Neutrophil Extracellular Traps Drive Dacryolithiasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Human Tissue Samples
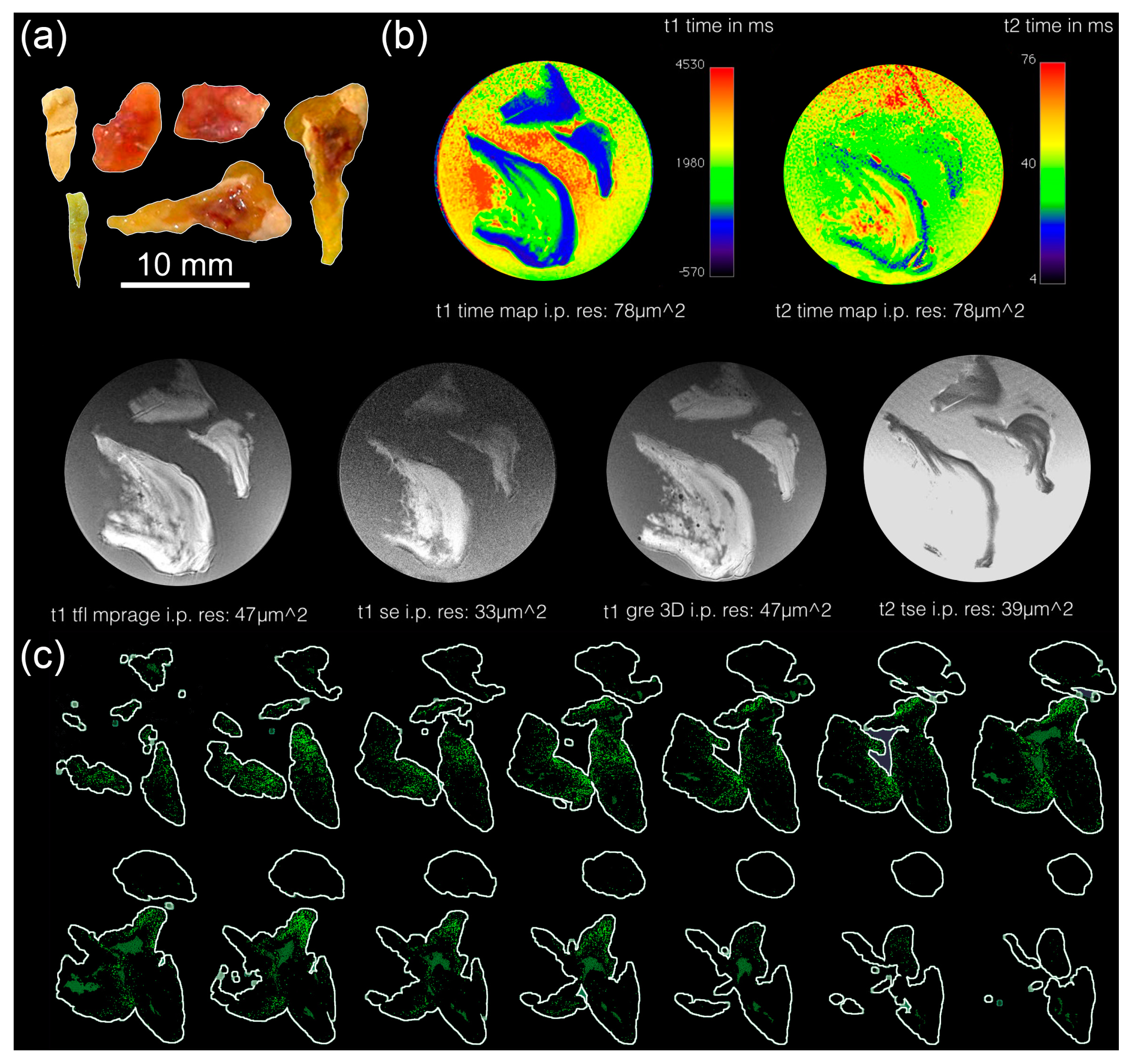
2.3. Magnetic Resonance Tomography (MRT)
2.4. Microcomputed Tomography (µCT)
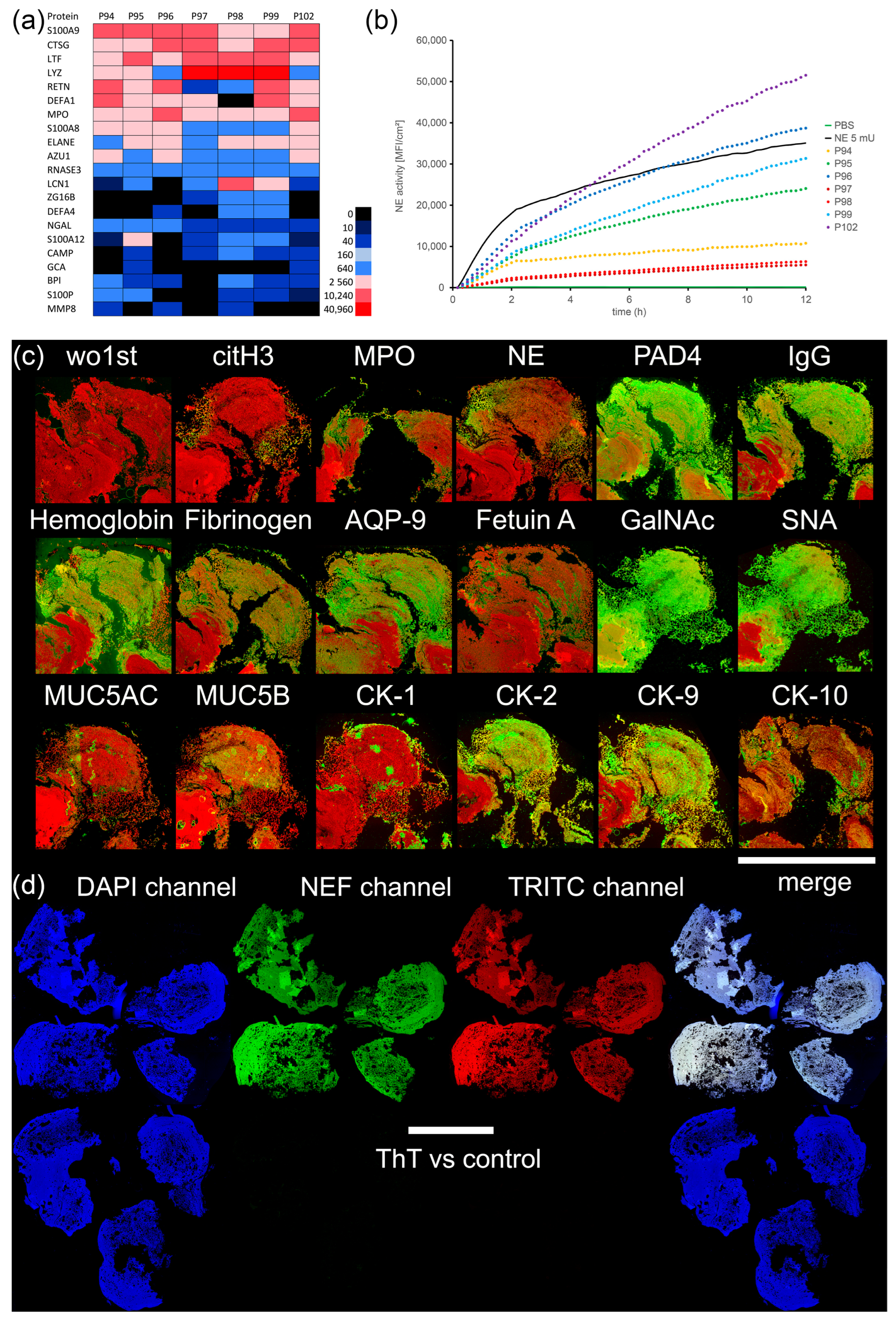
2.5. Mass Spectrometry (LC-ESI-MS)
2.6. Neutrophil Elastase (NE) Activity Measurement
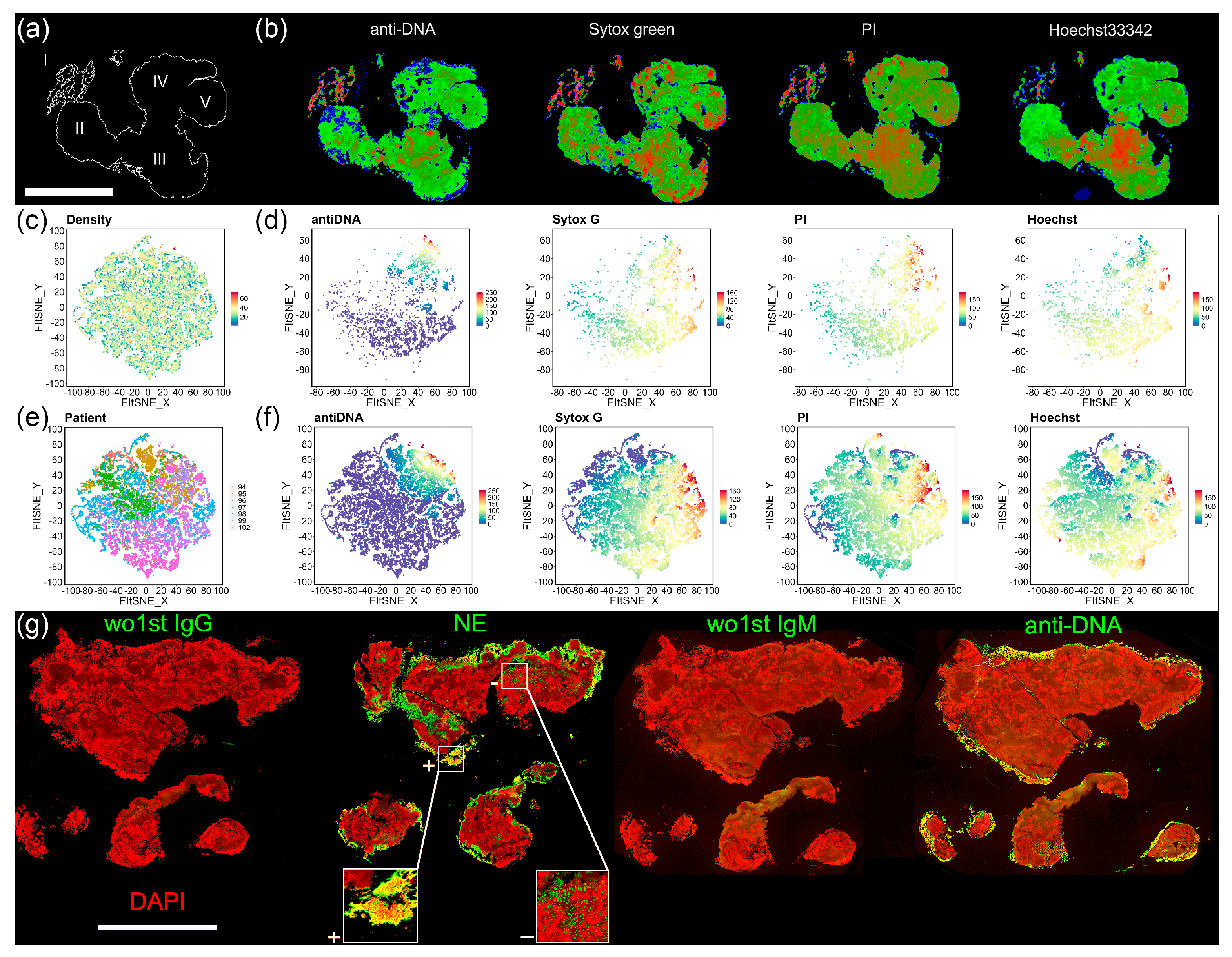
2.7. Histochemistry/Immune Fluorescence (IF)
2.8. Macroscopy and Fluorescence Microscopy
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulsen, F.P.; Schaudig, U.; Fabian, A.; Ehrich, D.; Sel, S. TFF peptides and mucins are major components of dacryoliths. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Hu, K.Y.; Kamal, S.; Andron, A.; Della Rocca, R.C.; Ali, M.J.; Nair, A.G. Dacryolithiasis: A Review. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kominek, P.; Doskarova, S.; Svagera, Z.; Lach, K.; Cervenka, S.; Zelenik, K.; Matousek, P. Lacrimal sac dacryoliths (86 samples): Chemical and mineralogic analyses. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Yazici, B.; Hammad, A.M.; Meyer, D.R. Lacrimal sac dacryoliths: Predictive factors and clinical characteristics. Ophthalmology 2001, 108, 1308–1312. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell. Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef]
