Sex Differences in Therapies against Myocardial Ischemia-Reperfusion Injury: From Basic Science to Clinical Perspectives
Abstract
1. Introduction
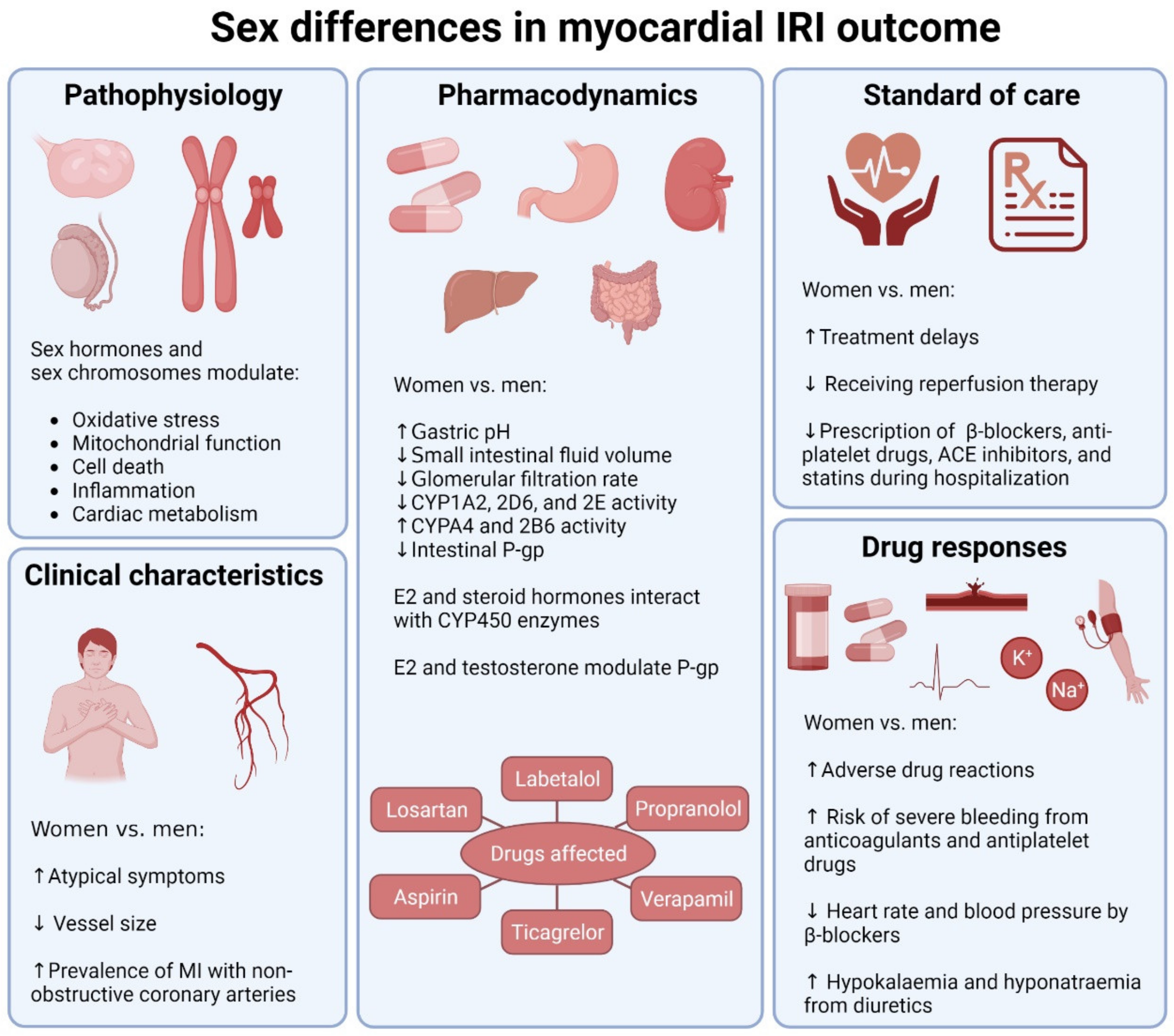
2. Pathophysiological Sex Differences in Myocardial IRI
2.1. Sex Differences in Experimental Models of Myocardial IRI
2.2. Roles of Sex Hormones
2.3. Roles of Sex Chromosomes
3. Sex Differences in Current MI Pharmacological Therapies
3.1. Sex Differences in Drug Responses
3.2. Sex Differences in Clinical Characteristics
3.3. Sex Differences in Standard of Care
4. Ongoing Clinical Trials for Drugs against Myocardial IRI: Potential Sex Differences
4.1. Drugs Targeting Oxidative Stress
4.2. Drugs Targeting Inflammation
4.3. Drugs Targeting Thrombosis
4.4. Drugs Targeting Lipid Metabolism
4.5. Drugs Targeting Glucose Metabolism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017; GBD 2017 Causes of Death Collaborators. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- George, J.; Rapsomaniki, E.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; Herrett, E.; Smeeth, L.; Timmis, A.; Hemingway, H. How Does Cardiovascular Disease First Present in Women and Men? Circulation 2015, 132, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Albrektsen, G.; Heuch, I.; Løchen, M.-L.; Thelle, D.S.; Wilsgaard, T.; Njølstad, I.; Bønaa, K.H. Lifelong Gender Gap in Risk of Incident Myocardial Infarction: The Tromsø Study. JAMA Intern. Med. 2016, 176, 1673–1679. [Google Scholar] [CrossRef]
- Leening, M.J.G.; Ferket, B.S.; Steyerberg, E.W.; Kavousi, M.; Deckers, J.W.; Nieboer, D.; Heeringa, J.; Portegies, M.L.P.; Hofman, A.; Ikram, M.A.; et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: Prospective population based cohort study. BMJ 2014, 349, g5992. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Cenko, E.; Yoon, J.; Kedev, S.; Stankovic, G.; Vasiljevic, Z.; Krljanac, G.; Kalpak, O.; Ricci, B.; Milicic, D.; Manfrini, O.; et al. Sex Differences in Outcomes After STEMI. JAMA Intern. Med. 2018, 178, 632–639. [Google Scholar] [CrossRef]
- Pancholy, S.B.; Shantha, G.P.S.; Patel, T.; Cheskin, L.J. Sex Differences in Short-term and Long-term All-Cause Mortality Among Patients With ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Intervention. JAMA Intern. Med. 2014, 174, 1822–1830. [Google Scholar] [CrossRef]
- Coughlan, J.; Räber, L.; Brugaletta, S.; Kufner, S.; Maeng, M.; Jensen, L.O.; Ortega-Paz, L.; Bär, S.; Laugwitz, K.-L.; Madsen, M.; et al. Sex Differences in 10-Year Outcomes After Percutaneous Coronary Intervention With Drug-Eluting Stents: Insights From the DECADE Cooperation. Circulation 2023, 147, 575–585. [Google Scholar] [CrossRef]
- Park, H.W.; Han, S.; Park, G.-M.; Ann, S.H.; Suh, J.; Kim, Y.-G.; Lee, S.-W.; Kim, Y.-H. Sex-related impacts on clinical outcomes after percutaneous coronary intervention. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the Decrease in U.S. Deaths from Coronary Disease, 1980–2000. N. Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, K.A.; O’flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Wang, Y.; Jones, S.B.; Leifheit-Limson, E.C.; Shaw, L.J.; Vaccarino, V.; Rumsfeld, J.S.; Krumholz, H.M.; Curtis, J.P. Age and sex differences in inhospital complication rates and mortality after percutaneous coronary intervention procedures: Evidence from the NCDR®. Am. Heart J. 2013, 167, 376–383. [Google Scholar] [CrossRef]
- Udell, J.A.; Koh, M.; Qiu, F.; Austin, P.C.; Wijeysundera, H.C.; Bagai, A.; Yan, A.T.; Goodman, S.G.; Tu, J.V.; Ko, D.T. Outcomes of Women and Men With Acute Coronary Syndrome Treated With and Without Percutaneous Coronary Revascularization. J. Am. Heart Assoc. 2017, 6, e004319. [Google Scholar] [CrossRef]
- Baehr, A.; Klymiuk, N.; Kupatt, C. Evaluating Novel Targets of Ischemia Reperfusion Injury in Pig Models. Int. J. Mol. Sci. 2019, 20, 4749. [Google Scholar] [CrossRef]
- Chi, H.-J.; Chen, M.-L.; Yang, X.-C.; Lin, X.-M.; Sun, H.; Zhao, W.-S.; Qi, D.; Dong, J.-L.; Cai, J. Progress in Therapies for Myocardial Ischemia Reperfusion Injury. Curr. Drug Targets 2017, 18, 1712–1721. [Google Scholar] [CrossRef]
- Haider, A.; Bengs, S.; Luu, J.; Osto, E.; Siller-Matula, J.M.; Muka, T.; Gebhard, C. Sex and gender in cardiovascular medicine: Presentation and outcomes of acute coronary syndrome. Eur. Heart J. 2019, 41, 1328–1336. [Google Scholar] [CrossRef]
- Palasubramaniam, J.; Wang, X.; Peter, K. Myocardial Infarction—From Atherosclerosis to Thrombosis: Uncovering New Diagnostic and Therapeutic Approaches. Arter. Thromb. Vasc. Biol. 2019, 39, e176–e185. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Eric Botker, H.; Dambrova, M.; et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J. Cell. Mol. Med. 2020, 24, 5937–5954. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Fels, J.A.; Manfredi, G. Sex Differences in Ischemia/Reperfusion Injury: The Role of Mitochondrial Permeability Transition. Neurochem. Res. 2019, 44, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Pullen, A.B.; Kain, V.; Serhan, C.N.; Halade, G.V. Molecular and Cellular Differences in Cardiac Repair of Male and Female Mice. J. Am. Heart Assoc. 2020, 9, e015672. [Google Scholar] [CrossRef]
- Ostadal, B.; Drahota, Z.; Houstek, J.; Milerova, M.; Ostadalova, I.; Hlavackova, M.; Kolar, F. Developmental and sex differences in cardiac tolerance to ischemia–reperfusion injury: The role of mitochondria. Can. J. Physiol. Pharmacol. 2019, 97, 808–814. [Google Scholar] [CrossRef]
- Ostadal, B.; Netuka, I.; Maly, J.; Besik, J.; Ostadalova, I. Gender Differences in Cardiac Ischemic Injury and Protection—Experimental Aspects. Exp. Biol. Med. 2009, 234, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Flaherty, M.P.; Wu, W.-J.; Tan, W.; Zhu, X.; Li, Q.; Bolli, R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: Results of a comprehensive analysis of determinants of infarct size in 1074 mice. Basic Res. Cardiol. 2012, 107, 1–24. [Google Scholar] [CrossRef]
- Bae, S.; Zhang, L. Gender Differences in Cardioprotection against Ischemia/Reperfusion Injury in Adult Rat Hearts: Focus on Akt and Protein Kinase C Signaling. Experiment 2005, 315, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S.; Moore, R.L.; Brown, D.A. Sex differences in myocardial infarct size are abolished by sarcolemmal KATPchannel blockade in rat. Am. J. Physiol. Circ. Physiol. 2006, 290, H2644–H2647. [Google Scholar] [CrossRef]
