Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples and Patients’ Selection
2.2. Isolation and Culture of Human Granulosa Lutein Cells
2.3. mLTC1 Cell Line
2.4. Dose-Finding Experiments for BPA Concentration
2.5. cAMP Production
2.6. Western Blotting
2.7. Gene Expression
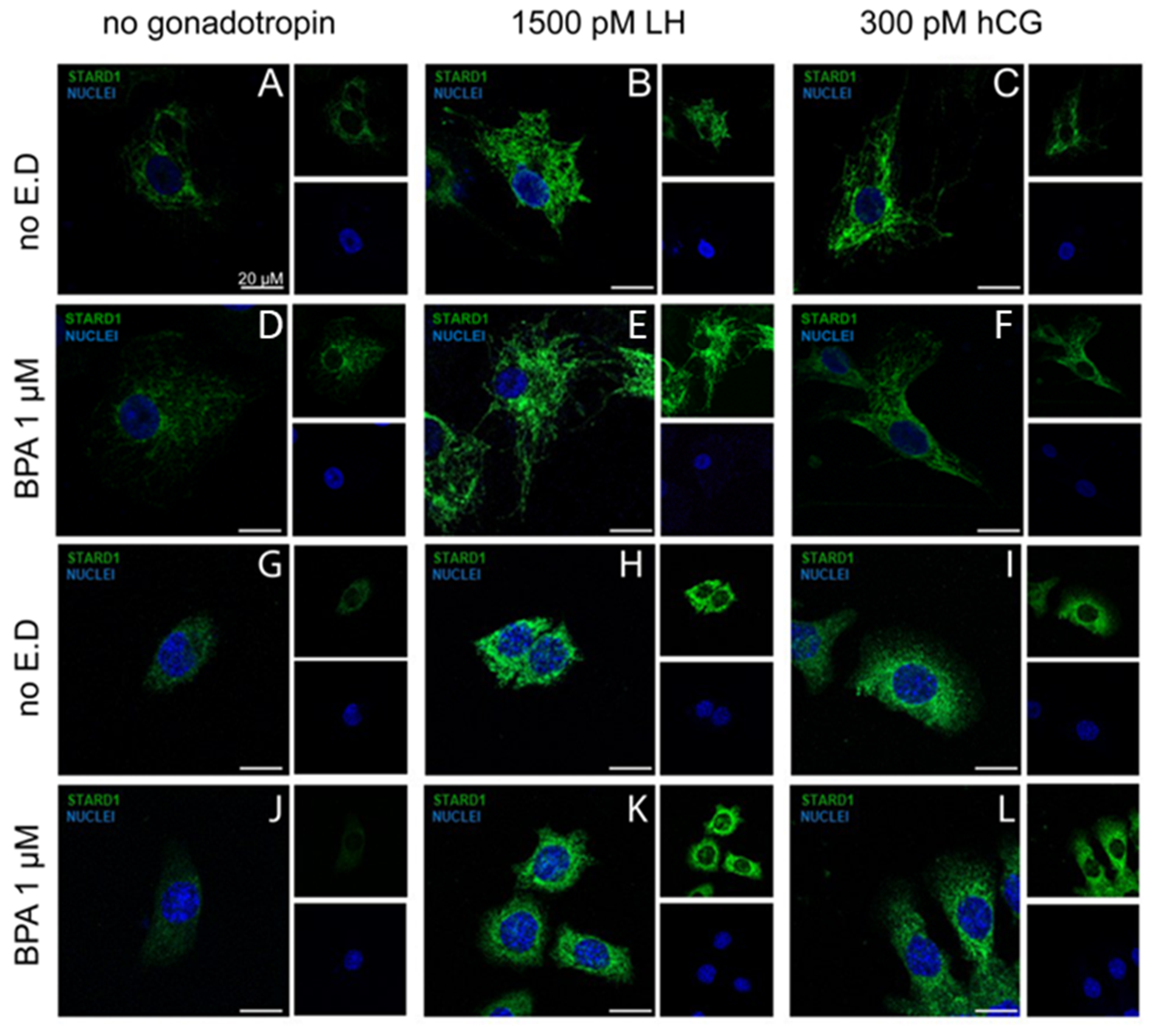
2.8. Immunofluorescence
2.9. Analysis of Steroidogenesis
2.10. Statistical Analysis
3. Results
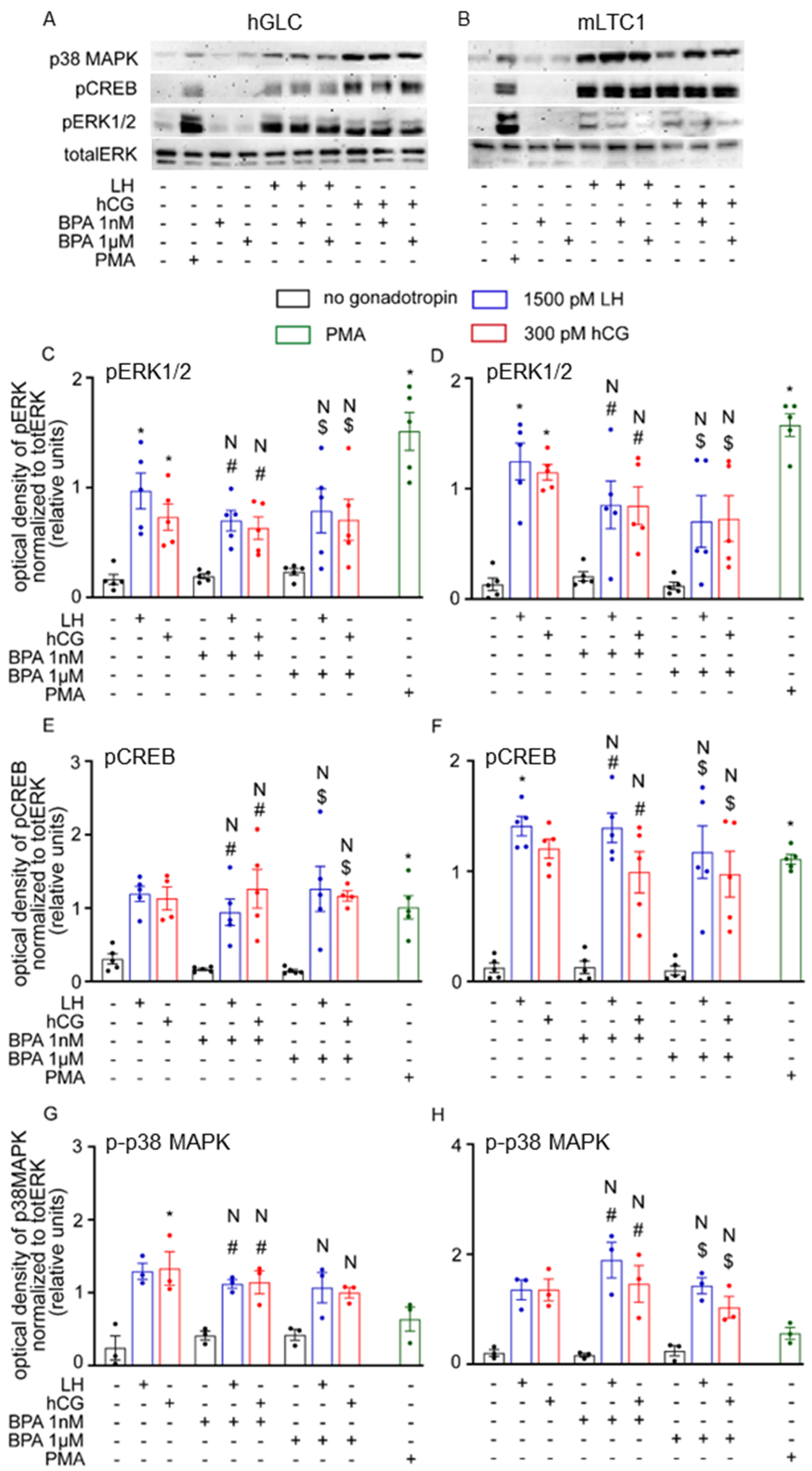
3.1. Cell Signalling Analysis
3.2. Gene Expression Analysis
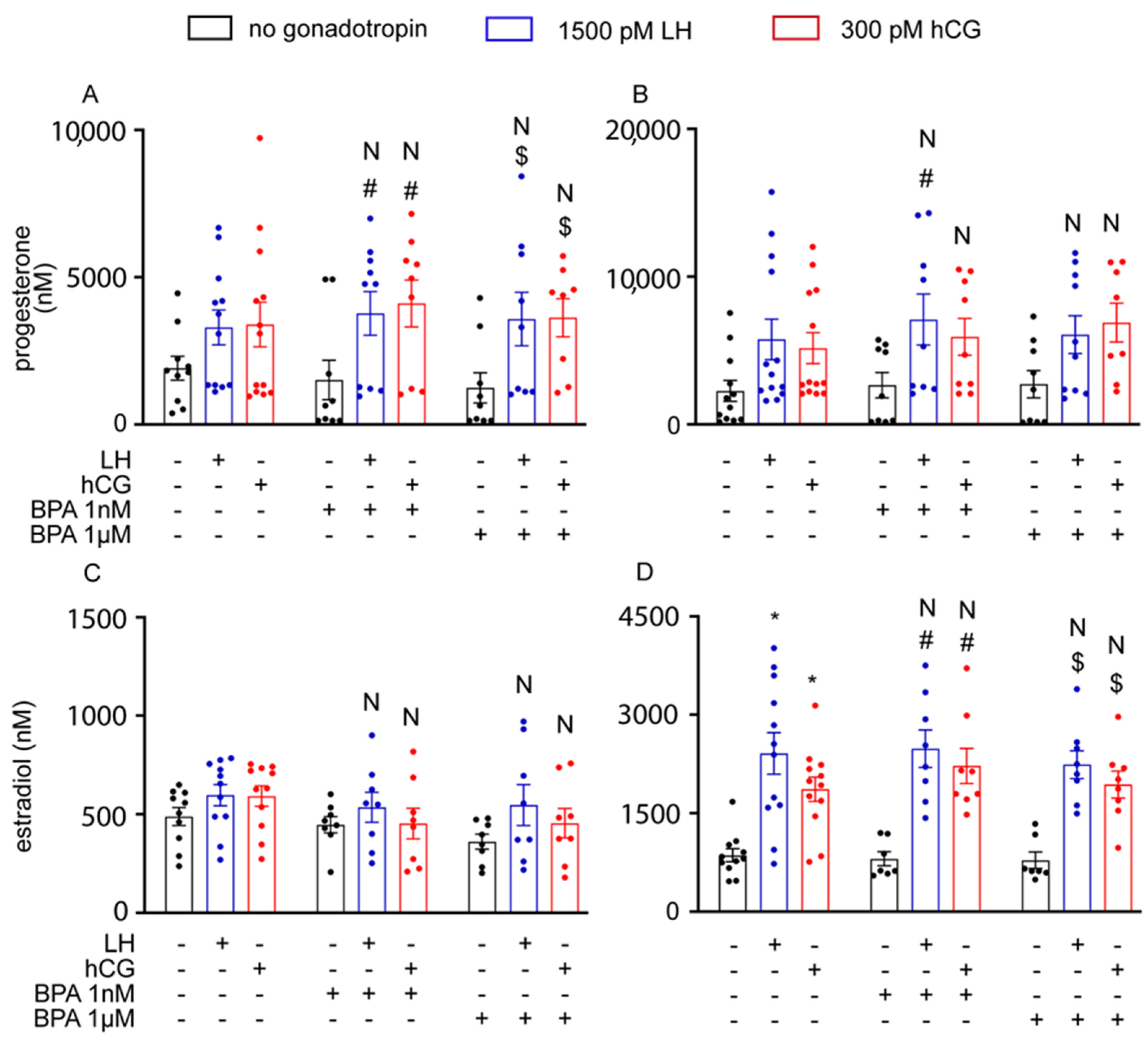
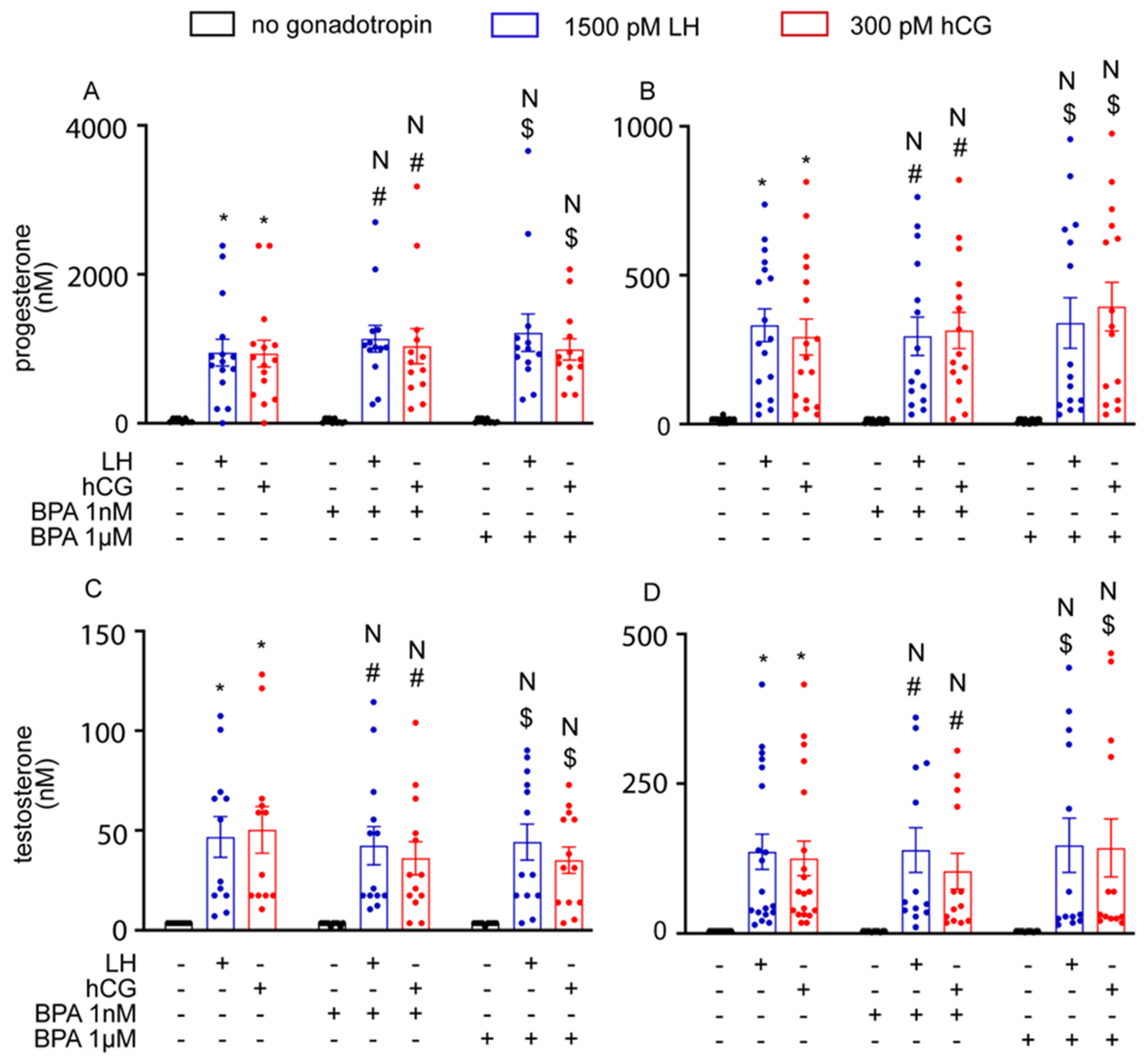
3.3. BPA Did Not Alter Steroidogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; Vandevoort, C.A.; et al. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Nam, S.H.; Seo, Y.M.; Kim, M.G. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 2010, 79, 949–952. [Google Scholar] [CrossRef]
- Lim, D.S.; Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Potential risk of bisphenol a migration from polycarbonate containers after heating, boiling, and microwaving. J. Toxicol. Environ. Health-Part A Curr. Issues 2009, 72, 1285–1291. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-a: An estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 1993, 132, 2279–2286. [Google Scholar] [CrossRef]
- Ougier, E.; Zeman, F.; Antignac, J.P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- Meeker, J.D.; Calafat, A.M.; Hauser, R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ. Sci. Technol. 2010, 44, 1458–1463. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, K.; Watanabe, S. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 780, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-Nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Mok-Lin, E.; Fujimoto, V.Y. Bisphenol A and ovarian steroidogenesis. Fertil. Steril. 2016, 106, 857–863. [Google Scholar] [CrossRef]
