FITC-Labeled RGD Peptides as Novel Contrast Agents for Functional Fluorescent Angiographic Detection of Retinal and Choroidal Neovascularization
Abstract
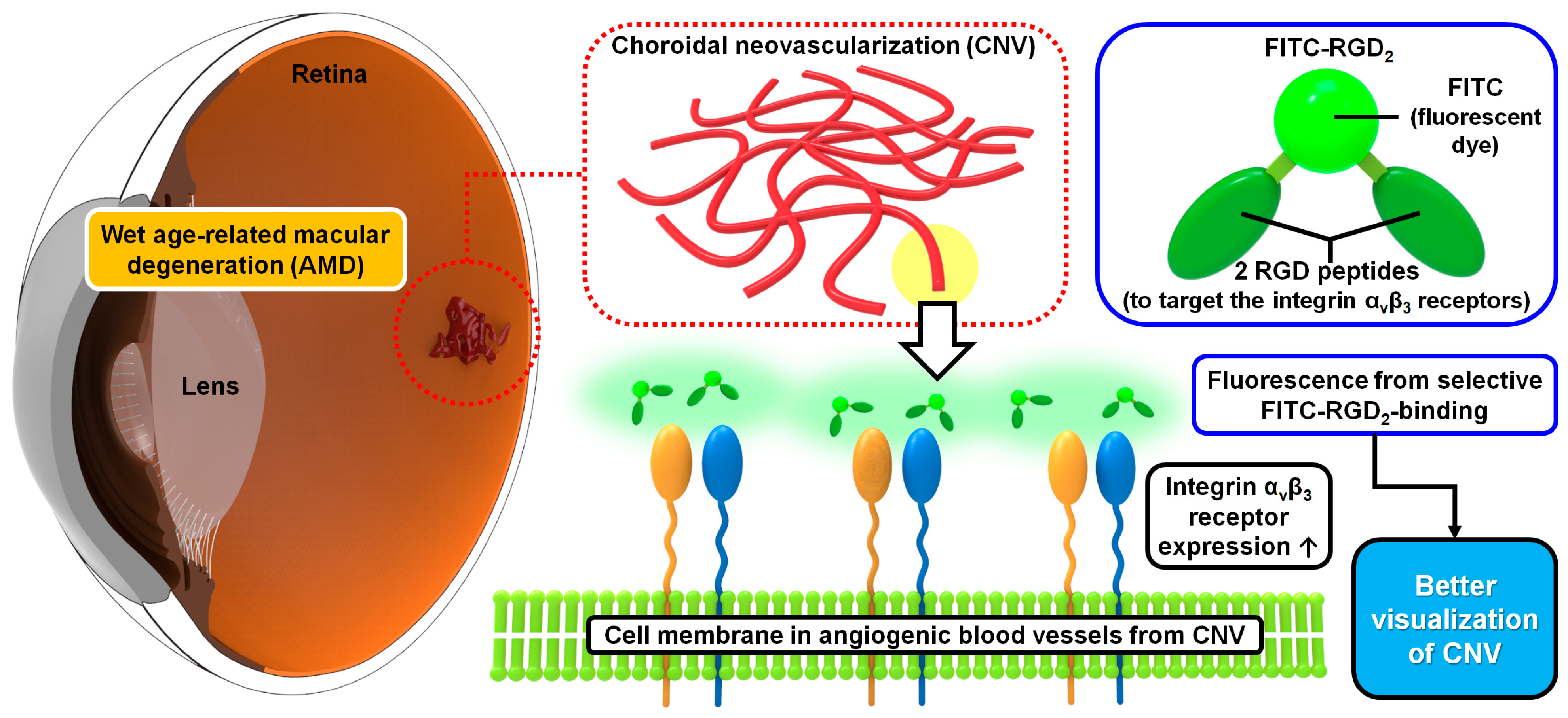
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Synthesis of AF488- and FITC-Labeled RGD Peptides (AF488-RGD2, FITC-RGD2)
2.3. In Vivo Confocal Microscopic Imaging and Angiographic Evaluation in Mice
3. Results
3.1. Characterization of the Interaction between Fluorescent Dye-Conjugated RGD Peptides and Integrins in Laser-Induced CNV Mouse Models and OIR Mouse Models
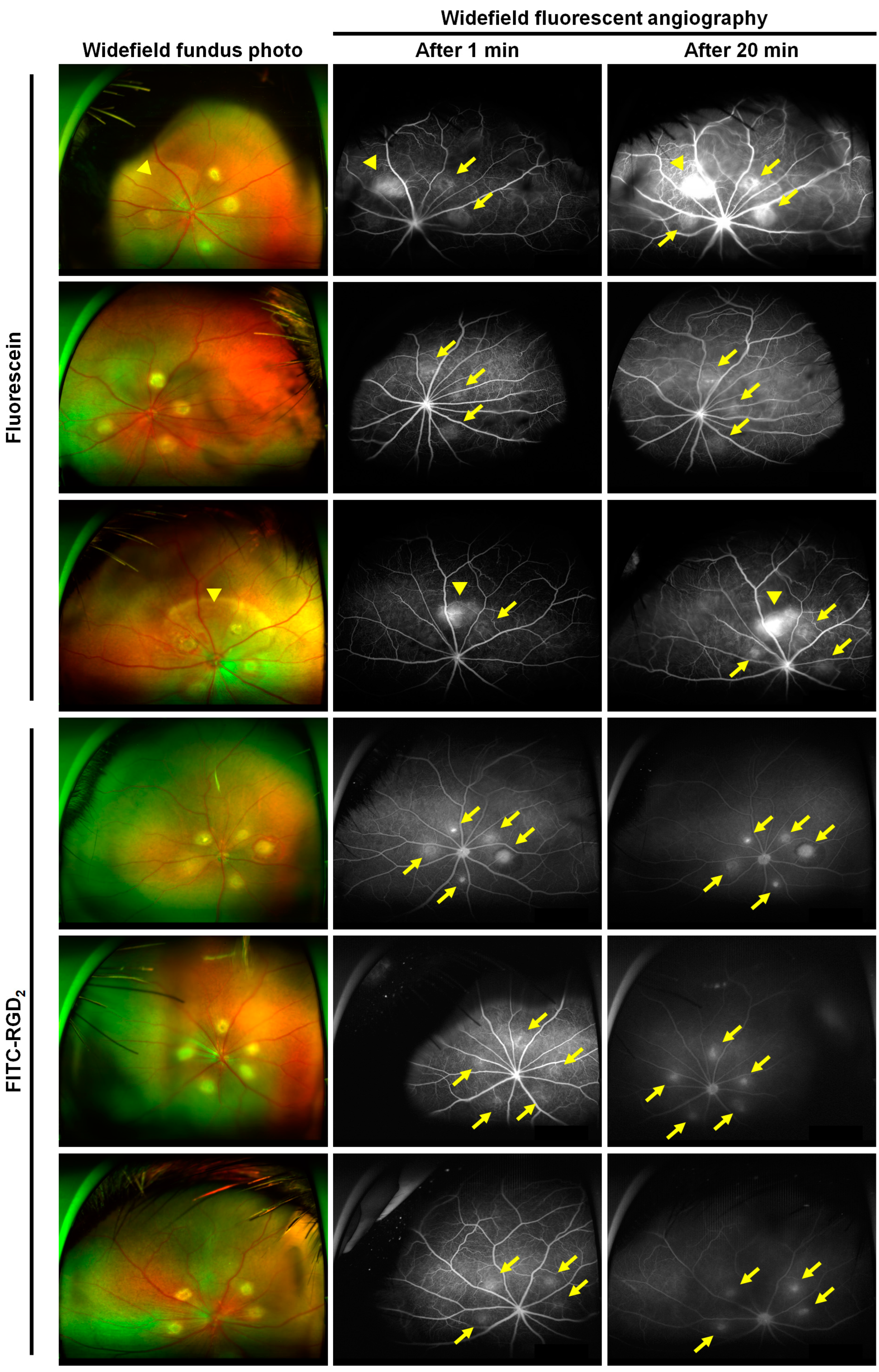
3.2. Comparison of CNV Visualization of Each Fluorescent Contrast Agent in the Laser-Induced CNV Mouse Models
3.3. Comparison of Ultrawide-Field Fluorescent Angiographic Results in Laser-Induced CNV Rat Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Hadziahmetovic, M.; Malek, G. Age-Related Macular Degeneration Revisited: From Pathology and Cellular Stress to Potential Therapies. Front. Cell Dev. Biol. 2020, 8, 612812. [Google Scholar] [CrossRef] [PubMed]
- Cimarolli, V.R.; Casten, R.J.; Rovner, B.W.; Heyl, V.; Sörensen, S.; Horowitz, A. Anxiety and depression in patients with advanced macular degeneration: Current perspectives. Clin. Ophthalmol. 2016, 10, 55–63. [Google Scholar] [CrossRef]
- Yuzawa, M.; Fujita, K.; Tanaka, E.; Wang, E.C. Assessing quality of life in the treatment of patients with age-related macular degeneration: Clinical research findings and recommendations for clinical practice. Clin. Ophthalmol. 2013, 7, 1325–1332. [Google Scholar] [CrossRef]
- Joo, K.; Mun, Y.S.; Park, S.J.; Park, K.H.; Woo, S.J. Ten-Year Progression from Intermediate to Exudative Age-Related Macular Degeneration and Risk Factors: Bundang AMD Cohort Study Report 1. Am. J. Ophthalmol. 2021, 224, 228–237. [Google Scholar] [CrossRef]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.; Loewenstein, A. Early detection of age related macular degeneration: Current status. Int. J. Retin. Vitr. 2015, 1, 20. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Brown, D.M.; Zhang, K.; Hudson, H.L.; Holz, F.G.; Shapiro, H.; Schneider, S.; Acharya, N.R. Ranibizumab for predominantly classic neovascular age-related macular degeneration: Subgroup analysis of first-year ANCHOR results. Am. J. Ophthalmol. 2007, 144, 850–857. [Google Scholar] [CrossRef]