- Le, T.Y.L.; Ashton, A.W.; Mardini, M.; Stanton, P.G.; Funder, J.W.; Handelsman, D.J.; Mihailidou, A.S. Role of Androgens in Sex Differences in Cardiac Damage During Myocardial Infarction. Endocrinology 2014, 155, 568–575. [Google Scholar] [CrossRef]
- Bouma, W.; Noma, M.; Kanemoto, S.; Matsubara, M.; Leshnower, B.G.; Hinmon, R.; Gorman, J.H.; Gorman, R.C. Sex-related resistance to myocardial ischemia-reperfusion injury is associated with high constitutive ARC expression. Am. J. Physiol. Circ. Physiol. 2010, 298, H1510–H1517. [Google Scholar] [CrossRef]
- Colom, B.; Oliver, J.; Roca, P.; Garciapalmer, F. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc. Res. 2007, 74, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Lagranha, C.J.; Deschamps, A.; Aponte, A.; Steenbergen, C.; Murphy, E. Sex Differences in the Phosphorylation of Mitochondrial Proteins Result in Reduced Production of Reactive Oxygen Species and Cardioprotection in Females. Circ. Res. 2010, 106, 1681–1691. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L.-X.; Dong, T.; Wang, G.-Q.; Wang, L.-H.; Zhou, X.-P.; Jiang, Y.; Murao, K.; Lu, S.-Q.; Chen, J.-W.; et al. Apoptosis and autophagy contribute to gender difference in cardiac ischemia–reperfusion induced injury in rats. Life Sci. 2013, 93, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tsai, B.M.; Kher, A.; Baker, L.B.; Wairiuko, G.M.; Meldrum, D.R. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am. J. Physiol. Circ. Physiol. 2005, 288, H221–H226. [Google Scholar] [CrossRef] [PubMed]
- Sivasinprasasn, S.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. Estrogenic Impact on Cardiac Ischemic/Reperfusion Injury. J. Cardiovasc. Transl. Res. 2016, 9, 23–39. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Zhai, P.; Eurell, T.E.; Cotthaus, R.P.; Jeffery, E.H.; Bahr, J.M.; Gross, D.R.; Regitz-Zagrosek, V.; Kararigas, G.; Battiprolu, P.K.; Rodnick, K.J.; et al. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am. J. Physiol. Circ. Physiol. 2000, 279, H2766–H2775. [Google Scholar] [CrossRef]
- Puglisi, R.; Mattia, G.; Carè, A.; Marano, G.; Malorni, W.; Matarrese, P. Non-genomic Effects of Estrogen on Cell Homeostasis and Remodeling With Special Focus on Cardiac Ischemia/Reperfusion Injury. Front. Endocrinol. 2019, 10, 733. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Pan, D.; Xu, T.; Luo, Y.; Wu, W.; Wu, P.; Zhu, H.; Li, D. Estrogen inhibits endoplasmic reticulum stress and ameliorates myocardial ischemia/reperfusion injury in rats by upregulating SERCA2a. Cell Commun. Signal. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Petrović, S.; Veličković, N.; Stanojević, I.; Milošević, M.; Drakulić, D.; Stanojlović, M.; Horvat, A. Inhibition of mitochondrial Na+-dependent Ca2+ efflux by 17β-estradiol in the rat hippocampus. Neuroscience 2011, 192, 195–204. [Google Scholar] [CrossRef]
- Weil, B.R.; Manukyan, M.C.; Herrmann, J.L.; Wang, Y.; Abarbanell, A.M.; Poynter, J.A.; Meldrum, D.R. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery 2010, 148, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bopassa, J.C.; Eghbali, M.; Toro, L.; Stefani, E.; Goncalves, G.K.; Scalzo, S.; Alves, A.P.; Agero, U.; Guatimosim, S.; Reis, A.M.; et al. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Circ. Physiol. 2010, 298, H16–H23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Chou, J.; Lin, M.; Ferrario, C.M.; Zapata-Sudo, G.; Groban, L. Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: A sex-specific gene profiling analysis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Liu, H.; Kim, J.K. Estrogen Protects the Female Heart from Ischemia/Reperfusion Injury through Manganese Superoxide Dismutase Phosphorylation by Mitochondrial p38β at Threonine 79 and Serine 106. PLoS ONE 2016, 11, e0167761. [Google Scholar] [CrossRef]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef]
- Xu, Y.; Arenas, I.A.; Armstrong, S.J.; Plahta, W.C.; Xu, H.; Davidge, S.T. Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-α. Cardiovasc. Res. 2006, 69, 836–844. [Google Scholar] [CrossRef]
- Wang, M.; Tsai, B.; Reiger, K.; Brown, J.; Meldrum, D. 17-β-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J. Mol. Cell. Cardiol. 2006, 40, 205–212. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Arias-Loza, P.-A.; Hu, K.; Dienesch, C.; Mehlich, A.M.; König, S.; Jazbutyte, V.; Neyses, L.; Hegele-Hartung, C.; Fritzemeier, K.H.; Pelzer, T.; et al. Both Estrogen Receptor Subtypes, α and β, Attenuate Cardiovascular Remodeling in Aldosterone Salt–Treated Rats. Hypertension 2007, 50, 432–438. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Pan, Q.; Allred, K.; Allred, C.; Sun, Y.; Threadgill, D.; Dostal, D.; Tong, C.; et al. Estrogen Protects Cardiac Function and Energy Metabolism in Dilated Cardiomyopathy Induced by Loss of Cardiac IRS1 and IRS2. Circ. Heart Fail. 2022, 15, e008758. [Google Scholar] [CrossRef]
- Liu, D.; Deschamps, A.; Korach, K.S.; Murphy, E. Estrogen-Enhanced Gene Expression of Lipoprotein Lipase in Heart Is Antagonized by Progesterone. Endocrinology 2007, 149, 711–716. [Google Scholar] [CrossRef][Green Version]
- Kararigas, G.; Bito, V.; Tinel, H.; Becher, E.; Baczko, I.; Knosalla, C.; Albrecht-Küpper, B.; Sipido, K.R.; Regitz-Zagrosek, V. Transcriptome Characterization of Estrogen-Treated Human Myocardium Identifies Myosin Regulatory Light Chain Interacting Protein as a Sex-Specific Element Influencing Contractile Function. J. Am. Coll. Cardiol. 2012, 59, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Pascale, J.V.; Lucchesi, P.A.; Garcia, V. Unraveling the Role of 12- and 20- HETE in Cardiac Pathophysiology: G-Protein–Coupled Receptors, Pharmacological Inhibitors, and Transgenic Approaches. J. Cardiovasc. Pharmacol. 2021, 77, 707–717. [Google Scholar] [CrossRef]
- Cai, W.; Liu, L.; Shi, X.; Liu, Y.; Wang, J.; Fang, X.; Chen, Z.; Ai, D.; Zhu, Y.; Zhang, X. Alox15/15-HpETE Aggravates Myocardial Ischemia-Reperfusion Injury by Promoting Cardiomyocyte Ferroptosis. Circulation 2023, 147, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Pollow, D.P.; Hoyer, P.B. The VCD Mouse Model of Menopause and Perimenopause for the Study of Sex Differences in Cardiovascular Disease and the Metabolic Syndrome. Physiology 2016, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Konhilas, J.P.; Sanchez, J.N.; Regan, J.A.; Constantopoulos, E.; Lopez-Pier, M.A.; Cannon, D.K.; Skaria, R.; McKee, L.A.; Chen, H.; Lipovka, Y.; et al. Using 4-vinylcyclohexene diepoxide as a model of menopause for cardiovascular disease. Am. J. Physiol. Circ. Physiol. 2020, 318, H1461–H1473. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M. Critical window hypothesis of hormone therapy and cognition. Menopause 2013, 20, 695–709. [Google Scholar] [CrossRef]