- Peretz, J.; Flaws, J.A. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2013, 271, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Peretz, J.; Gupta, R.K.; Singh, J.; Hernández-Ochoa, I.; Flaws, J.A. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the oestradiol biosynthesis pathway. Toxicol. Sci. 2011, 119, 209–217. [Google Scholar] [CrossRef]
- Grasselli, F.; Baratta, L.; Baioni, L.; Bussolati, S.; Ramoni, R.; Grolli, S.; Basini, G. Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 2010, 39, 34–39. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.Y.; Chung, J.Y.; Kim, Y.J.; Park, J.E.; Oh, S.; Yoon, Y.D.; Yoo, K.S.; Yoo, Y.H.; Kim, J.M. Bisphenol a exposure during adulthood causes augmentation of follicular atresia and luteal regression by Decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ. Health Perspect. 2013, 121, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, J.; Liao, L.; Han, S.; Liu, J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008, 283, 12–18. [Google Scholar] [CrossRef]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.M.; Taya, K.; Zhang, S.Y.; et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to oestradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef]
- Nanjappa, M.K.; Simon, L.; Akingbemi, B.T. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef]
- N’Tumba-Byn, T.; Moison, D.; Lacroix, M.; Lecureuil, C.; Lesage, L.; Prud’homme, S.M.; Pozzi-Gaudin, S.; Frydman, R.; Benachi, A.; Livera, G.; et al. Differential Effects of Bisphenol A and Diethylstilbestrol on Human, Rat and Mouse Fetal Leydig Cell Function. PLoS ONE 2012, 7, e51579. [Google Scholar] [CrossRef]
