A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development
Abstract
1. Introduction
2. The Protein Kinase Akt
3. Role of Akt in Cell Cycle Control
3.1. Akt Is Involved in Regulation of Mitosis
3.2. Akt Affects Progression of Meiosis
4. Role of Akt in Oogenesis and Folliculogenesis
4.1. Akt Regulates Follicle Development
4.2. Akt Promotes Survival of GCs
4.3. Activity of Akt in CCs
4.4. Akt Regulates Signaling between CCs and Oocytes
4.5. Role of Akt in Expansion of CCs
5. The Role of Akt in mRNA Translation
5.1. Akt Regulates mTOR Activity during Mitosis
5.2. Akt Affects mTORC1 Activity during Oocyte Meiosis
6. Akt in Zygotic Transition and Early Embryo Development
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, M.; Kihara, M.; Hennebold, J.D.; Eppig, J.J.; Viveiros, M.M.; Emery, B.R.; Carrell, D.T.; Kirkman, N.J.; Meczekalski, B.; Zhou, J.; et al. H1FOO Is Coupled to the Initiation of Oocytic Growth. Biol. Reprod. 2005, 72, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Z.; Kageyama, S.-I.; Aoki, F. Degradation of maternal mRNA in mouse embryos: Selective degradation of specific mRNAs after fertilization. Mol. Reprod. Dev. 2005, 72, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.D.; Lasko, P. Translational Control in Oocyte Development. Cold Spring Harb. Perspect. Biol. 2011, 3, a002758. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Sette, C. Post-Transcriptional Control of Gene Expression in Mouse Early Embryo Development: A View from the Tip of the Iceberg. Genes 2011, 2, 345–359. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Topisirovic, I.; Sonenberg, N. mRNA translation and energy metabolism in cancer: The role of the MAPK and mTORC1 pathways. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 355–367. [Google Scholar] [CrossRef]
- Kandel, E.S.; Skeen, J.; Majewski, N.; Di Cristofano, A.; Pandolfi, P.P.; Feliciano, C.S.; Gartel, A.; Hay, N. Activation of Akt/Protein Kinase B Overcomes a G2/M Cell Cycle Checkpoint Induced by DNA Damage. Mol. Cell. Biol. 2002, 22, 7831–7841. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef]
- Hanada, M.; Feng, J.; Hemmings, B.A. Structure, regulation and function of PKB/AKT—A major therapeutic target. Biochim. Biophys. Acta-Proteins Proteom. 2004, 1697, 3–16. [Google Scholar] [CrossRef]
- Hollander, M.C.; Maier, C.R.; Hobbs, E.A.; Ashmore, A.R.; Linnoila, R.I.; Dennis, P.A. Akt1 deletion prevents lung tumorigenesis by mutant K-ras. Oncogene 2011, 30, 1812–1821. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Downward, J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 1998, 10, 262–267. [Google Scholar] [CrossRef]
- Woodgett, J.R. Recent advances in the protein kinase B signaling pathway. Curr. Opin. Cell Biol. 2005, 17, 150–157. [Google Scholar] [CrossRef]
- Ebner, M.; Lučić, I.; Leonard, T.A.; Yudushkin, I. PI(3,4,5)P3 Engagement Restricts Akt Activity to Cellular Membranes. Mol. Cell 2017, 65, 416–431.e6. [Google Scholar] [CrossRef]
- Calleja, V.; Alcor, D.; Laguerre, M.; Park, J.; Vojnovic, B.; Hemmings, B.A.; Downward, J.; Parker, P.J.; Larijani, B. Intramolecular and Intermolecular Interactions of Protein Kinase B Define Its Activation In Vivo. PLoS Biol. 2007, 5, e95. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Lietzke, S.E.; Bose, S.; Cronin, T.; Klarlund, J.; Chawla, A.; Czech, M.P.; Lambright, D.G. Structural Basis of 3-Phosphoinositide Recognition by Pleckstrin Homology Domains. Mol. Cell 2000, 6, 385–394. [Google Scholar] [CrossRef]
- Bu, L.; Wang, H.; Pan, J.; Chen, L.; Xing, F.; Wu, J.; Li, S.; Guo, D. PTEN suppresses tumorigenesis by directly dephosphorylating Akt. Signal Transduct. Target. Ther. 2021, 6, 262. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Gao, T.; Furnari, F.; Newton, A.C. PHLPP: A Phosphatase that Directly Dephosphorylates Akt, Promotes Apoptosis, and Suppresses Tumor Growth. Mol. Cell 2005, 18, 13–24. [Google Scholar] [CrossRef]
