Systemic Inflammatory Response and Multiple Organ Dysfunction. World J. Surg. 2002, 26, 115–121. [Google Scholar] [CrossRef]
- Ward, B.; Mccarthy, A. Endothelial Cell “Swelling” in Ischaemia and Reperfusion. J. Mol. Cell. Cardiol. 1995, 27, 1293–1300. [Google Scholar] [CrossRef]
- Bychkov, R.; Pieper, K.; Ried, C.; Milosheva, M.; Bychkov, E.; Luft, F.C.; Haller, H. Hydrogen Peroxide, Potassium Currents, and Membrane Potential in Human Endothelial Cells. Circulation 1999, 99, 1719–1725. [Google Scholar] [CrossRef]
- Cantara, S.; Donnini, S.; Giachetti, A.; Thorpe, P.E.; Ziche, M. Exogenous BH4/Bcl-2 Peptide Reverts Coronary Endothelial Cell Apoptosis Induced by Oxidative Stress. J. Vasc. Res. 2004, 41, 202–207. [Google Scholar] [CrossRef]
- Hollander, M.R.; de Waard, G.A.; Konijnenberg, L.S.F.; Meijer-van Putten, R.M.E.; van den Brom, C.E.; Paauw, N.; de Vries, H.E.; van de Ven, P.M.; Aman, J.; Van Nieuw Amerongen, G.P.; et al. Correction: Dissecting the Effects of Ischemia and Reperfusion on the Coronary Microcirculation in a Rat Model of Acute Myocardial Infarction. PLoS ONE 2016, 11, e0166809. [Google Scholar] [CrossRef]
- Tsao, P.S.; Aoki, N.; Lefer, D.J.; Johnson, G.; Lefer, A.M. Time Course of Endothelial Dysfunction and Myocardial Injury during Myocardial Ischemia and Reperfusion in the Cat. Circulation 1990, 82, 1402–1412. [Google Scholar] [CrossRef]
- Ma, X.L.; Weyrich, A.S.; Lefer, D.J.; Lefer, A.M. Diminished Basal Nitric Oxide Release after Myocardial Ischemia and Reperfusion Promotes Neutrophil Adherence to Coronary Endothelium. Circ. Res. 1993, 72, 403–412. [Google Scholar] [CrossRef]
- Tsao, P.S.; Lefer, A.M. Time Course and Mechanism of Endothelial Dysfunction in Isolated Ischemic- and Hypoxic-Perfused Rat Hearts. Am. J. Physiol. Heart Circ. Physiol. 1990, 259, H1660–H1666. [Google Scholar] [CrossRef]
- Landmesser, U.; Cai, H.; Dikalov, S.; McCann, L.; Hwang, J.; Jo, H.; Holland, S.M.; Harrison, D.G. Role of P47phox in Vascular Oxidative Stress and Hypertension Caused by Angiotensin II. Hypertension 2002, 40, 511–515. [Google Scholar] [CrossRef]
- Mollnau, H.; Wendt, M.; Szöcs, K.; Lassègue, B.; Schulz, E.; Oelze, M.; Li, H.; Bodenschatz, M.; August, M.; Kleschyov, A.L.; et al. Effects of Angiotensin II Infusion on the Expression and Function of NAD(P)H Oxidase and Components of Nitric Oxide/CGMP Signaling. Circ. Res. 2002, 90, e58–e65. [Google Scholar] [CrossRef]
- Stasch, J.-P.; Schmidt, P.M.; Nedvetsky, P.I.; Nedvetskaya, T.Y.; Hs, A.K.; Meurer, S.; Deile, M.; Taye, A.; Knorr, A.; Lapp, H.; et al. Targeting the Heme-Oxidized Nitric Oxide Receptor for Selective Vasodilatation of Diseased Blood Vessels. J. Clin. Investig. 2006, 116, 2552–2561. [Google Scholar] [CrossRef]
- Kokura, S.; Wolf, R.E.; Yoshikawa, T.; Granger, D.N.; Aw, T.Y. Molecular Mechanisms of Neutrophil-Endothelial Cell Adhesion Induced by Redox Imbalance. Circ. Res. 1999, 84, 516–524. [Google Scholar] [CrossRef]
- Fukushima, S.; Coppen, S.R.; Varela-Carver, A.; Yamahara, K.; Sarathchandra, P.; Smolenski, R.T.; Yacoub, M.H.; Suzuki, K. A Novel Strategy for Myocardial Protection by Combined Antibody Therapy Inhibiting Both P-Selectin and Intercellular Adhesion Molecule-1 via Retrograde Intracoronary Route. Circulation 2006, 114, I-251–I-256. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. ATVB 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-Invasive Detection of Endothelial Dysfunction in Children and Adults at Risk of Atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Banitt, P.F.; Smits, P.; Williams, S.B.; Ganz, P.; Creager, M.A. Activation of ATP-Sensitive Potassium Channels Contributes to Reactive Hyperemia in Humans. Am. J. Physiol. Heart Circ. Physiol. 1996, 271, H1594–H1598. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in the Framingham Heart Study. Circulation 2008, 117, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kopel, T.; Kaufman, J.S.; Hamburg, N.; Sampalis, J.S.; Vita, J.A.; Dember, L.M. Endothelium-Dependent and -Independent Vascular Function in Advanced Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1588–1594. [Google Scholar] [CrossRef]