- Mannelli, C.; Szóstek, A.Z.; Lukasik, K.; Carotenuto, C.; Ietta, F.; Romagnoli, R.; Ferretti, C.; Paulesu, L.; Wołczynski, S.; Skarzynski, D.J. Bisphenol A modulates receptivity and secretory function of human decidual cells: An in vitro study. Reproduction 2015, 150, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nikula, H.; Talonpoika, T.; Kaleva, M.; Toppari, J. Inhibition of hCG-stimulated steroidogenesis in cultured mouse Leydig tumor cells by bisphenol A and octylphenols. Toxicol. Appl. Pharmacol. 1999, 157, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Franzo-Romain, M.; Damanpour, S.; Menon, K.M.J. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol. Endocrinol. 2011, 25, 282–290. [Google Scholar] [CrossRef]
- Palak, E.; Lebiedzinska, W.; Lupu, O.; Pulawska, K.; Anisimowicz, S.; Mieczkowska, A.N.; Sztachelska, M.; Niklinska, G.N.; Milewska, G.; Lukasiewicz, M.; et al. Molecular insights underlying the adverse effects of bisphenol A on gonadal somatic cells’ steroidogenic activity. Reprod. Biol. 2023, 23, 100766. [Google Scholar] [CrossRef]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; la Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG Action on the Same Receptor Results in Quantitatively and Qualitatively Different Intracellular Signalling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef]
- Casarini, L.; Moriondo, V.; Marino, M.; Adversi, F.; Capodanno, F.; Grisolia, C.; La Marca, A.; La Sala, G.B.; Simoni, M. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol. Cell. Endocrinol. 2014, 393, 83–91. [Google Scholar] [CrossRef]
- Sperduti, S.; Paradiso, E.; Anzivino, C.; Lazzaretti, C.; Limoncella, S.; D’Alessandro, S.; Roy, N.; Reggianini, F.; Ferrari, T.; Melli, B.; et al. LH increases the response to FSH in granulosa-lutein cells from sub/poor-responder patients in vitro. Hum. Reprod. 2023, 38, 103–112. [Google Scholar] [CrossRef]