- Brognard, J.; Sierecki, E.; Gao, T.; Newton, A.C. PHLPP and a Second Isoform, PHLPP2, Differentially Attenuate the Amplitude of Akt Signaling by Regulating Distinct Akt Isoforms. Mol. Cell 2007, 25, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.M.; Tabellini, G.; Bressanin, D.; Ognibene, A.; Goto, K.; Cocco, L.; Evangelisti, C. The emerging multiple roles of nuclear Akt. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.; Testa, J.R. Diverse Mechanisms of AKT Pathway Activation in Human Malignancy. Curr. Cancer Drug Targets 2013, 13, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Baldin, V.; Theis-Febvre, N.; Benne, C.; Froment, C.; Cazales, M.; Burlet-Schiltz, O.; Ducommun, B. PKB/Akt phosphorylates the CDC25B phosphatase and regulates its intracellular localisation. Biol. Cell 2003, 95, 547–554. [Google Scholar] [CrossRef]
- Ornelas, I.M.; Silva, T.M.; Fragel-Madeira, L.; Ventura, A.L.M. Inhibition of PI3K/Akt Pathway Impairs G2/M Transition of Cell Cycle in Late Developing Progenitors of the Avian Embryo Retina. PLoS ONE 2013, 8, e53517. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Flynn, D.C.; Zhang, Z.; Zhong, X.-S.; Walker, V.; Liu, K.J.; Shi, X.; Jiang, B.-H. G1 cell cycle progression and the expression of G 1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am. J. Physiol. Cell Physiol. 2004, 287, C281–C291. [Google Scholar] [CrossRef]
- Liang, J.; Slingerland, J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2003, 2, 339–345. [Google Scholar] [CrossRef]
- Maddika, S.; Ande, S.R.; Wiechec, E.; Hansen, L.L.; Wesselborg, S.; Los, M. Akt-mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J. Cell Sci. 2008, 121, 979–988. [Google Scholar] [CrossRef]
- Stern, A.D.; Smith, G.R.; Santos, L.C.; Sarmah, D.; Zhang, X.; Lu, X.; Iuricich, F.; Pandey, G.; Iyengar, R.; Birtwistle, M.R. Relating individual cell division events to single-cell ERK and Akt activity time courses. Sci. Rep. 2022, 12, 18077. [Google Scholar] [CrossRef]
- Rashid, M.S.; Mazur, T.; Ji, W.; Liu, S.T.; Taylor, W.R. Analysis of the role of GSK3 in the mitotic checkpoint. Sci. Rep. 2018, 8, 14259. [Google Scholar] [CrossRef]
- Leonard, M.; Hill, N.; Bubulya, P.; Kadakia, M. The PTEN-Akt pathway impacts the integrity and composition of mitotic centrosomes. Cell Cycle 2013, 12, 1406–1415. [Google Scholar] [CrossRef]
- Takegahara, N.; Kim, H.; Mizuno, H.; Sakaue-Sawano, A.; Miyawaki, A.; Tomura, M.; Kanagawa, O.; Ishii, M.; Choi, Y. Involvement of Receptor Activator of Nuclear Factor-κB Ligand (RANKL)-induced Incomplete Cytokinesis in the Polyploidization of Osteoclasts. J. Biol. Chem. 2016, 291, 3439–3454. [Google Scholar] [CrossRef]
- Maryu, G.; Matsuda, M.; Aoki, K. Multiplexed Fluorescence Imaging of ERK and Akt Activities and Cell-cycle Progression. Cell Struct. Funct. 2016, 41, 81–92. [Google Scholar] [CrossRef]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Ning, Y.; Halet, G.; Kaldis, P.; Liu, K. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum. Mol. Genet. 2012, 21, 2476–2484. [Google Scholar] [CrossRef]
- Diril, M.K.; Ratnacaram, C.K.; Padmakumar, V.C.; Du, T.; Wasser, M.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 3826–3831. [Google Scholar] [CrossRef]
- Katayama, K.; Fujita, N.; Tsuruo, T. Akt/Protein Kinase B-Dependent Phosphorylation and Inactivation of WEE1Hu Promote Cell Cycle Progression at G2/M Transition. Mol. Cell. Biol. 2005, 25, 5725–5737. [Google Scholar] [CrossRef]
- Wakefield, J.G.; Stephens, D.J.; Tavaré, J.M. A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. J. Cell Sci. 2003, 116, 637–646. [Google Scholar] [CrossRef]
- Kimura, T.; Tomooka, M.; Yamano, N.; Murayama, K.; Matoba, S.; Umehara, H.; Kanai, Y.; Nakano, T. AKT signaling promotes derivation of embryonic germ cells from primordial germ cells. Development 2008, 135, 869–879. [Google Scholar] [CrossRef]
- Tomek, W.; Smiljakovic, T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reproduction 2005, 130, 423–430. [Google Scholar] [CrossRef]