- Boyer, D.S.; Antoszyk, A.N.; Awh, C.C.; Bhisitkul, R.B.; Shapiro, H.; Acharya, N.R. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007, 114, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Heier, J.S.; Kim, J.E.; Garfinkel, R.; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology 2014, 121, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Tatlipinar, S.; Quinlan, E.; Sung, J.U.; Tabandeh, H.; Nguyen, Q.D.; Fahmy, A.S.; Zimmer-Galler, I.; Symons, R.C.A.; Cedarbaum, J.M.; et al. Dynamic and Quantitative Analysis of Choroidal Neovascularization by Fluorescein Angiography. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5460–5468. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, Z.; Liu, X. Diagnostic accuracy of optical coherence tomography angiography for choroidal neovascularization: A systematic review and meta-analysis. BMC Ophthalmol. 2019, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Soomro, T.; Talks, J. The use of optical coherence tomography angiography for detecting choroidal neovascularization, compared to standard multimodal imaging. Eye 2018, 32, 661–672. [Google Scholar] [CrossRef]
- Parekh, P.K.; Folk, J.C.; Gupta, P.; Russell, S.R.; Sohn, E.H.; Abràmoff, M.D. Fluorescein Angiography Does Not Alter the Initial Clinical Management of Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retin. 2018, 2, 659–666. [Google Scholar] [CrossRef]
- Malihi, M.; Jia, Y.; Gao, S.S.; Flaxel, C.; Lauer, A.K.; Hwang, T.; Wilson, D.J.; Huang, D.; Bailey, S.T. Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. Br. J. Ophthalmol. 2017, 101, 45–50. [Google Scholar] [CrossRef]
- Barteselli, G.; Chhablani, J.; Lee, S.N.; Wang, H.; El Emam, S.; Kozak, I.; Cheng, L.; Bartsch, D.-U.; Azen, S.; Freeman, W.R. Safety and efficacy of oral fluorescein angiography in detecting macular edema in comparison with spectral-domain optical coherence tomography. Retina 2013, 33, 1574–1583. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Y.-X.; Lin, Y.; Zhang, J.-S.; Yang, F.; Zhou, Q.-L.; Liao, Y.-Y. Advance of Molecular Imaging Technology and Targeted Imaging Agent in Imaging and Therapy. BioMed Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef]
- Achilefu, S. Introduction to Concepts and Strategies for Molecular Imaging. Chem. Rev. 2010, 110, 2575–2578. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, J.; Wang, J.; Parasuraman, P.; Busi, S.; Nauli, S.M.; Wáng, Y.X.J.; Pala, R.; Liu, G. Functional probes for cardiovascular molecular imaging. Quant. Imaging Med. Surg. 2018, 8, 838–852. [Google Scholar] [CrossRef]
- Fu, Y.; Ponce, M.L.; Thill, M.; Yuan, P.; Wang, N.S.; Csaky, K.G. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5184–5190. [Google Scholar] [CrossRef]