- Nordhoff, V.; Sonntag, B.; Von Tils, D.; Götte, M.; Schüring, A.N.; Gromoll, J.; Redmann, K.; Casarini, L.; Simoni, M. Effects of the FSH receptor gene polymorphism p.N680S on cAMP and steroid production in cultured primary human granulosa cells. Reprod. Biomed. Online 2011, 23, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Riccetti, L.; Limoncella, S.; Lazzaretti, C.; Barbagallo, F.; Pacifico, S.; Guerrini, R.; Tagliavini, S.; Trenti, T.; Simoni, M.; et al. Probing the effect of sildenafil on progesterone and testosterone production by an intracellular FRET/BRET combined approach. Biochemistry 2019, 58, 799–808. [Google Scholar] [CrossRef]
- Victor Rebois, R. Establishment of gonadotropin-responsive murine leydig tumor cell line. J. Cell Biol. 1982, 94, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human Luteinizing Hormone and Chorionic Gonadotropin Display Biased Agonism at the LH and LH/CG Receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef] [PubMed]
- Limoncella, S.; Lazzaretti, C.; Paradiso, E.; D’Alessandro, S.; Barbagallo, F.; Pacifico, S.; Guerrini, R.; Tagliavini, S.; Trenti, T.; Santi, D.; et al. Phosphodiesterase (PDE) 5 inhibitors sildenafil, tadalafil and vardenafil impact cAMP-specific PDE8 isoforms-linked second messengers and steroid production in a mouse Leydig tumor cell line. Mol. Cell. Endocrinol. 2022, 542, 111527. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Magagnoli, M.; Mezzullo, M.; Lispi, M.; Limoncella, S.; Tommasini, A.; Pelusi, C.; Santi, D.; Simoni, M.; Pagotto, U.; et al. Exploring the human chorionic gonadotropin induced steroid secretion profile of mouse Leydig tumor cell line 1 by a 20 steroid LC-MS/MS panel. J. Steroid Biochem. Mol. Biol. 2023, 229, 106270. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Baylor Steele, W.; Yates, B.S.; Breed, C.S.; Spencer Williams, E.; Brooks, B.W. Global assessment of bisphenol a in the environment: Review and analysis of its occurrence and bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Riccetti, L.; De Pascali, F.; Gilioli, L.; Potì, F.; Giva, L.B.; Marino, M.; Tagliavini, S.; Trenti, T.; Fanelli, F.; Mezzullo, M.; et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse Leydig cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Paradiso, E.; Lazzaretti, C.; Sperduti, S.; Antoniani, F.; Fornari, G.; Brigante, G.; Di Rocco, G.; Tagliavini, S.; Trenti, T.; Morini, D.; et al. Sphingosine-1 phosphate induces cAMP/PKA-independent phosphorylation of the cAMP response element-binding protein (CREB) in granulosa cells. Mol. Cell. Endocrinol. 2021, 520, 111082. [Google Scholar] [CrossRef]
- Casarini, L.; Lazzaretti, C.; Paradiso, E.; Limoncella, S.; Riccetti, L.; Sperduti, S.; Melli, B.; Marcozzi, S.; Anzivino, C.; Sayers, N.S.; et al. Membrane Estrogen Receptor (GPER) and Follicle-Stimulating Hormone Receptor (FSHR) Heteromeric Complexes Promote Human Ovarian Follicle Survival. iScience 2020, 23, 101812. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Horton, R.; Tait, J.F. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J. Clin. Investig. 1966, 45, 301–313. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Di Segni, N.; Mallozzi, M.; Giovanale, V.; Mantovani, A.; Marci, R.; Moscarini, M. Bisphenol a and the female reproductive tract: An overview of recent laboratory evidence and epidemiological studies. Reprod. Biol. Endocrinol. 2014, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.; Adir, M.; Yerushalmi, G.; Hourvitz, A.; Gitman, H.; Yung, Y.; Orvieto, R.; Machtinger, R. Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Hum. Reprod. 2016, 31, 1562–1569. [Google Scholar] [CrossRef]