- Gartlehner, G.; Patel, S.V.; Reddy, S.; Rains, C.; Schwimmer, M.; Kahwati, L. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Persons: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 1747–1765. [Google Scholar] [CrossRef]
- Pongkan, W.; Chattipakorn, S.C.; Chattipakorn, N. Roles of Testosterone Replacement in Cardiac Ischemia–Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2015, 21, 27–43. [Google Scholar] [CrossRef]
- Cavasin, M.A.; Tao, Z.-Y.; Yu, A.-L.; Yang, X.-P. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am. J. Physiol. Circ. Physiol. 2006, 290, H2043–H2050. [Google Scholar] [CrossRef]
- Pongkan, W.; Chattipakorn, S.C.; Chattipakorn, N. Chronic Testosterone Replacement Exerts Cardioprotection against Cardiac Ischemia-Reperfusion Injury by Attenuating Mitochondrial Dysfunction in Testosterone-Deprived Rats. PLoS ONE 2015, 10, e0122503. [Google Scholar] [CrossRef]
- Seara, F.A.; Barbosa, R.A.; Santos, M.V.; Domingos, A.E.; Monnerat, G.; Carvalho, A.B.; Olivares, E.L.; Mill, J.G.; Nascimento, J.H.; de Carvalho, A.C.C. Paradoxical effect of testosterone supplementation therapy on cardiac ischemia/reperfusion injury in aged rats. J. Steroid Biochem. Mol. Biol. 2019, 191, 105335. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S. Cardiovascular Disease Associated With Androgen-Deprivation Therapy: Time to Give It Due Respect. J. Clin. Oncol. 2015, 33, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Vigen, R.; O’donnell, C.I.; Barón, A.E.; Grunwald, G.K.; Maddox, T.M.; Bradley, S.M.; Barqawi, A.; Woning, G.; Wierman, M.E.; Plomondon, M.E.; et al. Association of Testosterone Therapy With Mortality, Myocardial Infarction, and Stroke in Men With Low Testosterone Levels. JAMA 2013, 310, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Shores, M.M.; Walsh, T.J.; Korpak, A.; Krakauer, C.; Forsberg, C.W.; Fox, A.E.; Moore, K.P.; Heckbert, S.R.; Thompson, M.L.; Smith, N.L.; et al. Association Between Testosterone Treatment and Risk of Incident Cardiovascular Events Among US Male Veterans With Low Testosterone Levels and Multiple Medical Comorbidities. J. Am. Heart Assoc. 2021, 10, e020562. [Google Scholar] [CrossRef]
- Lee, J.H.; Shah, P.H.; Uma, D.; Salvi, D.J.; Rabbani, R.; Hamid, P. Testosterone Replacement Therapy in Hypogonadal Men and Myocardial Infarction Risk: Systematic Review & Meta-Analysis. Cureus 2021, 13, e17475. [Google Scholar] [CrossRef]
- Brzozowska, M.; Lewiński, A. Changes of androgens levels in menopausal women. Menopausal Rev. 2020, 19, 151–154. [Google Scholar] [CrossRef]
- Awadji, F.B.; Huang, B.; Sasmita, B.R.; Obiegbusi, S.C.; Czika, A.; Xue, Y.; Luo, S.; Sowanou, A.; Liu, G. Association between Testosterone/Estradiol Ratio and Risk of Cardiometabolic Diseases in Women at Menopause Transition Age. Clin. Exp. Obstet. Gynecol. 2022, 49, 260. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ. Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; McClusky, R.; Ruiz-Sundstrom, M.; Itoh, Y.; Umar, S.; Arnold, A.P.; Eghbali, M. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: One X is better than two. Cardiovasc. Res. 2014, 102, 375–384. [Google Scholar] [CrossRef]
- Posynick, B.J.; Brown, C.J. Escape From X-Chromosome Inactivation: An Evolutionary Perspective. Front. Cell Dev. Biol. 2019, 7, 241. [Google Scholar] [CrossRef]
- Qi, S.; Al Mamun, A.; Ngwa, C.; Romana, S.; Ritzel, R.; Arnold, A.P.; McCullough, L.D.; Liu, F. X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. J. Neuroinflamm. 2021, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Walker, C.J.; Grim, J.C.; Schroeder, M.E.; Batan, D.; Vogt, B.J.; Rodriguez, A.G.; Schwisow, J.A.; Moulton, K.S.; Weiss, R.M.; et al. Genes That Escape X Chromosome Inactivation Modulate Sex Differences in Valve Myofibroblasts. Circulation 2022, 145, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Schupp, T.; Akin, I.; Behnes, M. Pharmacological Treatment Following Myocardial Infarction: How Large Is the Gap Between Guideline Recommendations and Routine Clinical Care? J. Am. Heart Assoc. 2021, 10, e021799. [Google Scholar] [CrossRef] [PubMed]
- Madla, C.M.; Gavins, F.K.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s talk about sex: Differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Fenech, A.G.; Magri, V.P. Gender Differences in Drug Therapy. Am. J. Ther. 2020, 547–570. [Google Scholar] [CrossRef]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef]
- Kalibala, J.; Pechère-Bertschi, A.; Desmeules, J. Gender Differences in Cardiovascular Pharmacotherapy—The Example of Hypertension: A Mini Review. Front. Pharmacol. 2020, 11, 564. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Von Moltke, L.L. Gender Has a Small but Statistically Significant Effect on Clearance of CYP3A Substrate Drugs. J. Clin. Pharmacol. 2008, 48, 1350–1355. [Google Scholar] [CrossRef]
- Maglich, J.M.; Stoltz, C.M.; Goodwin, B.; Hawkins-Brown, D.; Moore, J.T.; Kliewer, S.A. Nuclear Pregnane X Receptor and Constitutive Androstane Receptor Regulate Overlapping but Distinct Sets of Genes Involved in Xenobiotic Detoxification. Mol. Pharmacol. 2002, 62, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Kyo, S.; Kanaya, T.; Inoue, M.; Yokoi, T. Human CYP1B1 Is Regulated by Estradiol via Estrogen Receptor. Cancer Res. 2004, 64, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Amin, L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-Glycoprotein Transport System and Cardiovascular Drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef]
- Bebawy, M.; Chetty, M. Gender Differences in P-Glycoprotein Expression and Function: Effects on Drug Disposition and Outcome. Curr. Drug Metab. 2009, 10, 322–328. [Google Scholar] [CrossRef]