- Wu, T.-C.; Chen, Y.-H.; Chen, J.-W.; Chen, L.-C.; Lin, S.-J.; Ding, P.Y.-A.; Wang, S.-P.; Chang, M.-S. Impaired Forearm Reactive Hyperemia is Related to Late Restenosis after Coronary Stenting. Am. J. Cardiol. 2000, 85, 1071–1076. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Lerman, A. Endothelial Dysfunction and Coronary Artery Disease: Assessment, Prognosis, and Treatment. Coron. Artery Dis. 2014, 25, 713–724. [Google Scholar] [CrossRef]
- Anderson, T.J.; Uehata, A.; Gerhard, M.D.; Meredith, I.T.; Knab, S.; Delagrange, D.; Lieberman, E.H.; Ganz, P.; Creager, M.A.; Yeung, A.C.; et al. Close Relation of Endothelial Function in the Human Coronary and Peripheral Circulations. J. Am. Coll. Cardiol. 1995, 26, 1235–1241. [Google Scholar] [CrossRef]
- Takase, B.; Uehata, A.; Akima, T.; Nagai, T.; Nishioka, T.; Hamabe, A.; Satomura, K.; Ohsuzu, F.; Kurita, A. Endothelium-Dependent Flow-Mediated Vasodilation in Coronary and Brachial Arteries in Suspected Coronary Artery Disease. Am. J. Cardiol. 1998, 82, 1535–1539. [Google Scholar] [CrossRef]
- Yeboah, J.; Crouse, J.R.; Hsu, F.-C.; Burke, G.L.; Herrington, D.M. Brachial Flow-Mediated Dilation Predicts Incident Cardiovascular Events in Older Adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Méndez-Carmona, N.; Wyss, R.K.; Arnold, M.; Joachimbauer, A.; Segiser, A.; Fiedler, G.M.; Carrel, T.P.; Tevaearai Stahel, H.T.; Longnus, S.L. Differential Effects of Ischemia/Reperfusion on Endothelial Function and Contractility in Donation after Circulatory Death. J. Heart Lung Transplant. 2019, 38, 767–777. [Google Scholar] [CrossRef]
- Guensch, D.P.; Fischer, K.; Shie, N.; Lebel, J.; Friedrich, M.G. Hyperoxia Exacerbates Myocardial Ischemia in the Presence of Acute Coronary Artery Stenosis in Swine. Circ. Cardiovasc. Interv. 2015, 8, e002928. [Google Scholar] [CrossRef]
- Mace, E.H.; Kimlinger, M.J.; No, T.J.; Dikalov, S.I.; Hennessy, C.; Shotwell, M.S.; Billings, F.T.; Lopez, M.G. Soluble guanylyl cyclase activation rescues hyperoxia-induced dysfunction of vascular relaxation. Shock 2022, 58, 280–286. [Google Scholar] [CrossRef]
- Brueckl, C.; Kaestle, S.; Kerem, A.; Habazettl, H.; Krombach, F.; Kuppe, H.; Kuebler, W.M. Hyperoxia-Induced Reactive Oxygen Species Formation in Pulmonary Capillary Endothelial Cells in Situ. Am. J. Respir. Cell Mol. Biol. 2006, 34, 453–463. [Google Scholar] [CrossRef]
- Fessel, J.P.; Flynn, C.R.; Robinson, L.J.; Penner, N.L.; Gladson, S.; Kang, C.J.; Wasserman, D.H.; Hemnes, A.R.; West, J.D. Hyperoxia Synergizes with Mutant Bone Morphogenic Protein Receptor 2 to Cause Metabolic Stress, Oxidant Injury, and Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 49, 778–787. [Google Scholar] [CrossRef]
- Gao, Z.; Spilk, S.; Momen, A.; Muller, M.D.; Leuenberger, U.A.; Sinoway, L.I. Vitamin C Prevents Hyperoxia-Mediated Coronary Vasoconstriction and Impairment of Myocardial Function in Healthy Subjects. Eur. J. Appl. Physiol. 2012, 112, 483–492. [Google Scholar] [CrossRef]
- Hughes, C.G.; Morandi, A.; Girard, T.D.; Riedel, B.; Thompson, J.L.; Shintani, A.K.; Pun, B.T.; Ely, E.W.; Pandharipande, P.P. Association between Endothelial Dysfunction and Acute Brain Dysfunction during Critical Illness. Anesthesiology 2013, 118, 631–639. [Google Scholar] [CrossRef]
- Hughes, C.G.; Pandharipande, P.P.; Thompson, J.L.; Chandrasekhar, R.; Ware, L.B.; Ely, E.W.; Girard, T.D. Endothelial Activation and Blood-Brain Barrier Injury as Risk Factors for Delirium in Critically Ill Patients. Crit. Care Med. 2016, 44, e809–e817. [Google Scholar] [CrossRef]