- Kwintkiewicz, J.; Nishi, Y.; Yanase, T.; Giudice, L.C. Peroxisome proliferator-activated receptor-γ mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and oestradiol in human granulosa cells. Environ. Health Perspect. 2010, 118, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Pogrmic-Majkic, K.; Samardzija Nenadov, D.; Fa, S.; Stanic, B.; Trninic Pjevic, A.; Andric, N. BPA activates EGFR and ERK1/2 through PPARΓ to increase expression of steroidogenic acute regulatory protein in human cumulus granulosa cells. Chemosphere 2019, 229, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; He, H.; Jin, M.; Lu, Z.; Deng, Y.; Liu, C.; Shen, N.; Li, J.; Wang, H.; Ying, P.; et al. Genome-wide gene-bisphenol A, F and triclosan interaction analyses on urinary oxidative stress markers. Sci. Total Environ. 2022, 807, 150753. [Google Scholar] [CrossRef]
- Togola, A.; Desmarchais, A.; Téteau, O.; Vignault, C.; Maillard, V.; Buron, C.; Bristeau, S.; Guérif, F.; Binet, A.; Elis, S. Bisphenol S is present in culture media used for ART and cell culture. Hum. Reprod. 2021, 36, 1032–1042. [Google Scholar] [CrossRef]
- Sonavane, M.; Gassman, N.R. Bisphenol A co-exposure effects: A key factor in understanding BPA’s complex mechanism and health outcomes. Crit. Rev. Toxicol. 2019, 49, 371–386. [Google Scholar] [CrossRef]
- Shamhari, A. ‘Afifah; Hamid, Z.A.; Budin, S.B.; Shamsudin, N.J.; Taib, I.S. Bisphenol a and its analogues deteriorate the hormones physiological function of the male reproductive system: A mini-review. Biomedicines 2021, 9, 1744. [Google Scholar] [CrossRef]