- Khera, S.; Kolte, D.; Gupta, T.; Subramanian, K.S.; Khanna, N.; Aronow, W.S.; Ahn, C.; Timmermans, R.J.; Cooper, H.A.; Fonarow, G.C.; et al. Temporal Trends and Sex Differences in Revascularization and Outcomes of ST-Segment Elevation Myocardial Infarction in Younger Adults in the United States. J. Am. Coll. Cardiol. 2015, 66, 1961–1972. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Leifheit, E.C.; Safdar, B.; Bao, H.; Krumholz, H.M.; Lorenze, N.P.; Daneshvar, M.; Spertus, J.A.; D’onofrio, G. Sex Differences in the Presentation and Perception of Symptoms Among Young Patients With Myocardial Infarction. Circulation 2018, 137, 781–790. [Google Scholar] [CrossRef]
- Ng, V.G.; Baumbach, A.; Grinfeld, L.; Lincoff, A.M.; Mehran, R.; Stone, G.W.; Lansky, A.J. Impact of Bleeding and Bivalirudin Therapy on Mortality Risk in Women Undergoing Percutaneous Coronary Intervention (from the REPLACE-2, ACUITY, and HORIZONS-AMI Trials). Am. J. Cardiol. 2015, 117, 186–191. [Google Scholar] [CrossRef]
- Hiteshi, A.K.; Li, D.; Gao, Y.; Chen, A.; Flores, F.; Mao, S.S.; Budoff, M.J. Gender Differences in Coronary Artery Diameter Are Not Related to Body Habitus or Left Ventricular Mass. Clin. Cardiol. 2014, 37, 605–609. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjöld, A.; Gard, A.; Jernberg, T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Hirsh, J.; Califf, R.M.; Col, J.; White, H.D.; Betriu, A.; Woodlief, L.H.; Lee, K.L.; Bovill, E.G.; Simes, R.J.; et al. Activated Partial Thromboplastin Time and Outcome After Thrombolytic Therapy for Acute Myocardial Infarction: Results From the GUSTO-I Trial. Circulation 1996, 93, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Venetsanos, D.; Lawesson, S.S.; Fröbert, O.; Omerovic, E.; Henareh, L.; Robertsson, L.; Linder, R.; Götberg, M.; James, S.; Alfredsson, J.; et al. Sex-related response to bivalirudin and unfractionated heparin in patients with acute myocardial infarction undergoing percutaneous coronary intervention: A subgroup analysis of the VALIDATE-SWEDEHEART trial. Eur. Heart J. Acute Cardiovasc. Care 2018, 8, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Stehli, J.; Martin, C.; Brennan, A.; Dinh, D.T.; Lefkovits, J.; Zaman, S. Sex Differences Persist in Time to Presentation, Revascularization, and Mortality in Myocardial Infarction Treated With Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e012161. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.; Bebb, O.; Dondo, T.B.; Munyombwe, T.; Casadei, B.; Clarke, S.; Schiele, F.; Timmis, A.; Hall, M.; Gale, C.P. Sex differences in quality indicator attainment for myocardial infarction: A nationwide cohort study. Heart 2018, 105, 516–523. [Google Scholar] [CrossRef]
- Alabas, O.A.; Gale, C.P.; Hall, M.; Rutherford, M.J.; Szummer, K.; Lawesson, S.S.; Alfredsson, J.; Lindahl, B.; Jernberg, T. Sex Differences in Treatments, Relative Survival, and Excess Mortality Following Acute Myocardial Infarction: National Cohort Study Using the SWEDEHEART Registry. J. Am. Heart Assoc. 2017, 6, e007123. [Google Scholar] [CrossRef]
- Jneid, H.; Fonarow, G.C.; Cannon, C.P.; Hernandez, A.F.; Palacios, I.F.; Maree, A.O.; Wells, Q.; Bozkurt, B.; LaBresh, K.A.; Liang, L.; et al. Sex Differences in Medical Care and Early Death After Acute Myocardial Infarction. Circulation 2008, 118, 2803–2810. [Google Scholar] [CrossRef]
- Zhao, M.; Woodward, M.; Vaartjes, I.; Millett, E.R.C.; Klipstein-Grobusch, K.; Hyun, K.; Carcel, C.; Peters, S.A.E. Sex Differences in Cardiovascular Medication Prescription in Primary Care: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014742. [Google Scholar] [CrossRef]
- Smith, J.R.; Thomas, R.J.; Bonikowske, A.R.; Hammer, S.M.; Olson, T.P. Sex Differences in Cardiac Rehabilitation Outcomes. Circ. Res. 2022, 130, 552–565. [Google Scholar] [CrossRef]
- Khadanga, S.; Gaalema, D.E.; Savage, P.; Ades, P.A. Underutilization of Cardiac Rehabilitation in Women Barriers and Solutions. J. Cardiopulm. Rehabil. Prev. 2021, 41, 207–213. [Google Scholar] [CrossRef]
- Barp, J.; Araújo, A.; Fernandes, T.; Rigatto, K.; Llesuy, S.; Belló-Klein, A.; Singal, P. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz. J. Med. Biol. Res. 2002, 35, 1075–1081. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater Oxidative Stress in Healthy Young Men Compared With Premenopausal Women. Arter. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, L.-L.; Liu, T.-Y.; Wang, Z.-T. Evaluation of Gender-Related Differences in Various Oxidative Stress Enzymes in Mice. Chin. J. Physiol. 2012, 54, 385–390. [Google Scholar] [CrossRef]
- Vina, J.; Gambini, J.; Lopez-Grueso, R.; Abdelaziz, K.M.; Jove, M.; Borras, C. Females Live Longer than Males: Role of Oxidative Stress. Curr. Pharm. Des. 2011, 17, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Casin, K.M.; Mackowski, N.; Murphy, E.; Steenbergen, C.; Kohr, M.J. Adenosine A1 receptor activation increases myocardial protein S-nitrosothiols and elicits protection from ischemia-reperfusion injury in male and female hearts. PLoS ONE 2017, 12, e0177315. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Fallica, J.; Casin, K.M.; Murphy, E.; Steenbergen, C.; Kohr, M.J. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. Am. J. Physiol. Circ. Physiol. 2016, 310, H505–H515. [Google Scholar] [CrossRef]
- Giannakos, E.; Vardali, E.; Bartekova, M.; Fogarassyova, M.; Barancik, M.; Radosinska, J. Changes in Activities of Circulating MMP-2 and MMP-9 in Patients Suffering From Heart Failure in Relation to Gender, Hypertension and Treatment: A Cross-Sectional Study. Physiol. Res. 2016, 65, S149–S152. [Google Scholar] [CrossRef]
- Voloshenyuk, T.G.; Gardner, J.D. Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R683–R693. [Google Scholar] [CrossRef]
- Stewart, J.A., Jr.; Cashatt, D.O.; Borck, A.C.; Brown, J.E.; Carver, W.E. 17β-estradiol modulation of angiotensin II-stimulated response in cardiac fibroblasts. J. Mol. Cell. Cardiol. 2006, 41, 97–107. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Dworatzek, E.; Fritschka, S.; Pham, T.H.; Regitz-Zagrosek, V. 17β-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc. Res. 2009, 85, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Janicki, J.S.; McLarty, J.L.; Li, J.; Levick, S.P. Estrogen modulates the influence of cardiac inflammatory cells on function of cardiac fibroblasts. J. Inflamm. Res. 2013, 6, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Walsh-Wilkinson, É.; Drolet, M.; Roussel, E.; Melançon, N.; Fortier; Harpin, G.; Beaudoin, J.; Arsenault, M.; Couet, J. Testosterone deficiency reduces cardiac hypertrophy in a rat model of severe volume overload. Physiol. Rep. 2019, 7, e14088. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Murawski, N.; Wiesen, M.H.J.; Held, G.; Poeschel, V.; Zeynalova, S.; Wenger, M.; Nickenig, C.; Peter, N.; Lengfelder, E.; et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 2012, 119, 3276–3284. [Google Scholar] [CrossRef]