- Lopez, M.G.; Hughes, C.G.; DeMatteo, A.; O’Neal, J.B.; McNeil, J.B.; Shotwell, M.S.; Morse, J.; Petracek, M.R.; Shah, A.S.; Brown, N.J.; et al. Intraoperative Oxidative Damage and Delirium Following Cardiac Surgery. Anesthesiology 2020, 132, 551–561. [Google Scholar] [CrossRef]
- Lopez, M.G.; Pretorius, M.; Shotwell, M.S.; Deegan, R.; Eagle, S.S.; Bennett, J.M.; Sileshi, B.; Liang, Y.; Gelfand, B.J.; Kingeter, A.J.; et al. The Risk of Oxygen during Cardiac Surgery (ROCS) Trial: Study Protocol for a Randomized Clinical Trial. Trials 2017, 18, 295. [Google Scholar] [CrossRef]
- Hu, X.; De Silva, T.M.; Chen, J.; Faraci, F.M. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ. Res. 2017, 120, 449–471. [Google Scholar] [CrossRef]
- Santos-García, D.; Blanco, M.; Serena, J.; Arias, S.; Millán, M.; Rodríguez-Yáñez, M.; Leira, R.; Dávalos, A.; Castillo, J. Brachial Arterial Flow Mediated Dilation in Acute Ischemic Stroke. Eur. J. Neurol. 2009, 16, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.K.; Oka, F.; Kim, J.H.; Atochin, D.; Huang, P.L.; Ayata, C. Endothelial Dysfunction Abrogates the Efficacy of Normobaric Hyperoxia in Stroke. J. Neurosci. 2014, 34, 15200–15207. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Zhang, J.H.; Nanda, A.; Granger, D.N. Inflammatory Responses to Ischemia and Reperfusion in the Cerebral Microcirculation. Front. Biosci. 2004, 9, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, N.V.; Verin, A.D. The Role of Cytoskeleton in the Regulation of Vascular Endothelial Barrier Function. Microvasc. Res. 2008, 76, 202. [Google Scholar] [CrossRef]
- Yilmaz, G.; Granger, D.N. Cell Adhesion Molecules and Ischemic Stroke. Neurol. Res. 2008, 30, 783–793. [Google Scholar] [CrossRef]
- Elkins, J.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Chang, I.; Muralidharan, K.; et al. Safety and Efficacy of Natalizumab in Patients with Acute Ischaemic Stroke (ACTION): A Randomised, Placebo-Controlled, Double-Blind Phase 2 Trial. Lancet Neurol. 2017, 16, 217–226. [Google Scholar] [CrossRef]
- Elkind, M.S.V.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Kasliwal, R.; Elkins, J. Natalizumab in Acute Ischemic Stroke (ACTION II): A Randomized, Placebo-Controlled Trial. Neurology 2020, 95, e1091–e1104. [Google Scholar] [CrossRef]
- Kryl’skii, E.D.; Popova, T.N.; Safonova, O.A.; Stolyarova, A.O.; Razuvaev, G.A.; de Carvalho, M.A.P. Transcriptional Regulation of Antioxidant Enzymes Activity and Modulation of Oxidative Stress by Melatonin in Rats Under Cerebral Ischemia/Reperfusion Conditions. Neuroscience 2019, 406, 653–666. [Google Scholar] [CrossRef]
- Li, X.-S.; Uriuda, Y.; Wang, Q.-D.; Nordlander, R.; Sjöquist, P.-O.; Pernow, J. Role of L-Arginine in Preventing Myocardial and Endothelial Injury Following Ischaemia/Reperfusion in the Rat Isolated Heart. Acta Physiol. Scand. 1996, 156, 37–44. [Google Scholar] [CrossRef]
- Pernow, J.; Uriuda, Y.; Wang, Q.-D.; Li, X.-S.; Nordlander, R.; Rydeén, L. The Protective Effect of L-Arginine on Myocardial Injury and Endothelial Function Following Ischaemia and Reperfusion in the Pig. Eur. Heart J. 1994, 15, 1712–1719. [Google Scholar] [CrossRef]
- Siegfried, M.R.; Erhardt, J.; Rider, T.; Ma, X.L.; Lefer, A.M. Cardioprotection and Attenuation of Endothelial Dysfunction by Organic Nitric Oxide Donors in Myocardial Ischemia-Reperfusion. J. Pharmacol. Exp. Ther. 1992, 260, 668–675. [Google Scholar]
- Bice, J.S.; Keim, Y.; Stasch, J.-P.; Baxter, G.F. NO-Independent Stimulation or Activation of Soluble Guanylyl Cyclase during Early Reperfusion Limits Infarct Size. Cardiovasc. Res. 2014, 101, 220–228. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Mülsch, A. Explaining the Phenomenon of Nitrate Tolerance. Circ. Res. 2005, 97, 618–628. [Google Scholar] [CrossRef]