- Kalous, J.; Kubelka, M.; Šolc, P.; Šušor, A.; Motlík, J. AKT (protein kinase B) is implicated in meiotic maturation of porcine oocytes. Reproduction 2009, 138, 645–654. [Google Scholar] [CrossRef]
- Reddy, P.; Adhikari, D.; Zheng, W.; Liang, S.; Hämäläinen, T.; Tohonen, V.; Ogawa, W.; Noda, T.; Volarevic, S.; Huhtaniemi, I.; et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 2009, 18, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Vaccari, S.; Nedachi, T.; Andersen, C.B.; Kovacina, K.S.; Roth, R.A.; Conti, M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006, 25, 5716–5725. [Google Scholar] [CrossRef] [PubMed]
- Kalous, J.; Solc, P.; Baran, V.; Kubelka, M.; Schultz, R.M.; Motlik, J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol. Cell 2006, 98, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Newhall, K.J.; Criniti, A.R.; Cheah, C.S.; Smith, K.C.; Kafer, K.E.; Burkart, A.D.; McKnight, G.S. Dynamic Anchoring of PKA Is Essential during Oocyte Maturation. Curr. Biol. 2006, 16, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, D.; Aono, R.; Hanada, S.; Okumura, E.; Kishimoto, T. Two novel competing pathways establish the threshold for cyclin B-Cdk1 activation at the meiotic G2/M transition. J. Cell Sci. 2016, 129, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Okumura, E.; Fukuhara, T.; Yoshida, H.; Hanada, S.; Kozutsumi, R.; Mori, M.; Tachibana, K.; Kishimoto, T. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol. 2002, 4, 111–116. [Google Scholar] [CrossRef]
- Alcaráz, L.P.; Prellwitz, L.; Alves, G.; Souza-Fabjan, J.M.G.; Dias, A.J.B. Role of phosphoinositide 3-kinase/ protein kinase B/ phosphatase and tensin homologue (PI3K/AKT/PTEN) pathway inhibitors during in vitro maturation of mammalian oocytes on in vitro embryo production: A systematic review. Theriogenology 2022, 189, 42–52. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sato, E. Protein kinase B (PKB/Akt) is required for the completion of meiosis in mouse oocytes. Dev. Biol. 2008, 314, 215–223. [Google Scholar] [CrossRef]
- Andersen, C.B.; Roth, R.A.; Conti, M. Protein Kinase B/Akt Induces Resumption of Meiosis in Xenopus Oocytes. J. Biol. Chem. 1998, 273, 18705–18708. [Google Scholar] [CrossRef]
- Cecconi, S.; Rossi, G.; Santilli, A.; Di Stefano, L.; Hoshino, Y.; Sato, E.; Palmerini, M.G.; Macchiarelli, G. Akt expression in mouse oocytes matured in vivo and in vitro. Reprod. Biomed. Online 2010, 20, 35–41. [Google Scholar] [CrossRef]
- Procházka, R.; Bartková, A.; Němcová, L.; Murín, M.; Gad, A.; Marcollová, K.; Kinterová, V.; Lucas-Hahn, A.; Laurinčík, J. The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium. Int. J. Mol. Sci. 2021, 22, 11148. [Google Scholar] [CrossRef]
- Das, D.; Khan, P.P.; Maitra, S. Participation of PI3-kinase/Akt signalling in insulin stimulation of p34cdc2 activation in zebrafish oocyte: Phosphodiesterase 3 as a potential downstream target. Mol. Cell. Endocrinol. 2013, 374, 46–55. [Google Scholar] [CrossRef]
- Schuh, M.; Ellenberg, J. Self-Organization of MTOCs Replaces Centrosome Function during Acentrosomal Spindle Assembly in Live Mouse Oocytes. Cell 2007, 130, 484–498. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; Fu, J.; Kuang, Y.; Chen, B.; Gu, H.; Luo, Y.; Gu, R.; Zhang, M.; Li, W.; et al. The mechanism of acentrosomal spindle assembly in human oocytes. Science 2022, 378, eabq7361. [Google Scholar] [CrossRef]
- Clift, D.; Schuh, M. Restarting life: Fertilization and the transition from meiosis to mitosis. Nat. Rev. Mol. Cell Biol. 2013, 14, 549–562. [Google Scholar] [CrossRef]
- Gruss, O. Animal Female Meiosis: The Challenges of Eliminating Centrosomes. Cells 2018, 7, 73. [Google Scholar] [CrossRef]
- Tsuruta, F.; Masuyama, N.; Gotoh, Y. The Phosphatidylinositol 3-Kinase (PI3K)-Akt Pathway Suppresses Bax Translocation to Mitochondria. J. Biol. Chem. 2002, 277, 14040–14047. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, J.; Zhu, S.; Ahmed, J.Z.; Li, M.; Shi, D.; Huang, B. PI3K inhibitor reduces in vitro maturation and developmental competence of porcine oocytes. Theriogenology 2020, 157, 432–439. [Google Scholar] [CrossRef]