- Perry, L.J.; Jakobiec, F.A.; Zakka, F.R. Bacterial and mucopeptide concretions of the lacrimal drainage system: An analysis of 30 cases. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 126–133. [Google Scholar] [CrossRef]
- Kally, P.M.; Omari, A.; Schlachter, D.M.; Folberg, R.; Nesi-Eloff, F. Microbial profile of lacrimal system Dacryoliths in American Midwest patient population. Taiwan J. Ophthalmol. 2022, 12, 330–333. [Google Scholar] [CrossRef]
- Nomura, Y.; Nagata, Y.; Kashima, Y.; Hao, H. A rare case of a giant dacryolith removed by Dacryocystorhinostomy (DCR). Asian J. Surg. 2020, 43, 1010–1011. [Google Scholar] [CrossRef]
- Ali, M.J.; Heichel, J.; Paulsen, F. Immunohistochemical Analysis of the Lacrimal Sac Mucopeptide Concretions. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Schicht, M.; Heichel, J.; Nadimpalli, S.K.; Paulsen, F. Electron microscopic features of the lacrimal sac mucopeptide concretions. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Scholz, M.; Singh, S.; Heichel, J.; Paulsen, F. Etiopathogenesis of lacrimal sac mucopeptide concretions: Insights from cinematic rendering techniques. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Kuckleburg, C.J.; Newman, P.J. Neutrophil proteinase 3 acts on protease-activated receptor-2 to enhance vascular endothelial cell barrier function. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 275–284. [Google Scholar] [CrossRef]