- Van Hove, I.; Hu, T.-T.; Beets, K.; Van Bergen, T.; Etienne, I.; Stitt, A.W.; Vermassen, E.; Feyen, J.H. Targeting RGD-binding integrins as an integrative therapy for diabetic retinopathy and neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2021, 85, 100966. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Nagai, T.; Negi, A. Anti-angiogenic effects of non-peptide integrin αvβ3 specific antagonist on laser-induced choroidal neovascularization in mice. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 515–522. [Google Scholar] [CrossRef]
- Gonzalez-Salinas, R.; Hernández-Zimbrón, L.F.; Gulias-Cañizo, R.; Sánchez-Vela, M.A.; La Paz, L.O.-D.; Zamora, R.; Quiroz-Mercado, H. Current Anti-Integrin Therapy for Ocular Disease. Semin. Ophthalmol. 2018, 33, 634–642. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, H.Y.; Hong, H.K.; Jung, J.H.; Park, J.H.; Park, K.H.; Kim, S.E.; Woo, S.J.; Lee, B.C. Preclinical SPECT Imaging of Choroidal Neovascularization in Mice Using Integrin-Binding [(99m)Tc]IDA-D-[c(RGDfK)](2). Mol. Imaging Biol. 2019, 21, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Choi, W.; Hong, H.K.; Kim, Y.; Hwang, Y.; Woo, S.J.; Oh, W.-Y. Imaging Laser-Induced Choroidal Neovascularization in the Rodent Retina Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, Oct331–Oct340. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E.H. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef]
- Hong, H.K.; Park, Y.J.; Kim, D.K.; Ryoo, N.-K.; Ko, Y.-J.; Park, K.H.; Kim, H.M.; Woo, S.J. Preclinical Efficacy and Safety of VEGF-Grab, a Novel Anti-VEGF Drug, and Its Comparison to Aflibercept. Investig. Ophthalmol. Vis. Sci. 2020, 61, 22. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, Y.; Kim, J.H.; Kim, Y.S.; Jung, B.K.; Kim, P.; Lee, H. In Vivo Fluorescence Retinal Imaging Following AAV2-Mediated Gene Delivery in the Rat Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3390–3396. [Google Scholar] [CrossRef]
- Jeon, J.; Hwang, Y.; Lee, J.; Kong, E.; Moon, J.; Hong, S.; Kim, P. Intravital Imaging of Circulating Red Blood Cells in the Retinal Vasculature of Growing Mice. Transl. Vis. Sci. Technol. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, S.H.; Kong, E.; Kim, S.J.; Yang, J.M.; Lee, J.Y.; Lee, J.; Kim, Y.-M.; Kim, P. Establishment of the reproducible branch retinal artery occlusion mouse model and intravital longitudinal imaging of the retinal CX3CR1-GFP(+) cells after spontaneous arterial recanalization. Front. Med. 2022, 9, 897800. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yu, H.G.; Sohn, J. The anti-angiogenic effect of chlorogenic acid on choroidal neovascularization. Korean J. Ophthalmol. 2010, 24, 163–168. [Google Scholar] [CrossRef]