- De Felici, M.; Klinger, F.G. PI3K/PTEN/AKT Signaling Pathways in Germ Cell Development and Their Involvement in Germ Cell Tumors and Ovarian Dysfunctions. Int. J. Mol. Sci. 2021, 22, 9838. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Zhang, Z.; Xu, D.; Duan, J.; Li, X.; Yang, L.; Hua, R.; Cheng, J.; Li, Q. Isorhamnetin Promotes Estrogen Biosynthesis and Proliferation in Porcine Granulosa Cells via the PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2021, 69, 6535–6542. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef] [PubMed]
- Alberico, H.C.; Woods, D.C. Role of Granulosa Cells in the Aging Ovarian Landscape: A Focus on Mitochondrial and Metabolic Function. Front. Physiol. 2022, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wells, D. The human oocyte and cumulus cells relationship: New insights from the cumulus cell transcriptome. MHR Basic Sci. Reprod. Med. 2010, 16, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Iwase, A.; Ando, H.; Kurotsuchi, S.; Harata, T.; Kikkawa, F. PTEN and Akt expression during growth of human ovarian follicles. J. Assist. Reprod. Genet. 2007, 24, 541–546. [Google Scholar] [CrossRef]
- Brown, C.; LaRocca, J.; Pietruska, J.; Ota, M.; Anderson, L.; Duncan Smith, S.; Weston, P.; Rasoulpour, T.; Hixon, M.L. Subfertility Caused by Altered Follicular Development and Oocyte Growth in Female Mice Lacking PKBalpha/Akt11. Biol. Reprod. 2010, 82, 246–256. [Google Scholar] [CrossRef]
- Bezerra, M.É.S.; Barberino, R.S.; Menezes, V.G.; Gouveia, B.B.; Macedo, T.J.S.; Santos, J.M.S.; Monte, A.P.O.; Barros, V.R.P.; Matos, M.H.T. Insulin-like growth factor-1 (IGF-1) promotes primordial follicle growth and reduces DNA fragmentation through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signalling pathway. Reprod. Fertil. Dev. 2018, 30, 1503. [Google Scholar] [CrossRef]
- Alam, M.H.; Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 2020, 19, 13–23. [Google Scholar] [CrossRef]
- Alam, H.; Maizels, E.T.; Park, Y.; Ghaey, S.; Feiger, Z.J.; Chandel, N.S.; Hunzicker-Dunn, M. Follicle-stimulating Hormone Activation of Hypoxia-inducible Factor-1 by the Phosphatidylinositol 3-Kinase/AKT/Ras Homolog Enriched in Brain (Rheb)/Mammalian Target of Rapamycin (mTOR) Pathway Is Necessary for Induction of Select Protein Markers of Follic. J. Biol. Chem. 2004, 279, 19431–19440. [Google Scholar] [CrossRef]
- Zeleznik, A.J.; Saxena, D.; Little-Ihrig, L. Protein Kinase B Is Obligatory for Follicle-Stimulating Hormone-Induced Granulosa Cell Differentiation. Endocrinology 2003, 144, 3985–3994. [Google Scholar] [CrossRef]
- Bencomo, E.; Pérez, R.; Arteaga, M.-F.; Acosta, E.; Peña, O.; Lopez, L.; Avila, J.; Palumbo, A. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil. Steril. 2006, 85, 474–480. [Google Scholar] [CrossRef]
- Quirk, S.M.; Cowan, R.G.; Harman, R.M.; Hu, C.-L.; Porter, D.A. Ovarian follicular growth and atresia: The relationship between cell proliferation and survival. J. Anim. Sci. 2004, 82 (Suppl. 13), E40–E52. [Google Scholar] [CrossRef]
- Hu, C.-L.; Cowan, R.G.; Harman, R.M.; Quirk, S.M. Cell Cycle Progression and Activation of Akt Kinase Are Required for Insulin-Like Growth Factor I-Mediated Suppression of Apoptosis in Granulosa Cells. Mol. Endocrinol. 2004, 18, 326–338. [Google Scholar] [CrossRef]
- Johnson, A.L.; Bridgham, J.T.; Swenson, J.A. Activation of the Akt/Protein Kinase B Signaling Pathway Is Associated with Granulosa Cell Survival1. Biol. Reprod. 2001, 64, 1566–1574. [Google Scholar] [CrossRef]
- Demiray, S.B.; Goker, E.N.T.; Tavmergen, E.; Yilmaz, O.; Calimlioglu, N.; Soykam, H.O.; Oktem, G.; Sezerman, U. Differential gene expression analysis of human cumulus cells. Clin. Exp. Reprod. Med. 2019, 46, 76–86. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Shimada, M.; Ito, J.; Yamashita, Y.; Okazaki, T.; Isobe, N. Phosphatidylinositol 3-kinase in cumulus cells is responsible for both suppression of spontaneous maturation and induction of gonadotropin-stimulated maturation of porcine oocytes. J. Endocrinol. 2003, 179, 25–34. [Google Scholar] [CrossRef]