- Smith, R.P.; Kruszyna, H. Nitroprusside Produces Cyanide Poisoning via Reaction with Hemoglobin. J. Pharmacol. Exp. Ther. 1974, 191, 557–563. [Google Scholar]
- Bower, P.J.; Peterson, J.N. Methemoglobinemia after Sodium Nitroprusside Therapy. N. Engl. J. Med. 1975, 293, 865. [Google Scholar] [CrossRef]
- Wang-Rosenke, Y.; Mika, A.; Khadzhynov, D.; Loof, T.; Neumayer, H.-H.; Peters, H. Impact of Biological Gender and Soluble Guanylate Cyclase Stimulation on Renal Recovery After Relief of Unilateral Ureteral Obstruction. J. Urol. 2012, 188, 316–323. [Google Scholar] [CrossRef]
- Salloum, F.N.; Takenoshita, Y.; Ockaili, R.A.; Daoud, V.P.; Chou, E.; Yoshida, K.; Kukreja, R.C. Sildenafil and Vardenafil but Not Nitroglycerin Limit Myocardial Infarction through Opening of Mitochondrial KATP Channels When Administered at Reperfusion Following Ischemia in Rabbits. J. Mol. Cell. Cardiol. 2007, 42, 453–458. [Google Scholar] [CrossRef]
- Guazzi, M.; Vicenzi, M.; Arena, R.; Guazzi, M.D. PDE5 Inhibition with Sildenafil Improves Left Ventricular Diastolic Function, Cardiac Geometry, and Clinical Status in Patients with Stable Systolic Heart Failure: Results of a 1-Year, Prospective, Randomized, Placebo-Controlled Study. Circ. Heart Fail. 2011, 4, 8–17. [Google Scholar] [CrossRef]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; LeWinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.L.; et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2013, 309, 1268. [Google Scholar] [CrossRef]
- Andersen, M.J.; Ersbøll, M.; Axelsson, A.; Gustafsson, F.; Hassager, C.; Køber, L.; Borlaug, B.A.; Boesgaard, S.; Skovgaard, L.T.; Møller, J.E. Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction in Patients with Preserved Ejection Fraction: The Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction (SIDAMI) Trial. Circulation 2013, 127, 1200–1208. [Google Scholar] [CrossRef]
- Kumar, T.; Aujla, H.; Woźniak, M.; Dott, W.; Sullo, N.; Joel-David, L.; Pais, P.; Smallwood, D.; Miller, D.; Eagle-Hemming, B.; et al. Intravenous Sildenafil Citrate and Post-Cardiac Surgery Acute Kidney Injury: A Double-Blind, Randomised, Placebo-Controlled Trial. Br. J. Anaesth. 2020, 124, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.-P.; Pacher, P.; Evgenov, O.V. Soluble Guanylate Cyclase as an Emerging Therapeutic Target in Cardiopulmonary Disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Geschka, S.; Kretschmer, A.; Sharkovska, Y.; Evgenov, O.V.; Lawrenz, B.; Hucke, A.; Hocher, B.; Stasch, J.-P. Soluble Guanylate Cyclase Stimulation Prevents Fibrotic Tissue Remodeling and Improves Survival in Salt-Sensitive Dahl Rats. PLoS ONE 2011, 6, e21853. [Google Scholar] [CrossRef]
- Korkmaz, S.; Radovits, T.; Barnucz, E.; Hirschberg, K.; Neugebauer, P.; Loganathan, S.; Veres, G.; Páli, S.; Seidel, B.; Zöllner, S.; et al. Pharmacological Activation of Soluble Guanylate Cyclase Protects the Heart Against Ischemic Injury. Circulation 2009, 120, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Frankenreiter, S.; Groneberg, D.; Kuret, A.; Krieg, T.; Ruth, P.; Friebe, A.; Lukowski, R. Cardioprotection by Ischemic Postconditioning and Cyclic Guanosine Monophosphate-Elevating Agents Involves Cardiomyocyte Nitric Oxide-Sensitive Guanylyl Cyclase. Cardiovasc. Res. 2018, 114, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Salloum, F.N.; Das, A.; Samidurai, A.; Hoke, N.N.; Chau, V.Q.; Ockaili, R.A.; Stasch, J.-P.; Kukreja, R.C. Cinaciguat, a Novel Activator of Soluble Guanylate Cyclase, Protects against Ischemia/Reperfusion Injury: Role of Hydrogen Sulfide. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1347–H1354. [Google Scholar] [CrossRef]