- Giani, A.; Thanos, A.; Roh, M.I.; Connolly, E.; Trichonas, G.; Kim, I.; Gragoudas, E.; Vavvas, D.; Miller, J.W. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3880–3887. [Google Scholar] [CrossRef]
- Woodfin, A.; Voisin, M.B.; Nourshargh, S. PECAM-1: A multi-functional molecule in inflammation and vascular biology. Arter. Thromb. Vasc. Biol. 2007, 27, 2514–2523. [Google Scholar] [CrossRef] [PubMed]
- Vanchinathan, V.; Mirzamani, N.; Kantipudi, R.; Schwartz, E.J.; Sundram, U.N. The vascular marker CD31 also highlights histiocytes and histiocyte-like cells within cutaneous tumors. Am. J. Clin. Pathol. 2015, 143, 177–185, quiz 305. [Google Scholar] [CrossRef]
- Patel, V.; Syeda, S.; Zeiter, J.; Nassiri, N.; Kim, C.; Truhan, A.; Chapman, C.; Adam, C.; Parendo, A.; Savage, M.; et al. Randomized, Comparative Study of Full- and Half-Dose Fluorescein Angiography. J. Vitr. Dis. 2021, 5, 337–344. [Google Scholar] [CrossRef]
- Patel, C.; Goody, R.; Hu, W.; Kurian, A.; James, D.; Torres, R.; Christie, L.-A.; Hohman, T.; Lawrence, M. Primate model of chronic retinal neovascularization and vascular leakage. Exp. Eye Res. 2020, 195, 108031. [Google Scholar] [CrossRef]
- Livnat, T.; Weinberger, Y.; Fernández, J.A.; Bashir, A.; Ben-David, G.; Palevski, D.; Levy-Mendelovich, S.; Kenet, G.; Budnik, I.; Nisgav, Y.; et al. Activated Protein C (APC) and 3K3A-APC-Induced Regression of Choroidal Neovascularization (CNV) Is Accompanied by Vascular Endothelial Growth Factor (VEGF) Reduction. Biomolecules 2021, 11, 358. [Google Scholar] [CrossRef]
- Tolentino, M.J.; Husain, D.; Theodosiadis, P.; Gragoudas, E.S.; Connolly, E.; Kahn, J.; Cleland, J.; Adamis, A.P.; Cuthbertson, A.; Miller, J.W. Angiography of fluoresceinated anti-vascular endothelial growth factor antibody and dextrans in experimental choroidal neovascularization. Arch. Ophthalmol. 2000, 118, 78–84. [Google Scholar] [CrossRef]
- D’Amato, R.; Wesolowski, E.; Smith, L.E.H. Microscopic Visualization of the Retina by Angiography with High-Molecular-Weight Fluorescein-Labeled Dextrans in the Mouse. Microvasc. Res. 1993, 46, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Brancato, R.; Trabucchi, G. Fluorescein and indocyanine green angiography in vascular chorioretinal diseases. Semin. Ophthalmol. 1998, 13, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, P.M.; Flower, R.W. Ten years experience with choroidal angiography using indocyanine green dye: A new routine examination or an epilogue? Doc. Ophthalmol. 1985, 60, 235–291. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Cao, J.; Chan, R.; Ling, J.; Ye, J.Y. Optical properties of indocyanine green under ultrasound treatment. J. Photochem. Photobiol. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Fukushima, I.; Kusaka, K.; Takahashi, K.; Kishimoto, N.; Nishimura, T.; Ohkuma, H.; Uyama, M. Comparison of indocyanine green and fluorescein angiography of choroidal neovascularization. Jpn. J. Ophthalmol. 1997, 41, 284–296. [Google Scholar] [CrossRef]
- Coscas, G.J.; Lupidi, M.; Coscas, F.; Cagini, C.; Souied, E.H. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular generation: A New Diagnostic Challenge. Retina 2015, 35, 2219–2228. [Google Scholar] [CrossRef]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, M.M.; Fu, Y. Combination of AIBP, apoA-I, and Aflibercept Overcomes Anti-VEGF Resistance in Neovascular AMD by Inhibiting Arteriolar Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2022, 63, 2. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Suner, I.; Hernandez, E.P.; Frazier, W.D.; Csaky, K.G.; Cousins, S.W. Age as an Independent Risk Factor for Severity of Experimental Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1567–1573. [Google Scholar]
- Ning, A.; Cui, J.; Maberley, D.; Ma, P.; Matsubara, J. Expression of integrins in human proliferative diabetic retinopathy membranes. Can. J. Ophthalmol. 2008, 43, 683–688. [Google Scholar] [CrossRef]
- Ai, J.; Ma, J.; Chen, Z.-Q.; Sun, J.-H.; Yao, K. An Endostatin-lentivirus (ES-LV)-EPC gene therapy agent for suppression of neovascularization in oxygen-induced retinopathy rat model. BMC Mol. Cell Biol. 2020, 21, 57. [Google Scholar] [CrossRef]
- Yu, L.; Fu, J.; Yu, N.; Wu, Y.; Han, N. Long noncoding RNA MALAT1 participates in the pathological angiogenesis of diabetic retinopathy in an oxygen-induced retinopathy mouse model by sponging miR-203a-3p. Can. J. Physiol. Pharmacol. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Li, X.H.; Wang, L.F.; Kuang, X.; Hang, Z.-X.; Deng, Y.; Du, J.-R. Therapeutic efficacy of a novel non-peptide αvβ3 integrin antagonist for pathological retinal angiogenesis in mice. Exp. Eye Res. 2014, 129, 119–126. [Google Scholar] [CrossRef]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Suzuma, K.; Otani, A.; Oh, H.; Koyama, S.; Ohashi, H.; Watanabe, D.; Ojima, T.; Suganami, E.; Honda, Y. Role of vitronectin receptor-type integrins and osteopontin in ischemia-induced retinal neovascularization. Jpn. J. Ophthalmol. 2002, 46, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Maberley, D.; Samad, A.; Ma, P.; Ning, A.; Matsubara, J.A.; Baciu, P. Expression of integrins on human choroidal neovascular membranes. J. Ocul. Biol. Dis. Inf. 2009, 2, 12–19. [Google Scholar] [CrossRef]