- Coticchio, G.; Sereni, E.; Serrao, L.; Mazzone, S.; Iadarola, I.; Borini, A. What criteria for the definition of oocyte quality? Ann. N. Y. Acad. Sci. 2004, 1034, 132–144. [Google Scholar] [CrossRef]
- Artini, P.G.; Tatone, C.; Sperduti, S.; D’Aurora, M.; Franchi, S.; Di Emidio, G.; Ciriminna, R.; Vento, M.; Di Pietro, C.; Stuppia, L.; et al. Cumulus cells surrounding oocytes with high developmental competence exhibit down-regulation of phosphoinositol 1,3 kinase/protein kinase B (PI3K/AKT) signalling genes involved in proliferation and survival. Hum. Reprod. 2017, 32, 2474–2484. [Google Scholar] [CrossRef]
- Haghighat, N.; Van Winkle, L.J. Developmental change in follicular cell-enhanced amino acid uptake into mouse oocytes that depends on intact gap junctions and transport system gly. J. Exp. Zool. 1990, 253, 71–82. [Google Scholar] [CrossRef]
- El-Hayek, S.; Yang, Q.; Abbassi, L.; FitzHarris, G.; Clarke, H.J. Mammalian Oocytes Locally Remodel Follicular Architecture to Provide the Foundation for Germline-Soma Communication. Curr. Biol. 2018, 28, 1124–1131.e3. [Google Scholar] [CrossRef]
- Baena, V.; Terasaki, M. Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle. Sci. Rep. 2019, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.; Goodenough, D.; Sosinsky, G. Three-Dimensional Structure of the Gap Junction Connexon. Biophys. J. 1997, 72, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shang, W.; Wei, D.-L.; Zeng, S.-M. Cited2 protein level in cumulus cells is a biomarker for human embryo quality and pregnancy outcome in one in vitro fertilization cycle. Fertil. Steril. 2016, 105, 1351–1359.e4. [Google Scholar] [CrossRef] [PubMed]
- Gatta, V.; Tatone, C.; Ciriminna, R.; Vento, M.; Franchi, S.; D’Aurora, M.; Sperduti, S.; Cela, V.; Borzì, P.; Palermo, R.; et al. Gene expression profiles of cumulus cells obtained from women treated with recombinant human luteinizing hormone + recombinant human follicle-stimulating hormone or highly purified human menopausal gonadotropin versus recombinant human follicle-stimulatin. Fertil. Steril. 2013, 99, 2000–2008.e1. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Kidder, G.M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Hum. Reprod. Updat. 2015, 21, 340–352. [Google Scholar] [CrossRef]
- Dunn, C.A.; Lampe, P.D. Injury-triggered Akt phosphorylation of Cx43: A ZO-1-driven molecular switch that regulates gap junction size. J. Cell Sci. 2013, 127, 455–464. [Google Scholar] [CrossRef]
- Shimada, M.; Terada, T. Phosphorylation of Connexin-43, Gap Junctional Protein, in Cumulus Cells is Regulated by Mitogen-Activated Protein Kinase and Phosphatidylinositol 3-Kinase during In Vitro Meiotic Resumption in Porcine Follicular Oocytes. J. Mamm. Ova Res. 1999, 16, 37–42. [Google Scholar] [CrossRef][Green Version]
- Shimada, M.; Terada, T. Phosphatidylinositol 3-kinase in cumulus cells and oocytes is responsible for activation of oocyte mitogen-activated protein kinase during meiotic progression beyond the meiosis I stage in pigs. Biol. Reprod. 2001, 64, 1106–1114. [Google Scholar] [CrossRef][Green Version]
- Camaioni, A.; Salustri, A.; Yanagishita, M.; Hascall, V.C. Proteoglycans and Proteins in the Extracellular Matrix of Mouse Cumulus Cell–Oocyte Complexes. Arch. Biochem. Biophys. 1996, 325, 190–198. [Google Scholar] [CrossRef]
- Nagyova, E.; Scsukova, S.; Kalous, J.; Mlynarcikova, A. Effects of RU486 and indomethacin on meiotic maturation, formation of extracellular matrix, and progesterone production by porcine oocyte-cumulus complexes. Domest. Anim. Endocrinol. 2014, 48, 7–14. [Google Scholar] [CrossRef]