- Brandt, K.; Lundell, K.; Brismar, K. Neutrophil-derived azurocidin cleaves insulin-like growth factor-binding protein-1, -2 and -4. Growth Horm. IGF Res. 2011, 21, 167–173. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a Novel Host Defense Peptide of Innate Immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, W. Defensins in innate immunity. Curr. Opin. Hematol. 2014, 21, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Boettcher, M.; Dolling, M.; Heuer, A.; Hohberger, B.; Leppkes, M.; Naschberger, E.; Schapher, M.; Schauer, C.; Schoen, J.; et al. Moonlighting chromatin: When DNA escapes nuclear control. Cell Death Differ. 2023, 30, 861–875. [Google Scholar] [CrossRef]
- Wang, H.; Stehr, A.M.; Singh, J.; Zlatar, L.; Hartmann, A.; Evert, K.; Naschberger, E.; von Stillfried, S.; Boor, P.; Munoz, L.E.; et al. Anti-DNA-IgM Favors the Detection of NET-Associated Extracellular DNA. Int. J. Mol. Sci. 2023, 24, 4101. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martinez-Guzman, M.A.; Iniguez-Gutierrez, L.; Garcia-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhofer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Munoz, L.E.; Boeltz, S.; Bilyy, R.; Schauer, C.; Mahajan, A.; Widulin, N.; Gruneboom, A.; Herrmann, I.; Boada, E.; Rauh, M.; et al. Neutrophil Extracellular Traps Initiate Gallstone Formation. Immunity 2019, 51, 443–450.e4. [Google Scholar] [CrossRef]