- Methner, C.; Buonincontri, G.; Hu, C.-H.; Vujic, A.; Kretschmer, A.; Sawiak, S.; Carpenter, A.; Stasch, J.-P.; Krieg, T. Riociguat Reduces Infarct Size and Post-Infarct Heart Failure in Mouse Hearts: Insights from MRI/PET Imaging. PLoS ONE 2013, 8, e83910. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, B.; Shalamu, A.; Gao, T.; Ge, J. Soluble Guanylate Cyclase (SGC) Stimulator Vericiguat Alleviates Myocardial Ischemia-Reperfusion Injury by Improving Microcirculation. Ann. Transl. Med. 2022, 10, 662. [Google Scholar] [CrossRef]
- Radovits, T.; Korkmaz, S.; Miesel-Gröschel, C.; Seidel, B.; Stasch, J.-P.; Merkely, B.; Karck, M.; Szabó, G. Pre-Conditioning with the Soluble Guanylate Cyclase Activator Cinaciguat Reduces Ischaemia–Reperfusion Injury after Cardiopulmonary Bypass. Eur. J. Cardio Thorac. Surg. 2011, 39, 248–255. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.-R.; Cho, H.; Woo, J.S.; Kang, J.H.; Jeong, Y.-M.; Cheng, X.W.; Kim, W.-S.; Kim, W. Cardioprotective Effects of PKG Activation by Soluble GC Activator, BAY 60-2770, in Ischemia-Reperfusion-Injured Rat Hearts. PLoS ONE 2017, 12, e0180207. [Google Scholar] [CrossRef]
- Kasseckert, S.A.; Schafer, C.; Kluger, A.; Gligorievski, D.; Tillmann, J.; Schluter, K.-D.; Noll, T.; Sauer, H.; Piper, H.M.; Abdallah, Y. Stimulation of CGMP Signalling Protects Coronary Endothelium against Reperfusion-Induced Intercellular Gap Formation. Cardiovasc. Res. 2009, 83, 381–387. [Google Scholar] [CrossRef]
- Langhauser, F.; Casas, A.I.; Dao, V.-T.-V.; Guney, E.; Menche, J.; Geuss, E.; Kleikers, P.W.M.; López, M.G.; Barabási, A.-L.; Kleinschnitz, C.; et al. A Diseasome Cluster-Based Drug Repurposing of Soluble Guanylate Cyclase Activators from Smooth Muscle Relaxation to Direct Neuroprotection. NPJ Syst. Biol. Appl. 2018, 4, 8. [Google Scholar] [CrossRef]
- Correia, S.S.; Liu, G.; Jacobson, S.; Bernier, S.G.; Tobin, J.V.; Schwartzkopf, C.D.; Atwater, E.; Lonie, E.; Rivers, S.; Carvalho, A.; et al. The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CYR119 Attenuates Markers of Inflammation in the Central Nervous System. J. Neuroinflammation 2021, 18, 213. [Google Scholar] [CrossRef]
- Hall, K.C.; Bernier, S.G.; Jacobson, S.; Liu, G.; Zhang, P.Y.; Sarno, R.; Catanzano, V.; Currie, M.G.; Masferrer, J.L. SGC Stimulator Praliciguat Suppresses Stellate Cell Fibrotic Transformation and Inhibits Fibrosis and Inflammation in Models of NASH. Proc. Natl. Acad. Sci. USA 2019, 116, 11057–11062. [Google Scholar] [CrossRef]
- Foussard, N.; Rouault, P.; Cornuault, L.; Reynaud, A.; Buys, E.S.; Chapouly, C.; Gadeau, A.-P.; Couffinhal, T.; Mohammedi, K.; Renault, M.-A. Praliciguat Promotes Ischemic Leg Reperfusion in Leptin Receptor-Deficient Mice. Circ. Res. 2023, 132, 34–48. [Google Scholar] [CrossRef]
- Egemnazarov, B.; Sydykov, A.; Schermuly, R.T.; Weissmann, N.; Stasch, J.-P.; Sarybaev, A.S.; Seeger, W.; Grimminger, F.; Ghofrani, H.A. Novel Soluble Guanylyl Cyclase Stimulator BAY 41-2272 Attenuates Ischemia-Reperfusion-Induced Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L462–L469. [Google Scholar] [CrossRef]
- Costell, M.H.; Ancellin, N.; Bernard, R.E.; Zhao, S.; Upson, J.J.; Morgan, L.A.; Maniscalco, K.; Olzinski, A.R.; Ballard, V.L.T.; Herry, K.; et al. Comparison of Soluble Guanylate Cyclase Stimulators and Activators in Models of Cardiovascular Disease Associated with Oxidative Stress. Front. Pharmacol. 2012, 3, 128. [Google Scholar] [CrossRef]
- Tchernychev, B.; Li, H.; Lee, S.-K.; Gao, X.; Ramanarasimhaiah, R.; Liu, G.; Hall, K.C.; Bernier, S.G.; Jones, J.E.; Feil, S.; et al. Olinciguat, a Stimulator of Soluble Guanylyl Cyclase, Attenuates Inflammation, Vaso-Occlusion and Nephropathy in Mouse Models of Sickle Cell Disease. Br. J. Pharmacol. 2021, 178, 3463–3475. [Google Scholar] [CrossRef]