- Keane, P.A.; de Salvo, G.; Sim, D.A.; Goverdhan, S.; Agrawal, R.; Tufail, A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 353–366. [Google Scholar] [CrossRef]
- Atkinson, E.G.; Jones, S.; Ellis, B.A.; Dumonde, D.C.; Graham, E. Molecular size of retinal vascular leakage determined by FITC-dextran angiography in patients with posterior uveitis. Eye 1991, 5 Pt 4, 440–446. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef]
- Alipour, M.; Baneshi, M.; Hosseinkhani, S.; Mahmoudi, R.; Arabzadeh, A.J.; Akrami, M.; Mehrzad, J.; Bardania, H. Recent progress in biomedical applications of RGD-based ligand: From precise cancer theranostics to biomaterial engineering: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 839–850. [Google Scholar] [CrossRef]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar] [PubMed]
- Mena, E.; Owenius, R.; Turkbey, B.; Sherry, R.; Bratslavsky, G.; Macholl, S.; Miller, M.P.; Somer, E.J.; Lindenberg, L.; Shih, J.; et al. [¹⁸F]fluciclatide in the in vivo evaluation of human melanoma and renal tumors expressing αvβ 3 and α vβ 5 integrins. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1879–1888. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.W.; Hong, H.K.; Jeon, J.; Choi, J.Y.; Kim, M.; Kim, P.; Lee, B.C.; Woo, S.J. FITC-Labeled RGD Peptides as Novel Contrast Agents for Functional Fluorescent Angiographic Detection of Retinal and Choroidal Neovascularization. Cells 2023, 12, 1902. https://doi.org/10.3390/cells12141902
Choi SW, Hong HK, Jeon J, Choi JY, Kim M, Kim P, Lee BC, Woo SJ. FITC-Labeled RGD Peptides as Novel Contrast Agents for Functional Fluorescent Angiographic Detection of Retinal and Choroidal Neovascularization. Cells. 2023; 12(14):1902. https://doi.org/10.3390/cells12141902
Chicago/Turabian StyleChoi, Seung Woo, Hye Kyoung Hong, Jehwi Jeon, Ji Young Choi, Minah Kim, Pilhan Kim, Byung Chul Lee, and Se Joon Woo. 2023. "FITC-Labeled RGD Peptides as Novel Contrast Agents for Functional Fluorescent Angiographic Detection of Retinal and Choroidal Neovascularization" Cells 12, no. 14: 1902. https://doi.org/10.3390/cells12141902
APA StyleChoi, S. W., Hong, H. K., Jeon, J., Choi, J. Y., Kim, M., Kim, P., Lee, B. C., & Woo, S. J. (2023). FITC-Labeled RGD Peptides as Novel Contrast Agents for Functional Fluorescent Angiographic Detection of Retinal and Choroidal Neovascularization. Cells, 12(14), 1902. https://doi.org/10.3390/cells12141902