- Schapher, M.; Koch, M.; Weidner, D.; Scholz, M.; Wirtz, S.; Mahajan, A.; Herrmann, I.; Singh, J.; Knopf, J.; Leppkes, M.; et al. Neutrophil Extracellular Traps Promote the Development and Growth of Human Salivary Stones. Cells 2020, 9, 2139. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef]
- Jarva, M.A.; Lingford, J.P.; John, A.; Soler, N.M.; Scott, N.E.; Goddard-Borger, E.D. Trefoil factors share a lectin activity that defines their role in mucus. Nat. Commun. 2020, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Coleman, C.; Ionescu-Zanetti, C.; Carter, S.A.; Krishna, V.; Grover, R.K.; Roy, R.; Singh, S. Mechanism of thioflavin T binding to amyloid fibrils. J. Struct. Biol. 2005, 151, 229–238. [Google Scholar] [CrossRef]
- Hawe, A.; Sutter, M.; Jiskoot, W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm. Res. 2008, 25, 1487–1499. [Google Scholar] [CrossRef]
- Mano, F.; Takimoto, H.; Oe, M.; Chang, K.C.; Mano, T.; Yoshida, Y. Proteomic Analysis of Dacryoliths from Patients with or without Topical Rebamipide Treatment. Biomed. Hub 2018, 3, 1–11. [Google Scholar] [CrossRef]

| Core | Surface | |
|---|---|---|
| MUC5B (57%) | 0.14 | 0.29 |
| Fetuin A (57%) | 0.43 | 0.57 |
| citH3 (43%) | 0.14 | 0.71 |
| CK-10 (100%) | 0.71 | 1.00 |
| NE (43%) | 0.86 | 1.00 |
| MPO (71%) | 0.29 | 1.14 |
| MUC5AC (86%) | 0.57 | 1.14 |
| CK-1 (86%) | 0.57 | 2.00 |
| Fibrinogen (86%) | 0.86 | 1.86 |