- Zanini, G.M.; Cabrales, P.; Barkho, W.; Frangos, J.A.; Carvalho, L.J. Exogenous Nitric Oxide Decreases Brain Vascular Inflammation, Leakage and Venular Resistance during Plasmodium Berghei ANKA Infection in Mice. J. Neuroinflammation 2011, 8, 66. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Zhang, L.; Zhang, Z.; Tsang, W.; Lu, M.; Zhang, L.; Chopp, M. Sildenafil (Viagra) Induces Neurogenesis and Promotes Functional Recovery After Stroke in Rats. Stroke 2002, 33, 2675–2680. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Lian, G.; Wang, X.; Zhang, H.; Yao, X.; Yang, J.; Wu, C. Sildenafil Attenuates LPS-Induced pro-Inflammatory Responses through down-Regulation of Intracellular ROS-Related MAPK/NF-κB Signaling Pathways in N9 Microglia. Int. Immunopharmacol. 2011, 11, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, B.; Daniel, C.; Wagner, A.; Stasch, J.-P.; Hugo, C. Stimulation of Soluble Guanylyl Cyclase Inhibits Mesangial Cell Proliferation and Matrix Accumulation in Experimental Glomerulonephritis. Am. J. Physiol. Ren. Physiol. 2005, 288, F685–F693. [Google Scholar] [CrossRef] [PubMed]
- Sharkovska, Y.; Kalk, P.; Lawrenz, B.; Godes, M.; Hoffmann, L.S.; Wellkisch, K.; Geschka, S.; Relle, K.; Hocher, B.; Stasch, J.-P. Nitric Oxide-Independent Stimulation of Soluble Guanylate Cyclase Reduces Organ Damage in Experimental Low-Renin and High-Renin Models. J. Hypertens. 2010, 28, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Grimminger, F.; Weimann, G.; Frey, R.; Voswinckel, R.; Thamm, M.; Bolkow, D.; Weissmann, N.; Muck, W.; Unger, S.; Wensing, G.; et al. First Acute Haemodynamic Study of Soluble Guanylate Cyclase Stimulator Riociguat in Pulmonary Hypertension. Eur. Respir. J. 2009, 33, 785–792. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Bonderman, D.; Ghio, S.; Felix, S.B.; Ghofrani, H.-A.; Michelakis, E.; Mitrovic, V.; Oudiz, R.J.; Boateng, F.; Scalise, A.-V.; Roessig, L.; et al. Riociguat for Patients with Pulmonary Hypertension Caused by Systolic Left Ventricular Dysfunction: A Phase IIb Double-Blind, Randomized, Placebo-Controlled, Dose-Ranging Hemodynamic Study. Circulation 2013, 128, 502–511. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Halank, M.; Wilkens, H.; Günther, A.; Weimann, G.; Gebert, I.; Leuchte, H.H.; Behr, J. Riociguat for Interstitial Lung Disease and Pulmonary Hypertension: A Pilot Trial. Eur. Respir. J. 2013, 41, 853–860. [Google Scholar] [CrossRef]
- Lapp, H.; Mitrovic, V.; Franz, N.; Heuer, H.; Buerke, M.; Wolfertz, J.; Mueck, W.; Unger, S.; Wensing, G.; Frey, R. Cinaciguat (BAY 58-2667) Improves Cardiopulmonary Hemodynamics in Patients with Acute Decompensated Heart Failure. Circulation 2009, 119, 2781–2788. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Greene, S.J.; Filippatos, G.; Erdmann, E.; Ferrari, R.; Levy, P.D.; Maggioni, A.; Nowack, C.; Mebazaa, A.; on behalf of the COMPOSE Investigators and Coordinator. Cinaciguat, a Soluble Guanylate Cyclase Activator: Results from the Randomized, Controlled, Phase IIb COMPOSE Programme in Acute Heart Failure Syndromes. Eur. J. Heart Fail. 2012, 14, 1056–1066. [Google Scholar] [CrossRef]
- Erdmann, E.; Semigran, M.J.; Nieminen, M.S.; Gheorghiade, M.; Agrawal, R.; Mitrovic, V.; Mebazaa, A. Cinaciguat, a Soluble Guanylate Cyclase Activator, Unloads the Heart but Also Causes Hypotension in Acute Decompensated Heart Failure. Eur. Heart J. 2013, 34, 57–67. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of Vericiguat vs. Placebo on Quality of Life in Patients with Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA 2020, 324, 1512. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Hoeper, M.M.; Halank, M.; Meyer, F.J.; Staehler, G.; Behr, J.; Ewert, R.; Weimann, G.; Grimminger, F. Riociguat for Chronic Thromboembolic Pulmonary Hypertension and Pulmonary Arterial Hypertension: A Phase II Study. Eur. Respir. J. 2010, 36, 792–799. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; Hoeper, M.M.; Halank, M.; Meyer, F.J.; Staehler, G.; Behr, J.; Ewert, R.; Binnen, T.; Weimann, G.; Grimminger, F. Riociguat For Chronic Thromboembolic Pulmonary Hypertension And Pulmonary Arterial Hypertension: First Long-Term Extension Data from A Phase II Study. In Proceedings of the B20. New Treatment Approaches for Lung Disease: Late Breaking Abstracts, American Thoracic Society, Washington, DC, USA, 19–24 May 2010; p. A6770. [Google Scholar]