- Jäger, U.; Fridrik, M.; Zeitlinger, M.; Heintel, D.; Hopfinger, G.; Burgstaller, S.; Mannhalter, C.; Oberaigner, W.; Porpaczy, E.; Skrabs, C.; et al. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica 2012, 97, 1431–1438. [Google Scholar] [CrossRef]
- Perna, A.; Ruggiero, B.; Podestà, M.A.; Perico, L.; Orisio, S.; Debiec, H.; Remuzzi, G.; Ruggenenti, P. Sexual dimorphic response to rituximab treatment: A longitudinal observational study in a large cohort of patients with primary membranous nephropathy and persistent nephrotic syndrome. Front. Pharmacol. 2022, 13, 958136. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, B.; Liu, X.; Li, H.; Xie, L.; Gao, Y.; Duan, R.; Li, Z.; Zhang, J.; Zheng, Y.; et al. Effects of sex and aging on the immune cell landscape as assessed by single-cell transcriptomic analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023216118. [Google Scholar] [CrossRef]
- Farkouh, A.; Baumgärtel, C.; Gottardi, R.; Hemetsberger, M.; Czejka, M.; Kautzky-Willer, A. Sex-Related Differences in Drugs with Anti-Inflammatory Properties. J. Clin. Med. 2021, 10, 1441. [Google Scholar] [CrossRef]
- Ayyar, V.S.; DuBois, D.C.; Nakamura, T.; Almon, R.R.; Jusko, W.J. Modeling Corticosteroid Pharmacokinetics and Pharmacodynamics, Part II: Sex Differences in Methylprednisolone Pharmacokinetics and Corticosterone Suppression. Experiment 2019, 370, 327–336. [Google Scholar] [CrossRef]
- Duma, D.; Collins, J.B.; Chou, J.W.; Cidlowski, J.A. Sexually Dimorphic Actions of Glucocorticoids Provide a Link to Inflammatory Diseases with Gender Differences in Prevalence. Sci. Signal. 2010, 3, ra74. [Google Scholar] [CrossRef]
- Thompson, K.; Venkatesh, B.; Hammond, N.; Taylor, C.; Finfer, S. Sex differences in response to adjunctive corticosteroid treatment for patients with septic shock. Intensiv. Care Med. 2021, 47, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mehran, R.; Grinfeld, L.; Xu, K.; Nikolsky, E.; Brodie, B.R.; Witzenbichler, B.; Kornowski, R.; Dangas, G.D.; Lansky, A.J.; et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: Three year results from the HORIZONS-AMI trial. Catheter. Cardiovasc. Interv. 2014, 85, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.P.; Chen, A.Y.; Newby, L.K.; Schwartz, J.B.; Redberg, R.F.; Hochman, J.S.; Roe, M.T.; Gibler, W.B.; Ohman, E.M.; Peterson, E.D.; et al. Sex Differences in Major Bleeding With Glycoprotein IIb/IIIa Inhibitors. Circulation 2006, 114, 1380–1387. [Google Scholar] [CrossRef]
- Gremmel, T.; Kopp, C.W.; Eichelberger, B.; Koppensteiner, R.; Panzer, S. Sex differences of leukocyte–platelet interactions and on-treatment platelet reactivity in patients with atherosclerosis. Atherosclerosis 2014, 237, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Aloisio, T.; Dedda, U.D.; Menicanti, L.; De Vincentiis, C.; Baryshnikova, E. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS ONE 2019, 14, e0225771. [Google Scholar] [CrossRef]
- Leng, X.-H.; Hong, S.Y.; Larrucea, S.; Zhang, W.; Li, T.-T.; López, J.A.; Bray, P.F. Platelets of Female Mice Are Intrinsically More Sensitive to Agonists Than Are Platelets of Males. Arter. Thromb. Vasc. Biol. 2004, 24, 376–381. [Google Scholar] [CrossRef]
- Waissi, F.; Dekker, M.; Bank, I.E.M.; Korporaal, S.J.A.; Urbanus, R.T.; de Borst, G.J.; Pasterkamp, G.; Scholtens, A.M.; Grobbee, D.E.; Mosterd, A.; et al. Sex differences in flow cytometry–based platelet reactivity in stable outpatients suspected of myocardial ischemia. Res. Pract. Thromb. Haemost. 2020, 4, 879–885. [Google Scholar] [CrossRef]
- Berlin, G.; Hammar, M.; Tapper, L.; Tynngård, N. Effects of age, gender and menstrual cycle on platelet function assessed by impedance aggregometry. Platelets 2018, 30, 473–479. [Google Scholar] [CrossRef]
- Alzahrani, F.; Hassan, F. Modulation of Platelet Functions Assessment during Menstruation and Ovulatory Phases. J. Med. Life 2019, 12, 296–300. [Google Scholar] [CrossRef]
- Dupuis, M.; Severin, S.; Esclassan, N.; Arnal, J.-F.; Payrastre, B.; Valéra, M.-C. Effects of Estrogens on Platelets and Megakaryocytes. Int. J. Mol. Sci. 2019, 20, 3111. [Google Scholar] [CrossRef]
- Johnson, M.; Ramey, E.; Ramwell, P.W. Sex and age differences in human platelet aggregation. Nature 1975, 253, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Roşca, A.E.; Vlădăreanu, A.-M.; Mititelu, A.; Popescu, B.O.; Badiu, C.; Căruntu, C.; Voiculescu, S.E.; Onisâi, M.; Gologan, Ş.; Mirica, R.; et al. Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review. J. Clin. Med. 2021, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, S.-J.; Kim, B.-K.; Lee, Y.-J.; Hong, S.-J.; Ahn, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; Hong, M.-K.; et al. Sex Differences in Outcomes of Ticagrelor Therapy With or Without Aspirin After Percutaneous Coronary Intervention in Patients With Acute Coronary Syndrome: A Post Hoc Secondary Analysis of the TICO Randomized Clinical Trial. Arter. Thromb. Vasc. Biol. 2023, 43, e218–e226. [Google Scholar] [CrossRef]
- Daugherty, S.L.; Thompson, L.E.; Kim, S.; Rao, S.V.; Subherwal, S.; Tsai, T.T.; Messenger, J.C.; Masoudi, F.A. Patterns of Use and Comparative Effectiveness of Bleeding Avoidance Strategies in Men and Women Following Percutaneous Coronary Interventions: An Observational Study from the National Cardiovascular Data Registry®. J. Am. Coll. Cardiol. 2013, 61, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Baber, U.; Cohen, D.J.; Sartori, S.; Sharma, S.K.; Angiolillo, D.J.; Farhan, S.; Goel, R.; Zhang, Z.; Briguori, C.; et al. Sex Differences Among Patients With High Risk Receiving Ticagrelor With or Without Aspirin After Percutaneous Coronary Intervention: A Subgroup Analysis of the TWILIGHT Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Sandner, S.; Kastrati, A.; Niessner, A.; Böning, A.; Zeymer, U.; Conradi, L.; Danner, B.; Zimpfer, D.; Färber, G.; Manville, E.; et al. Sex differences among patients receiving ticagrelor monotherapy or aspirin after coronary bypass surgery: A prespecified subgroup analysis of the TiCAB trial. Int. J. Cardiol. 2022, 370, 129–135. [Google Scholar] [CrossRef]