- Nagyova, E.; Kalous, J.; Nemcova, L. Increased expression of pentraxin 3 after in vivo and in vitro stimulation with gonadotropins in porcine oocyte-cumulus complexes and granulosa cells. Domest. Anim. Endocrinol. 2016, 56, 29–35. [Google Scholar] [CrossRef]
- Němcová, L.; Nagyová, E.; Petlach, M.; Tománek, M.; Procházka, R. Molecular Mechanisms of Insulin-Like Growth Factor 1 Promoted Synthesis and Retention of Hyaluronic Acid in Porcine Oocyte-Cumulus Complexes1. Biol. Reprod. 2007, 76, 1016–1024. [Google Scholar] [CrossRef]
- Procházka, R.; Petlach, M.; Nagyová, E.; Němcová, L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: Comparison with gonadotropins. Reproduction 2011, 141, 425–435. [Google Scholar] [CrossRef]
- Blaha, M.; Prochazka, R.; Adamkova, K.; Nevoral, J.; Nemcova, L. Prostaglandin E2 stimulates the expression of cumulus expansion-related genes in pigs: The role of protein kinase B. Prostaglandins Other Lipid Mediat. 2017, 130, 38–46. [Google Scholar] [CrossRef]
- Guo, J.; Shi, L.; Gong, X.; Jiang, M.; Yin, Y.; Zhang, X.; Yin, H.; Li, H.; Emori, C.; Sugiura, K.; et al. Oocyte-dependent activation of MTOR in cumulus cells controls the development and survival of cumulus-oocyte complexes. J. Cell Sci. 2016, 129, 3091–3103. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Starkman, B.G.; Cravero, J.D.; Delcarlo, M.; Loeser, R.F. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem. J. 2005, 389, 723–729. [Google Scholar] [CrossRef]
- Varma, S.; Shrivastav, A.; Changela, S.; Khandelwal, R.L. Long-term effects of rapamycin treatment on insulin mediated phosphorylation of Akt/PKB and glycogen synthase activity. Exp. Cell Res. 2008, 314, 1281–1291. [Google Scholar] [CrossRef]
- Kapp, L.D.; Lorsch, J.R. The Molecular Mechanics of Eukaryotic Translation. Annu. Rev. Biochem. 2004, 73, 657–704. [Google Scholar] [CrossRef]
- Dowling, R.J.O.; Topisirovic, I.; Fonseca, B.D.; Sonenberg, N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 433–439. [Google Scholar] [CrossRef]
- Nitta, N.; Nakasu, S.; Shima, A.; Nozaki, K. mTORC1 signaling in primary central nervous system lymphoma. Surg. Neurol. Int. 2016, 7, 475. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, T.; Hall, M.N. TOR, a Central Controller of Cell Growth. Cell 2000, 103, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.-C.; Kennedy, S.G.; O’Leary, M.A.; Sonenberg, N.; Hay, N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998, 12, 502–513. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.C.; Ruggero, D. Targeting Eukaryotic Translation Initiation Factor 4E (eIF4E) in Cancer. Clin. Cancer Res. 2010, 16, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- Kovacina, K.S.; Park, G.Y.; Bae, S.S.; Guzzetta, A.W.; Schaefer, E.; Birnbaum, M.J.; Roth, R.A. Identification of a Proline-rich Akt Substrate as a 14-3-3 Binding Partner. J. Biol. Chem. 2003, 278, 10189–10194. [Google Scholar] [CrossRef]
- Wang, L.; Harris, T.E.; Roth, R.A.; Lawrence, J.C. PRAS40 Regulates mTORC1 Kinase Activity by Functioning as a Direct Inhibitor of Substrate Binding. J. Biol. Chem. 2007, 282, 20036–20044. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. United at last: The tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem. Soc. Trans. 2003, 31, 573–578. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Tee, A.R.; Manning, B.D.; Roux, P.P.; Cantley, L.C.; Blenis, J. Tuberous Sclerosis Complex Gene Products, Tuberin and Hamartin, Control mTOR Signaling by Acting as a GTPase-Activating Protein Complex toward Rheb. Curr. Biol. 2003, 13, 1259–1268. [Google Scholar] [CrossRef]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Wang, X. Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. EMBO J. 2001, 20, 4370–4379. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, T.; Guo, Y.; Sun, T.; Li, H.; Zhang, X.; Yin, H.; Cao, G.; Yin, Y.; Wang, H.; et al. Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E5326–E5333. [Google Scholar] [CrossRef]