- Murono, E.P.; Derk, R.C.; De León, J.H. Differential effects of octylphenol, 17β-estradiol, endosulfan, or bisphenol A on the steroidogenic competence of cultured adult rat Leydig cells. Reprod. Toxicol. 2001, 15, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Lambard, S.; Huhtaniemi, I.T. Effect of bisphenol A on human chorionic gonadotrophin-stimulated gene expression of cultured mouse Leydig tumour cells. Reprod. Toxicol. 2007, 24, 265–275. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, N.; Lazzaretti, C.; Paradiso, E.; Capponi, C.; Ferrari, T.; Reggianini, F.; Sperduti, S.; Baschieri, L.; Mascolo, E.; Perri, C.; et al. Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro. Cells 2023, 12, 1537. https://doi.org/10.3390/cells12111537
Roy N, Lazzaretti C, Paradiso E, Capponi C, Ferrari T, Reggianini F, Sperduti S, Baschieri L, Mascolo E, Perri C, et al. Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro. Cells. 2023; 12(11):1537. https://doi.org/10.3390/cells12111537
Chicago/Turabian StyleRoy, Neena, Clara Lazzaretti, Elia Paradiso, Chiara Capponi, Tommaso Ferrari, Francesca Reggianini, Samantha Sperduti, Lara Baschieri, Elisa Mascolo, Carmela Perri, and et al. 2023. "Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro" Cells 12, no. 11: 1537. https://doi.org/10.3390/cells12111537
APA StyleRoy, N., Lazzaretti, C., Paradiso, E., Capponi, C., Ferrari, T., Reggianini, F., Sperduti, S., Baschieri, L., Mascolo, E., Perri, C., Varani, M., Canu, G., Trenti, T., Nicoli, A., Morini, D., Iannotti, F., Villani, M. T., Vicini, E., Simoni, M., & Casarini, L. (2023). Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro. Cells, 12(11), 1537. https://doi.org/10.3390/cells12111537