- Lau, E.S.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Bonaca, M.P.; Husted, S.; James, S.K.; Wallentin, L.; Clemmensen, P.; Roe, M.T.; et al. Potent P2Y 12 Inhibitors in Men Versus Women. J. Am. Coll. Cardiol. 2017, 69, 1549–1559. [Google Scholar] [CrossRef]
- Kent, D.M.; Price, L.L.; Ringleb, P.; Hill, M.D.; Selker, H.P. Sex-Based Differences in Response to Recombinant Tissue Plasminogen Activator in Acute Ischemic Stroke. Stroke 2005, 36, 62–65. [Google Scholar] [CrossRef]
- Noseda, R.; Rea, F.; Pagnamenta, A.; Agazzi, P.; Bianco, G.; Sihabdeen, S.; Seiffge, D.; Michel, P.; Nedeltchev, K.; Bonati, L.; et al. Sex Differences in Outcomes of Intravenous Thrombolysis in Acute Ischemic Stroke Patients with Preadmission Use of Antiplatelets. CNS Drugs 2023, 37, 351–361. [Google Scholar] [CrossRef]
- Stauffer, B.L.; Hoetzer, G.L.; Van Guilder, G.P.; Smith, D.T.; DeSouza, C.A. Gender Differences in Endothelial Tissue-Type Plasminogen Activator Release in Middle-Aged Adults. J. Am. Coll. Cardiol. 2005, 45, 1547–1549. [Google Scholar] [CrossRef][Green Version]
- Graf, S.; Huber-Beckmann, R.; Zorn, G.; Lang, I.; Kneussl, M.; Binder, B.R.; Huber, K.; Christ, G. Impairment of the Plasmin Activation System in Primary Pulmonary Hypertension: Evidence for Gender Differences. Thromb. Haemost. 2001, 86, 557–562. [Google Scholar] [CrossRef]
- van der Weerd, N.; van Os, H.J.A.; Ali, M.; Schoones, J.W.; Maagdenberg, A.M.J.M.v.D.; Kruyt, N.D.; Siegerink, B.; Wermer, M.J.H. Sex Differences in Hemostatic Factors in Patients With Ischemic Stroke and the Relation With Migraine—A Systematic Review. Front. Cell. Neurosci. 2021, 15, 711604. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.-K.; Kang, Y.-H.; Oh, W.-J.; Lee, D.-Y.; Kim, D.-K.; Kessel, B.; Kang, C.-D. Effects of 17β-Estradiol on the Plasminogen Activator System in Vascular Smooth Muscle Cells Treated with Lysophophatidylcholine. J. Menopausal Med. 2020, 26, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.B.; Agle, Z.W.; Zhang, P.; Reue, K. Chromosomal and gonadal sex drive sex differences in lipids and hepatic gene expression in response to hypercholesterolemia and statin treatment. Biol. Sex Differ. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Fei, S.-F.; Tong, D.-B.; Xue, C.; Li, J.-J. Sex difference in circulating PCSK9 and its clinical implications. Front. Pharmacol. 2022, 13, 953845. [Google Scholar] [CrossRef]
- Fu, W.; Gao, X.-P.; Zhang, S.; Dai, Y.-P.; Zou, W.-J.; Yue, L.-M. 17β-Estradiol Inhibits PCSK9-Mediated LDLR Degradation Through GPER/PLC Activation in HepG2 Cells. Front. Endocrinol. 2020, 10, 930. [Google Scholar] [CrossRef]
- Starr, A.E.; Lemieux, V.; Noad, J.; Moore, J.I.; Dewpura, T.; Raymond, A.; Chrétien, M.; Figeys, D.; Mayne, J. β-Estradiol results in a proprotein convertase subtilisin/kexin type 9-dependent increase in low-density lipoprotein receptor levels in human hepatic HuH7 cells. FEBS J. 2015, 282, 2682–2696. [Google Scholar] [CrossRef]
- Galema-Boers, A.M.; Mulder, J.W.; Steward, K.; van Lennep, J.E.R. Sex differences in efficacy and safety of PCSK9 monoclonal antibodies: A real-world registry. Atherosclerosis 2023. [Google Scholar] [CrossRef]
- Cordero, A.; del Olmo, M.R.F.; Quiroga, G.A.C.; Romero-Menor, C.; Fácila, L.; Seijas-Amigo, J.; Fornovi, A.; Murillo, J.R.; Rodríguez-Mañero, M.; Mora, M.C.B.; et al. Sex Differences in Low-Density Lipoprotein Cholesterol Reduction With PCSK9 Inhibitors in Real-world Patients: The LIPID-REAL Registry. J. Cardiovasc. Pharmacol. 2021, 79, 523–529. [Google Scholar] [CrossRef]
- Paquette, M.; Faubert, S.; Saint-Pierre, N.; Baass, A.; Bernard, S. Sex differences in LDL-C response to PCSK9 inhibitors: A real world experience. J. Clin. Lipidol. 2022, 17, 142–149. [Google Scholar] [CrossRef]
- Rachamin, Y.; Grischott, T.; Rosemann, T.; Meyer, M.R. Inferior control of low-density lipoprotein cholesterol in women is the primary sex difference in modifiable cardiovascular risk: A large-scale, cross-sectional study in primary care. Atherosclerosis 2021, 324, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Vrhovac, I.; Eror, D.B.; Gerasimova, M.; Rose, M.; Breljak, D.; Ljubojević, M.; Brzica, H.; Sebastiani, A.; Thal, S.C.; Sauvant, C.; et al. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Physiol. 2012, 302, C1174–C1188. [Google Scholar] [CrossRef]
- Yoshida, S.; Konishi, H. Gender differences in the gene expression profiles of glucose transporter GLUT class I and SGLT in mouse tissues. Die Pharm.—Int. J. Pharm. Sci. 2014, 69, 856–859. [Google Scholar] [CrossRef]
- Rivera, F.B.; Tang, V.A.S.; De Luna, D.V.; Lerma, E.V.; Vijayaraghavan, K.; Kazory, A.; Shah, N.S.; Volgman, A.S. Sex differences in cardiovascular outcomes of SGLT-2 inhibitors in heart failure randomized controlled trials: A systematic review and meta-analysis. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 26, 100261. [Google Scholar] [CrossRef] [PubMed]
- Adlam, D.; Zarebinski, M.; Uren, N.G.; Ptaszynski, P.; Oldroyd, K.G.; Munir, S.; Zaman, A.; Contractor, H.; Kiss, R.G.; Édes, I.; et al. A Randomized, double-blind, dose ranging clinical trial of intravenous FDY-5301 in acute STEMI patients undergoing primary PCI. Int. J. Cardiol. 2021, 347, 1–7. [Google Scholar] [CrossRef]
- Berne, R.M. The role of adenosine in the regulation of coronary blood flow. Circ. Res. 1980, 47, 807–813. [Google Scholar] [CrossRef]
- Wagner, D.R.; McTiernan, C.; Sanders, V.J.; Feldman, A.M. Adenosine Inhibits Lipopolysaccharide-Induced Secretion of Tumor Necrosis Factor-α in the Failing Human Heart. Circulation 1998, 97, 521–524. [Google Scholar] [CrossRef]
- Sullivan, G.W.; Rieger, J.M.; Scheld, W.M.; Macdonald, T.L.; Linden, J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2Areceptor agonists. Br. J. Pharmacol. 2001, 132, 1017–1026. [Google Scholar] [CrossRef]
- Sato, H.; Williams, M.W.; Fernandez, A.Z.; Moses, M.A.; Addison, P.D.; Neligan, P.C.; Ashrafpour, H.; Huang, N.; McAllister, S.E.; Lipa, J.E.; et al. Adenosine A2-receptor activation inhibits neutrophil-mediated injury to coronary endothelium. Am. J. Physiol. Heart Circ. Physiol. 1996, 271, H1456–H1464. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef]