- Susor, A.; Jansova, D.; Cerna, R.; Danylevska, A.; Anger, M.; Toralova, T.; Malik, R.; Supolikova, J.; Cook, M.S.; Oh, J.S.; et al. Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR–eIF4F pathway. Nat. Commun. 2015, 6, 6078. [Google Scholar] [CrossRef]
- Šušor, A.; Jelínková, L.; Karabínová, P.; Torner, H.; Tomek, W.; Kovářová, H.; Kubelka, M. Regulation of cap-dependent translation initiation in the early stage porcine parthenotes. Mol. Reprod. Dev. 2008, 75, 1716–1725. [Google Scholar] [CrossRef]
- Kogasaka, Y.; Hoshino, Y.; Hiradate, Y.; Tanemura, K.; Sato, E. Distribution and association of mTOR with its cofactors, raptor and rictor, in cumulus cells and oocytes during meiotic maturation in mice. Mol. Reprod. Dev. 2013, 80, 334–348. [Google Scholar] [CrossRef]
- Jansova, D.; Koncicka, M.; Tetkova, A.; Cerna, R.; Malik, R.; del Llano, E.; Kubelka, M.; Susor, A. Regulation of 4E-BP1 activity in the mammalian oocyte. Cell Cycle 2017, 16, 927–939. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.-L.; Kong, I.-K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- Li, Y.; Chandrakanthan, V.; Day, M.L.; O’Neill, C. Direct Evidence for the Action of Phosphatidylinositol (3,4,5)-Trisphosphate-Mediated Signal Transduction in the 2-Cell Mouse Embryo1. Biol. Reprod. 2007, 77, 813–821. [Google Scholar] [CrossRef]
- Jin, X.L.; Chandrakanthan, V.; Morgan, H.D.; O’Neill, C. Preimplantation Embryo Development in the Mouse Requires the Latency of TRP53 Expression, Which Is Induced by a Ligand-Activated PI3 Kinase/AKT/MDM2-Mediated Signaling Pathway1. Biol. Reprod. 2009, 80, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, J.; Ji, X.; Hua, M.-M.; Liu, M.; Chang, L.; Gu, Y.; Shi, C.; Ni, W.; Liu, J.; et al. Regulation of the mammalian maternal-to-embryonic transition by eukaryotic translation initiation factor 4E. Development 2021, 148, dev190793. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.; Morris, M.B.; Day, M.L. Amino acid supplementation of a simple inorganic salt solution supports efficient in vitro maturation (IVM) of bovine oocytes. Sci. Rep. 2019, 9, 11739. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.C.; Biggers, J.D. Chemically defined media and the culture of mammalian preimplantation embryos: Historical perspective and current issues. Hum. Reprod. Updat. 2003, 9, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Zamfirescu, R.C.; Day, M.L.; Morris, M.B. mTORC1/2 signaling is downregulated by amino acid-free culture of mouse preimplantation embryos and is only partially restored by amino acid readdition. Am. J. Physiol. Cell Physiol. 2021, 320, C30–C44. [Google Scholar] [CrossRef]
- Song, B.-S.; Jeong, P.-S.; Lee, J.-H.; Lee, M.-H.; Yang, H.-J.; Choi, S.-A.; Lee, H.-Y.; Yoon, S.-B.; Park, Y.-H.; Jeong, K.-J.; et al. The effects of kinase modulation on in vitro maturation according to different cumulus-oocyte complex morphologies. PLoS ONE 2018, 13, e0205495. [Google Scholar] [CrossRef]
- Baran, V.; Fabian, D.; Rehak, P. Akt/PKB plays role of apoptosis relay on entry into first mitosis of mouse embryo. Zygote 2013, 21, 406–416. [Google Scholar] [CrossRef]
- Fiorenza, M.T.; Torcia, S.; Canterini, S.; Bevilacqua, A.; Narducci, M.G.; Ragone, G.; Croce, C.M.; Russo, G.; Mangia, F. TCL1 promotes blastomere proliferation through nuclear transfer, but not direct phosphorylation, of AKT/PKB in early mouse embryos. Cell Death Differ. 2008, 15, 420–422. [Google Scholar] [CrossRef]
- Fiorenza, M.T.; Russo, G.; Narducci, M.G.; Bresin, A.; Mangia, F.; Bevilacqua, A. Protein kinase Akt2/PKBβ is involved in blastomere proliferation of preimplantation mouse embryos. J. Cell. Physiol. 2020, 235, 3393–3401. [Google Scholar] [CrossRef]
- Chen, J.; Lian, X.; Du, J.; Xu, S.; Wei, J.; Pang, L.; Song, C.; He, L.; Wang, S. Inhibition of phosphorylated Ser473-Akt from translocating into the nucleus contributes to 2-cell arrest and defective zygotic genome activation in mouse preimplantation embryogenesis. Dev. Growth Differ. 2016, 58, 280–292. [Google Scholar] [CrossRef]