| AQP-9 (86%) | 1.00 | 1.86 |
| CK-9 (86%) | 1.57 | 1.71 |
| Hemoglobin (100%) | 1.86 | 1.71 |
| CK-2 (100%) | 1.43 | 2.14 |
| SNA (100%) | 1.57 | 2.57 |
| IgG (100%) | 2.00 | 2.57 |
| PAD4 (100%) | 2.14 | 2.71 |
| GalNAc (100%) | 2.43 | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlatar, L.; Timm, T.; Lochnit, G.; Bilyy, R.; Bäuerle, T.; Munoz-Becerra, M.; Schett, G.; Knopf, J.; Heichel, J.; Ali, M.J.; et al. Neutrophil Extracellular Traps Drive Dacryolithiasis. Cells 2023, 12, 1857. https://doi.org/10.3390/cells12141857
Zlatar L, Timm T, Lochnit G, Bilyy R, Bäuerle T, Munoz-Becerra M, Schett G, Knopf J, Heichel J, Ali MJ, et al. Neutrophil Extracellular Traps Drive Dacryolithiasis. Cells. 2023; 12(14):1857. https://doi.org/10.3390/cells12141857
Chicago/Turabian StyleZlatar, Leticija, Thomas Timm, Günter Lochnit, Rostyslav Bilyy, Tobias Bäuerle, Marco Munoz-Becerra, Georg Schett, Jasmin Knopf, Jens Heichel, Mohammad Javed Ali, and et al. 2023. "Neutrophil Extracellular Traps Drive Dacryolithiasis" Cells 12, no. 14: 1857. https://doi.org/10.3390/cells12141857
APA StyleZlatar, L., Timm, T., Lochnit, G., Bilyy, R., Bäuerle, T., Munoz-Becerra, M., Schett, G., Knopf, J., Heichel, J., Ali, M. J., Schapher, M., Paulsen, F., & Herrmann, M. (2023). Neutrophil Extracellular Traps Drive Dacryolithiasis. Cells, 12(14), 1857. https://doi.org/10.3390/cells12141857