- Hoeper, M.M.; Halank, M.; Wilkens, H.; Guenther, A.; Weimann, G.; Gebert, I.; Leuchte, H.; Behr, J. Riociguat For Patients With Pulmonary Hypertension Associated With Interstitial Lung Disease. In Proceedings of the C106. Pulmonary Hypertension in Chronic Obstructive Pulmonary Disease And Interstitial Lung Disease, American Thoracic Society, Washington, DC, USA, 19–24 May 2010; p. A5262. [Google Scholar]
- Khaybullina, D.; Patel, A.; Zerilli, T. Riociguat (Adempas): A Novel Agent for the Treatment of Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Pharm. Ther. 2014, 39, 749–758. [Google Scholar]
- Markham, A.; Duggan, S. Vericiguat: First Approval. Drugs 2021, 81, 721–726. [Google Scholar] [CrossRef]
| Citation | Drug | Model | Findings |
|---|---|---|---|
| Korkmaz et al. [94] | Cinaciguat | Global cardiac ischemia | Cinaciguat reduced oxidative stress, reduced inflammatory marker expression, improved histopathological lesions, and improved cardiac performance. |
| Korkmaz et al. [94] | Cinaciguat | Coronary oxidation injury | Cinaciguat improved endothelium-dependent relaxation and reduced vascular inflammation |
| Frankenreiter et al. [95] | Cinaciguat | Cardiac IR | Intraperitoneal cinaciguat prior to LAD clamping reduced cardiac injury in sGC WT mice but not sGC KO mice. |
| Salloum et al. [96] | Cinaciguat | Cardiac IR | sGC treatment prior to ischemia reduced infarct size more than sGC treatment during reperfusion. Cinaciguat increased PKG activity and H2S generation. |
| Methner et al. [97] | Riociguat | Cardiac IR | Riociguat reduced infarct size after LAD ligation and improved LV systolic function preservation. Effects persisted at 28 days after injury. |
| Bice et al. [82] | BAY 41-2272, BAY 60-2770 | Cardiac IR | sGC stimulators and activators reduced infarct size in rat hearts after reversible LCA occlusion, but simultaneous treatment with stimulators and activators did not further improve efficacy. eNOS inhibition attenuated the protective effect of sGC stimulation. cGMP levels did not correlate with the degree of LV protection. eNOS inhibition eliminated the rise in cGMP after sGC stimulation but did not eliminate its protective effect. |
| Cai et al. [98] | Vericiguat | Cardiac IR | sGC stimulation reduced myocardial IR injury by improving coronary microcirculation. |
| Radovitz et al. [99] | Cinaciguat | Cardiac IR | Pre-incubation of coronary rings with cinaciguat reduced peroxynitrite-induced endothelial dysfunction and restored vasodilatory responses to acetylcholine. Cinaciguat infusion prior to CPB increased LV and RV contractility recovery and increased coronary blood flow. |
| Lee et al. [100] | BAY 60-2770 | Cardiac IR | sGC activation decreased mitochondrial superoxide production and decreased myocardial injury via cGMP-activation of PKG. |
| Kasseckert et al. [101] | HMR1766 | Cardiac IR | sGC activation reduced endothelial intercellular gap formation and reduced myocardial edema. |
| Langhauser et al. [102] | BAY 60-2770, BAY 58-2667 | Cerebral IR | sGC activator treatment reduced cerebral infarct volume at 24 h in transient but not permanent MCA occlusion. It also reduced BBB leakage and increased post-stroke cerebral blood flow without affecting systemic BP. |
| Correia et al. [103] | CYR119 | Neuro-inflammation | sGC stimulator treatment reduced inflammatory gene expression and cytokine production in LPS and diet-induced neuroinflammation. |