- Ashry, M.; Rajput, S.K.; Folger, J.K.; Knott, J.G.; Hemeida, N.A.; Kandil, O.M.; Ragab, R.S.; Smith, G.W. Functional role of AKT signaling in bovine early embryonic development: Potential link to embryotrophic actions of follistatin. Reprod. Biol. Endocrinol. 2018, 16, 1. [Google Scholar] [CrossRef]
- Riley, J.K.; Carayannopoulos, M.O.; Wyman, A.H.; Chi, M.; Ratajczak, C.K.; Moley, K.H. The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Dev. Biol. 2005, 284, 377–386. [Google Scholar] [CrossRef]
- Buttrick, G.J.; Beaumont, L.M.A.; Leitch, J.; Yau, C.; Hughes, J.R.; Wakefield, J.G. Akt regulates centrosome migration and spindle orientation in the early Drosophila melanogaster embryo. J. Cell Biol. 2008, 180, 537–548. [Google Scholar] [CrossRef]
- Xu, S.; Pang, L.; Liu, Y.; Lian, X.; Mo, K.; Lv, R.; Zhu, H.; Lv, C.; Lin, J.; Sun, J.; et al. Akt plays indispensable roles during the first cell lineage differentiation of mouse. J. Mol. Histol. 2019, 50, 369–374. [Google Scholar] [CrossRef]
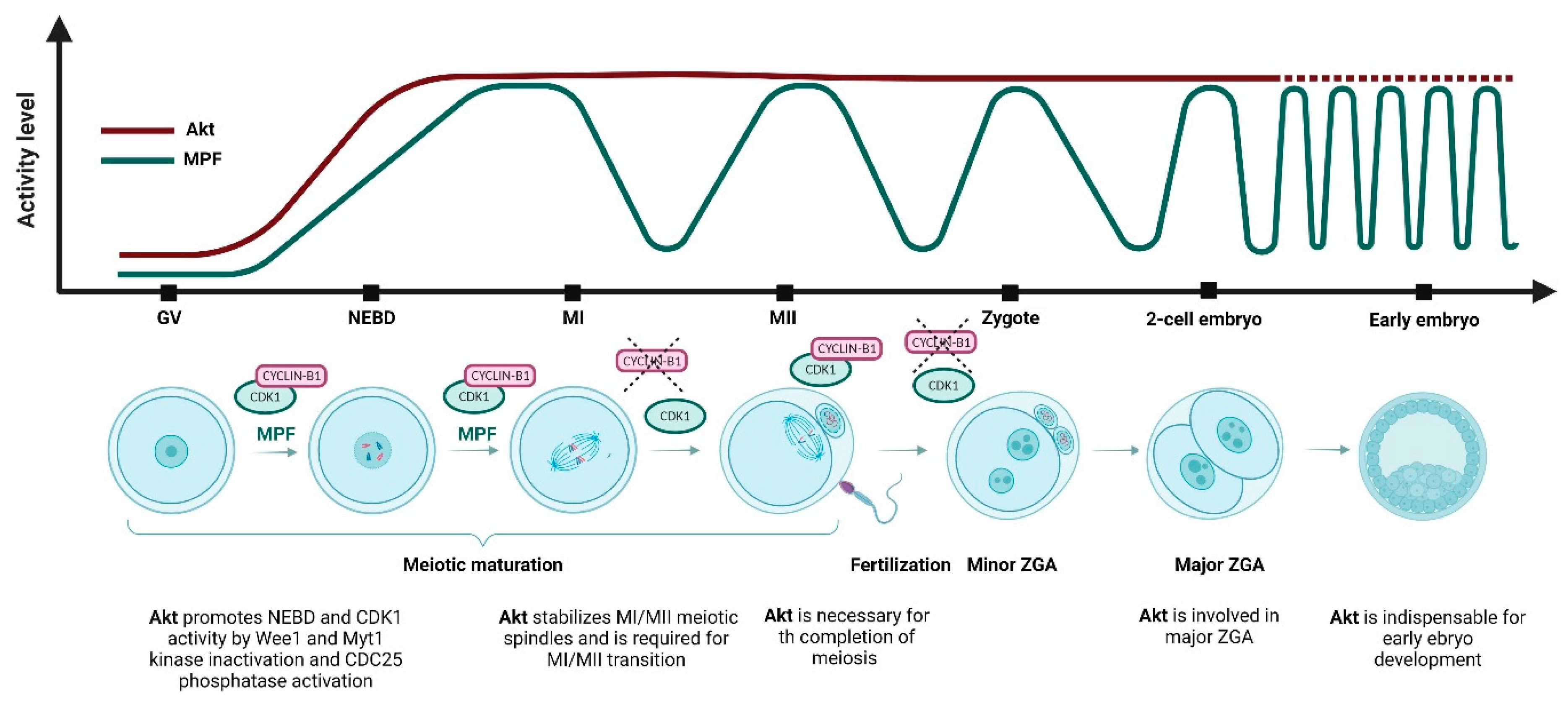
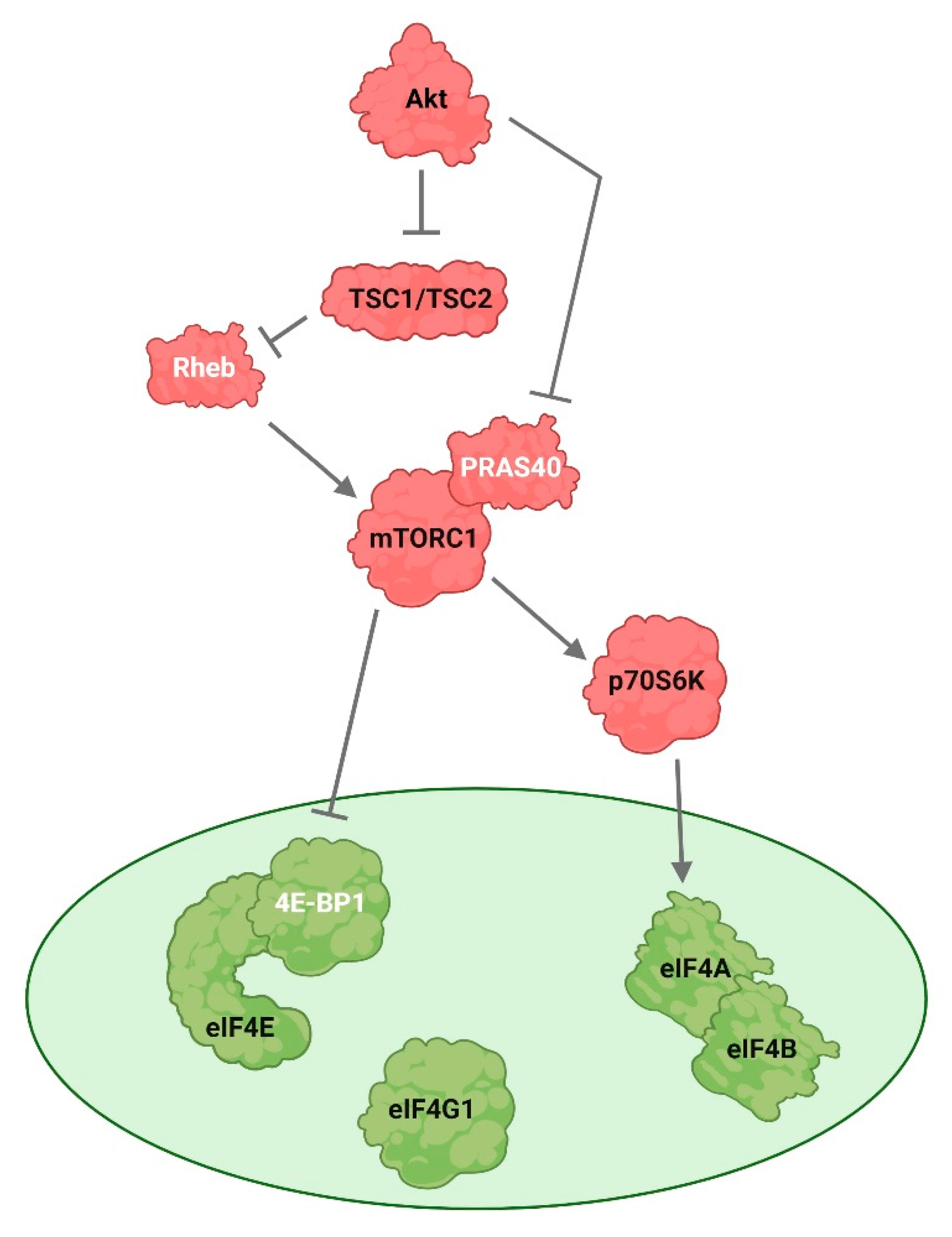
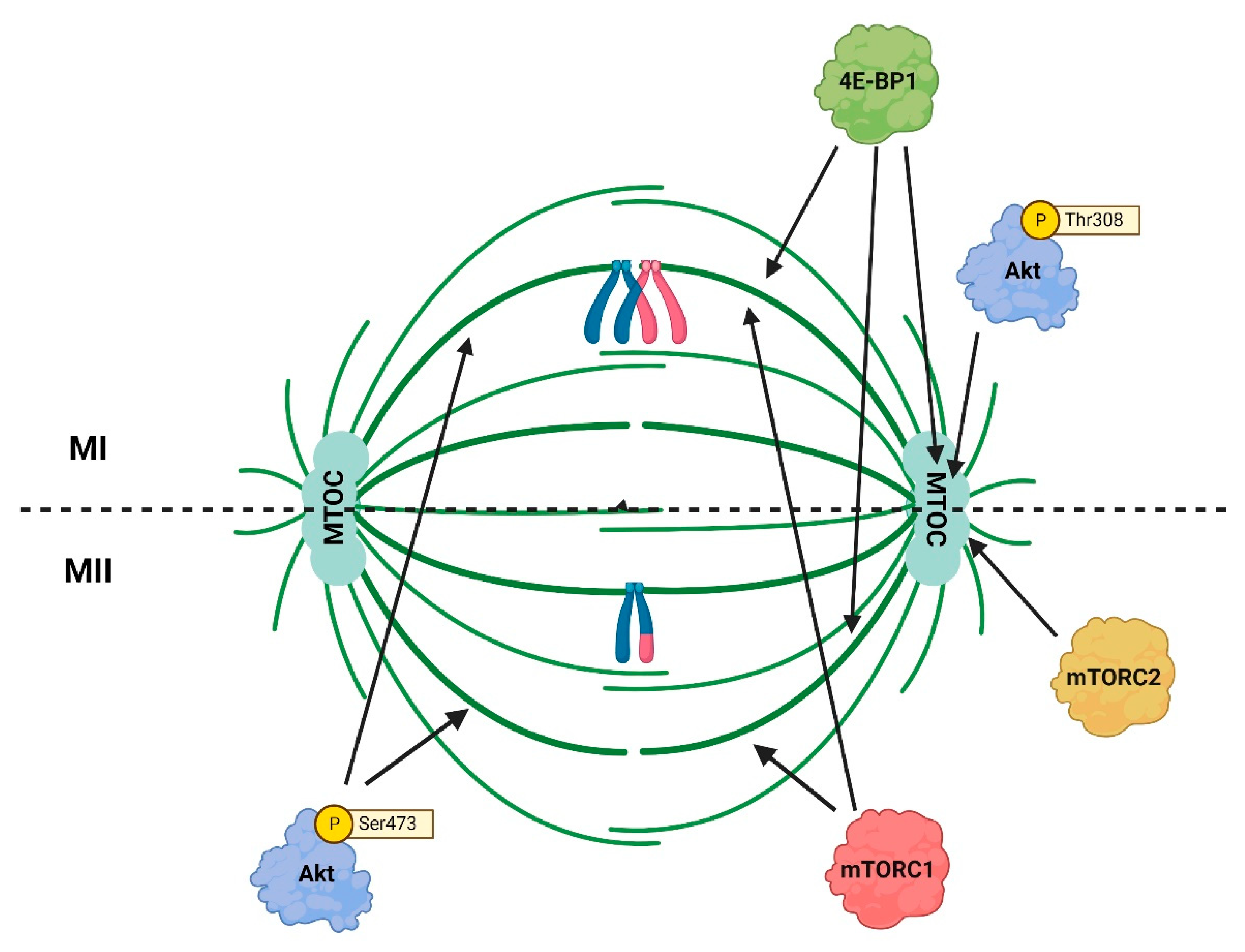
| Cell Cycle Stage | Akt Role in Mitosis | References |
|---|---|---|
| G1 phase | Akt/mTOR stimulates expression of cyclin D1, CDK4 and CDC25A, that are involved in cell growth and proliferation | [26] |
| G1/S | Akt promotes inactivation of p21WAF1 and p27kip1, the CDK2 inhibitors | [27,28] |
| S/G2 | Enhanced Akt activity indicates a role for Akt in the S/G2 transition | [29] |
| G2/M | Akt regulates cell cycle progression by direct phosphorylation and inactivation of Wee1 and Myt1 kinases and activates the CDC25 phosphatase | [23,24,25] |
| M-phase | Akt is involved in the control of the mitotic spindle checkpoint affects the integrity and composition of mitotic centrosomes | [30,31] |
| Cytokinesis | Akt participates in the regulation of cytokinesis | [32] |
| Meiosis Stage | Akt Role in Meiosis | References |
|---|---|---|
| Prophase of 1st meiosis | Akt is involved in CDK1 activation and GVBD induction during meiosis resumption | [42,43,44,45,46] |
| MI/MII transition | Akt is required for the transition from meiotic metaphase I (MI) to metaphase II (MII) | [39,43,47,48,49] |
| MI and MII-phase | Akt participates in the formation and stabilization of the MI and MII meiotic spindles | [43,48,50] |
| MI and MII-phase | Akt contributes to centrosome integrity in oocytes | [43] |
| Meiosis completion | Akt is necessary for completion of meiosis | [47,48,51] |
| Stage of Early Embryo | Role of Akt in the Early Embryo Development | References |
|---|---|---|
| 1-cell | Akt is essential for the entry of 1-cell mouse embryos into the first mitosis | [127,128] |
| 2-cell, ZGA | pSer473-Akt is localized to the nuclei of 2-cell embryos, Akt is possibly involved in the major ZGA of 2-cell mouse embryos | [129,130] |
| 8- to 16-cell | Akt is important for mouse embryo development to the 8- to 16-cells | [131] |
| Blastocyst | Akt is necessary for embryo development to the blastocyst stage | [120,126,128,131,132] |
| Early embryo | In the early Drosophila melanogaster embryo, Akt regulates centrosome migration, promotes mitotic spindle orientation and proper spindle morphology | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalous, J.; Aleshkina, D.; Anger, M. A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development. Cells 2023, 12, 1830. https://doi.org/10.3390/cells12141830
Kalous J, Aleshkina D, Anger M. A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development. Cells. 2023; 12(14):1830. https://doi.org/10.3390/cells12141830
Chicago/Turabian StyleKalous, Jaroslav, Daria Aleshkina, and Martin Anger. 2023. "A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development" Cells 12, no. 14: 1830. https://doi.org/10.3390/cells12141830
APA StyleKalous, J., Aleshkina, D., & Anger, M. (2023). A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development. Cells, 12(14), 1830. https://doi.org/10.3390/cells12141830