- Yetgin, T.; Uitterdijk, A.; Hekkert, M.T.L.; Merkus, D.; Krabbendam-Peters, I.; van Beusekom, H.M.; Falotico, R.; Serruys, P.W.; Manintveld, O.C.; van Geuns, R.-J.M.; et al. Limitation of Infarct Size and No-Reflow by Intracoronary Adenosine Depends Critically on Dose and Duration. JACC Cardiovasc. Interv. 2015, 8, 1990–1999. [Google Scholar] [CrossRef]
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.L. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog. Mol. Biol. Transl. Sci. 2017, 147, 75–100. [Google Scholar] [PubMed]
- Hughes, B.G.; Schulz, R. Targeting MMP-2 to treat ischemic heart injury. Basic Res. Cardiol. 2014, 109, 424. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; Pasini, E.; Schulze, C.J.; Ferrari-Vivaldi, M.; Ferrari-Vivaldi, G.; Bachetti, T.; Schulz, R. Ischaemia–reperfusion injury activates matrix metalloproteinases in the human heart. Eur. Heart J. 2004, 26, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Manfrinato, M.C.; Castellazzi, M.; Bellini, T. Sex-Related Differences of Matrix Metalloproteinases (MMPs): New Perspectives for These Biomarkers in Cardiovascular and Neurological Diseases. J. Pers. Med. 2022, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, M.A.; Tao, Z.; Menon, S.; Yang, X.-P. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004, 75, 2181–2192. [Google Scholar] [CrossRef]
- Cerisano, G.; Buonamici, P.; Gori, A.M.; Valenti, R.; Sciagrà, R.; Giusti, B.; Sereni, A.; Raspanti, S.; Colonna, P.; Gensini, G.F.; et al. Matrix metalloproteinases and their tissue inhibitor after reperfused ST-elevation myocardial infarction treated with doxycycline. Insights from the TIPTOP trial. Int. J. Cardiol. 2015, 197, 147–153. [Google Scholar] [CrossRef]
- Feng, Q.; Li, Q.; Zhou, H.; Sun, L.; Lin, C.; Jin, Y.; Wang, D.; Guo, G. The role of major immune cells in myocardial infarction. Front. Immunol. 2023, 13, 1084460. [Google Scholar] [CrossRef]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- Zhao, T.X.; Aetesam-Ur-Rahman, M.; Sage, A.P.; Victor, S.; Kurian, R.; Fielding, S.; Ait-Oufella, H.; Chiu, Y.-D.; Binder, C.J.; Mckie, M.; et al. Rituximab in patients with acute ST-elevation myocardial infarction: An experimental medicine safety study. Cardiovasc. Res. 2021, 118, 872–882. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Schaafsma, D.; Amrani, Y.; Koziol-White, C.; Ostrom, R.; Tliba, O. Non-genomic Effects of Glucocorticoids: An Updated View. Trends Pharmacol. Sci. 2019, 40, 38–49. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Kunapuli, S.P. Central role of the P2Y12 receptor in platelet activation. J. Clin. Investig. 2004, 113, 340–345. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 1–22. [Google Scholar] [CrossRef]
- Newby, D.E.; Wright, R.A.; Labinjoh, C.; Ludlam, C.A.; Fox, K.A.A.; Boon, N.A.; Webb, D.J. Endothelial Dysfunction, Impaired Endogenous Fibrinolysis, and Cigarette Smoking: A mechanism for arterial thrombosis and myocardial infarction. Circulation 1999, 99, 1411–1415. [Google Scholar] [CrossRef]
- Huynh, T.; Perron, S.; O’Loughlin, J.; Joseph, L.; Labrecque, M.; Tu, J.V.; Théroux, P. Comparison of Primary Percutaneous Coronary Intervention and Fibrinolytic Therapy in ST-Segment-Elevation Myocardial Infarction. Circulation 2009, 119, 3101–3109. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Gershlick, A.H.; Goldstein, P.; Wilcox, R.; Danays, T.; Lambert, Y.; Sulimov, V.; Ortiz, F.R.; Ostojic, M.; Welsh, R.C.; et al. Fibrinolysis or Primary PCI in ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2013, 368, 1379–1387. [Google Scholar] [CrossRef]
- Middeldorp, S.; Tekelenburg, W.; Ende, A.E.v.D.; Tans, G.; Prins, M.H.; Rosing, J.; Büller, H.R.; Bouma, B.N.; Meijers, J. Increased Fibrinolytic Activity during Use of Oral Contraceptives Is Counteracted by an Enhanced Factor XI-independent down Regulation of Fibrinolysis. Thromb. Haemost. 2000, 84, 9–14. [Google Scholar] [CrossRef]
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond Lipid-Lowering: Effects of Statins on Cardiovascular and Cerebrovascular Diseases and Cancer. Pharmaceuticals 2022, 15, 151. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Tavori, H.; Fazio, S. PCSK9. Circ. Res. 2018, 122, 1420–1438. [Google Scholar] [CrossRef]
- Coppinger, C.; Movahed, M.R.; Azemawah, V.; Peyton, L.; Gregory, J.; Hashemzadeh, M. A Comprehensive Review of PCSK9 Inhibitors. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 107424842211001. [Google Scholar] [CrossRef]
- Hussain, Y.; Ding, Q.; Connelly, P.W.; Brunt, J.H.; Ban, M.R.; McIntyre, A.D.; Huff, M.W.; Gros, R.; Hegele, R.A.; Feldman, R.D. G-Protein Estrogen Receptor as a Regulator of Low-Density Lipoprotein Cholesterol Metabolism: Cellular and population genetic studies. Arter. Thromb. Vasc. Biol. 2015, 35, 213–221. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: A systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 181–187. [Google Scholar] [CrossRef]
- Sharma, A.; Wood, S.; Bell, J.S.; De Blasio, M.J.; Ilomäki, J.; Ritchie, R.H. Sex differences in risk of cardiovascular events and mortality with sodium glucose co-transporter-2 inhibitors versus glucagon-like peptide 1 receptor agonists in Australians with type 2 diabetes: A population-based cohort study. Lancet Reg. Health West. Pac. 2023, 33, 100692. [Google Scholar] [CrossRef]
- Raparelli, V.; Elharram, M.; Moura, C.S.; Abrahamowicz, M.; Bernatsky, S.; Behlouli, H.; Pilote, L. Sex Differences in Cardiovascular Effectiveness of Newer Glucose-Lowering Drugs Added to Metformin in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e012940. [Google Scholar] [CrossRef]
- Pruett, J.E.; Lirette, S.T.; Romero, D.G.; Cardozo, L.L.Y. Sodium-Glucose Cotransporter-2 Inhibition Benefits in Cardiorenal Risk in Men and Women. J. Endocr. Soc. 2022, 7, bvac191. [Google Scholar] [CrossRef]
- Wang, X.; Vaduganathan, M.; Claggett, B.L.; Hegde, S.M.; Pabon, M.; Kulac, I.J.; Vardeny, O.; O’meara, E.; Zieroth, S.; Katova, T.; et al. Sex Differences in Characteristics, Outcomes, and Treatment Response With Dapagliflozin Across the Range of Ejection Fraction in Patients With Heart Failure: Insights From DAPA-HF and DELIVER. Circulation 2023, 147, 624–634. [Google Scholar] [CrossRef]
- Rådholm, K.; Zhou, Z.; Clemens, K.; Neal, B.; Woodward, M. Author response for “Effects of SGLT2 inhibitors in type 2 diabetes, comparing women to men”. Diabetes Obes. Metab. 2019, 22, 263–266. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