| Geschka et al. [93] | Riociguat | Hypertension | sGC stimulation prevented renal fibrotic tissue remodeling in systemic hypertension. |
| Hall et al. [104] | Praliciguat | Cirrhosis | sGC stimulation inhibited hepatic fibrosis and inflammation in NASH cirrhosis. |
| Foussard et al. [105] | Praliciguat | Limb ischemia | sGC stimulation improved perfusion and foot function as well as increased arteriole diameter and reduced ICAM-1 expression in ischemic limb injuries. |
| Egemnazarov et al. [106] | BAY 41-2272 | Pulmonary IR | sGC stimulation reduced IR-induced lung injury by decreasing vascular permeability and ROS production. |
| Mace et al. [62] | Cinaciguat | Renal IR | Hyperoxic conditions increased superoxide levels and impaired endothelium-dependent and -independent relaxation, while sGC activation was unaffected. |
| Citation | Drug | Model | Findings |
|---|---|---|---|
| Grimminger et al. [114] | Riociguat | PH | Riociguat reduced PVR and improved PAP and cardiac index to a greater extent than inhaled NO and was well tolerated. |
| Ghofrani et al. [115] | Riociguat | CTEPH | Riociguat significantly improved exercise capacity as well as PVR, NT-proBNP level, and WHO functional class. |
| Ghofrani et al. [116] | Riociguat | PAH | Riociguat significantly improved exercise capacity as well as PVR, NT-proBNP levels, WHO functional class, time to clinical worsening, and Borg dyspnea score. |
| Bonderman et al. [117] | Riociguat | PH caused by LV systolic dysfunction | Riociguat did not affect the primary endpoint, mPAP, but it increased cardiac index and stroke volume index and decreased PVR and SVR. Riociguat improved health-related quality of life but did not affect clinical worsening events and change in functional class. |
| Hoeper et al. [118] | Riociguat | PH due to ILD | Riociguat improved cardiac output and pulmonary vascular resistance but not mPAP, 6 min walk duration, functional class, and quality of life measures. |
| Lapp et al. [119] | Cinaciguat | Acute decompensated heart failure | Cinaciguat reduced cardiac preload and afterload and increased cardiac output while preserving renal function. |
| Gheorghiade et al. [120] | Cinaciguat | Heart failure | Constant-dose cinaciguat infusions caused hypotension without improving dyspnea, cardiac index, or renal function. Trial stopped early. |
| Erdmann et al. [121] | Cinaciguat | Heart failure | Titratable-dose cinaciguat infusions caused hypotension at moderate and high doses. Trial stopped early. |
| Armstrong et al. [122] | Vericiguat | HFrEF | Vericiguat reduced the composite endpoint of all-cause mortality or hospitalization for heart failure without causing symptomatic hypotension. |
| Armstrong et al. [123] | Vericiguat | HFpEF | Vericiguat did not improve the physical limitation score compared to placebo after 24 weeks of treatment for patients with recent decompensation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mace, E.H.; Kimlinger, M.J.; Billings, F.T., IV; Lopez, M.G. Targeting Soluble Guanylyl Cyclase during Ischemia and Reperfusion. Cells 2023, 12, 1903. https://doi.org/10.3390/cells12141903
Mace EH, Kimlinger MJ, Billings FT IV, Lopez MG. Targeting Soluble Guanylyl Cyclase during Ischemia and Reperfusion. Cells. 2023; 12(14):1903. https://doi.org/10.3390/cells12141903
Chicago/Turabian StyleMace, Eric H., Melissa J. Kimlinger, Frederic T. Billings, IV, and Marcos G. Lopez. 2023. "Targeting Soluble Guanylyl Cyclase during Ischemia and Reperfusion" Cells 12, no. 14: 1903. https://doi.org/10.3390/cells12141903
APA StyleMace, E. H., Kimlinger, M. J., Billings, F. T., IV, & Lopez, M. G. (2023). Targeting Soluble Guanylyl Cyclase during Ischemia and Reperfusion. Cells, 12(14), 1903. https://doi.org/10.3390/cells12141903