| Target | Clinical Trial | Drug and Administration Route | Proposed Mechanism | Possible Sex Differences |
|---|---|---|---|---|
| Oxidative stress | NCT04837001 | FDY-5301, i.v. delivery before a PCI | Anti-peroxidant, promotes the conversion of hydrogen peroxide to water and oxygen | ↑ SOD activity in hearts of F rats [102] ↓ SOD expression after gonadectomy in both sexes [102] ↑ Catalase activity in F rat kidneys [102,103,104] ↓ GPx activity in F rodents [102,103,104] Estrogen phenolic hydroxyl group scavenges free radicals [102] E2 promotes Mn-SOD and GPx gene expression [105] |
| NCT05014061 | Adenosine, i.v. delivery before a PCI | Antioxidative, vasodilatation, anti-inflammatory, regulation of calcium homeostasis | ↑ Cardiac adenosine A1 receptor-induced eNOS phosphorylation in M [106,107] | |
| Inflammation | NCT03508232 | Doxycycline, oral immediately after a PCI and 7 days after a PCI | MMP-2 inhibition | ↓ Activity of serum pro-MMP-2 activity in F HF patients [108] ↑ LV MMP-2 activity in M mice after MI [60] ↑ Cardiac MMP-2 activity in healthy ovariectomized F rats [109] ↓ Cardiac MMP-2 expression upon volume overload in ovariectomized F rats [109] E2 inhibits MMP-2 expression in rat cardiac fibroblasts and cardiac inflammatory cells [110,111,112] ↓ Cardiac MMP-2 expression upon volume overload in castrated M rats [113] |
| NCT05211401 | Ritixumab, i.v. within 3 h of PCI | CD20 antibody, B cell depletion, anti-inflammatory | ↑ Rituximab clearance and poorer treatment outcomes in M DLBCL patients [114,115] ↑ Complete or partial remission following Rituximab therapy in F nephropathy patients [116] ↑ Baseline B-cell-activated signaling in peripheral immune cells in F [117] | |
| NCT05462730 | Methylprednisolone, single i.v. bolus in the prehospital setting before PCI | Glucocorticoid, anti-inflammatory, antioxidant, promoted mitochondrial function, regulation of calcium homeostasis | ↑ Methylprednisolone clearance in F patients [118] ↑ Methylprednisolone clearance in M rats [119] Sensitivity of basophils to methylprednisolone treatment to plasma E2 in F [118] ↑ Survival rate in ovariectomized endotoxemic F rats treated with dexamethasone [120] Castration in M rats had no effect on dexamethasone treatment of endotoxemia [120] ↓ Length of mechanical ventilation and ICU stay by hydrocortisone treatment only in M septic shock patients [121] Dexamethasone treatment in rats promotes sex-specific glucocorticoid-regulated gene expression in the liver [120] | |
| Thrombosis | NCT04825743 | Zalunfiban, administered by the ambulance staff prior to a PCI | Platelet αIIbβ3 receptor inhibitor, inhibition of platelet aggregation | ↑ Bleeding in F after GpIIb/IIIa inhibitor treatment [122,123] ↑ Platelet count and reactivity in F [124,125,126,127] Menstrual cycle affects platelet reactivity [128,129] Contradictory effects of E2 and testosterone on platelet activation [130,131,132] |
| NCT05149560 | Ticagrelor, oral after PCI for 12 months | Platelet P2Y12 receptor inhibitor, inhibition of platelet aggregation | ↑ Bleeding in F after Ticagrelor treatment [133,134,135,136] Less pronounced benefits of Ticagrelor on MACE in F vs. M [133,134,135,136] No difference in efficacy and safety of P2Y12 inhibitors between M and F [137] | |
| NCT03998319, NCT03335839, and NCT02894138 | Tenecteplase or Alteplase, intracoronary administration immediately following a PCI | Recombinant tPA, promotion of fibrinolysis | Less pronounced benefits of recombinant tPA in M ischemic stroke patients [138] Better functional outcomes after recombinant tPA in M ischemic stroke patients [139] ↑ Endothelial tPA antigen release in F [140] ↑ tPA antigen, ↑ PAI-1 activity, and ↓ tPA levels in pulmonary arteries of F PAH patients [141] ↑ PAI-1 in F stroke with migraine patients, no differences in tPA levels between M and F [142] E2 represses PAI-1 expression via ER in vascular smooth muscle cells [143] | |
| Lipid metabolism | NCT04974814 | Rosuvastatin and Atorvastatin, single high-dose preloading before a PCI | Anti-inflammatory, antioxidant, inhibition of platelet aggregation, improve endothelial function | ↑ Statin-induced reductions in cholesterol levels in mice with testes than mice with ovaries [144] Sex chromosome complement impacts hepatic transcriptional response to statins [144] |
| NCT05284747 and NCT04951856 | Evolocumab, s.c. immediately after a PCI and biweekly after | PCSK9 antibody, promotion of revascularization | ↑ Circulating PCSK9 in F [145] PCSK9-LDLR complex internalization inhibited by E2 and GPR30 [146,147] PCSK9 antibodies were less effective in lowering LDL-C in F [148,149,150,151] | |
| Glucose metabolism | NCT05305911, NCT04363697, and NCT04509674 | Dapagliflozin or Empagliflozin, oral after a PCI once daily for two or six months | SGLT2 inhibitor, promotion of cardiac energy metabolism, enhanced circulating progenitor cells, erythropoiesis, decreased blood pressure, anti-inflammatory | ↑ SGLT2 mRNA in F rats, ↑ SGLT2 protein in M rats [152,153] Less pronounced benefits of SGLT2 inhibitors on HF risk in F vs. M [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medzikovic, L.; Azem, T.; Sun, W.; Rejali, P.; Esdin, L.; Rahman, S.; Dehghanitafti, A.; Aryan, L.; Eghbali, M. Sex Differences in Therapies against Myocardial Ischemia-Reperfusion Injury: From Basic Science to Clinical Perspectives. Cells 2023, 12, 2077. https://doi.org/10.3390/cells12162077
Medzikovic L, Azem T, Sun W, Rejali P, Esdin L, Rahman S, Dehghanitafti A, Aryan L, Eghbali M. Sex Differences in Therapies against Myocardial Ischemia-Reperfusion Injury: From Basic Science to Clinical Perspectives. Cells. 2023; 12(16):2077. https://doi.org/10.3390/cells12162077
Chicago/Turabian StyleMedzikovic, Lejla, Tara Azem, Wasila Sun, Parmis Rejali, Leana Esdin, Shadie Rahman, Ateyeh Dehghanitafti, Laila Aryan, and Mansoureh Eghbali. 2023. "Sex Differences in Therapies against Myocardial Ischemia-Reperfusion Injury: From Basic Science to Clinical Perspectives" Cells 12, no. 16: 2077. https://doi.org/10.3390/cells12162077
APA StyleMedzikovic, L., Azem, T., Sun, W., Rejali, P., Esdin, L., Rahman, S., Dehghanitafti, A., Aryan, L., & Eghbali, M. (2023). Sex Differences in Therapies against Myocardial Ischemia-Reperfusion Injury: From Basic Science to Clinical Perspectives. Cells, 12(16), 2077. https://doi.org/10.3390/cells12162077







