Abstract
High spinal cord injuries (SCIs) lead to permanent functional deficits, including respiratory dysfunction. Patients living with such conditions often rely on ventilatory assistance to survive, and even those that can be weaned continue to suffer life-threatening impairments. There is currently no treatment for SCI that is capable of providing complete recovery of diaphragm activity and respiratory function. The diaphragm is the main inspiratory muscle, and its activity is controlled by phrenic motoneurons (phMNs) located in the cervical (C3–C5) spinal cord. Preserving and/or restoring phMN activity following a high SCI is essential for achieving voluntary control of breathing. In this review, we will highlight (1) the current knowledge of inflammatory and spontaneous pro-regenerative processes occurring after SCI, (2) key therapeutics developed to date, and (3) how these can be harnessed to drive respiratory recovery following SCIs. These therapeutic approaches are typically first developed and tested in relevant preclinical models, with some of them having been translated into clinical studies. A better understanding of inflammatory and pro-regenerative processes, as well as how they can be therapeutically manipulated, will be the key to achieving optimal functional recovery following SCIs.
1. Introduction
Spinal cord injuries (SCIs) have an occurrence of approximately 700,000 new cases worldwide each year [1], and they lead to sensory, motor, and autonomic deficiencies [2,3]. In most cases, SCIs occur in high spinal cord segments, which compromises respiratory function. Patients with mid-to-high cervical injuries often rely on ventilatory assistance to survive, but this significantly increases the risk of respiratory infections and pneumonia. Even those that recover voluntary control of breathing are at risk of respiratory failure. Accordingly, impaired breathing after a cervical SCI is a leading cause of death for these individuals [4,5]. To date, no therapies can completely restore respiratory function post-SCI [6,7,8,9], but some limited spontaneous recovery does occur, which can be attributed to neuroplasticity occurring through the remaining spinal fibers [10]. There is an urgent need for new therapeutics to improve respiratory recovery; a number of laboratories worldwide are working on this critically important problem. In this review, we will provide an overview of the key therapeutics that have been developed and tested in relevant preclinical SCI models, as well as the treatments that have been translated into human studies. All of these therapeutics are based on a comprehensive understanding of the deleterious and beneficial processes occurring post-SCI. Indeed, understanding and harnessing these diverse inflammatory and neuroplastic mechanisms will likely be a key to effective spinal cord repair.
1.1. Inflammation
The initial mechanical trauma of an SCI results in extensive tissue destruction at the level of injury, including vascular compromise, cell loss, and direct disruption of neural pathways [11]. These initial events are followed by a cascade of inflammatory and other pathological processes, which are collectively referred to as the secondary injury [11]. This includes vascular trauma and hemorrhage, which lead to progressive blood and fluid accumulation (edema) [12]. Ischemic damage of intact tissues from blood vessel disruption and any potential tissue compression (e.g., from surrounding bone) contributes to additional cell death and tissue loss. This can be exacerbated if sympathetic descending pathways are impacted and vascular function is impaired [13].
There is also a rapid increase in the concentration of damage-associated molecular patterns (DAMPs), which are endogenous molecules that are released by damaged cells [14]. They activate inflammatory pathways by stimulating pro-inflammatory cytokine production by glial cells [14,15]. For example, adenosine triphosphate (ATP) is a DAMP that acts as a chemoattractant for microglia through purinergic receptors, promotes migration to the injured area, and limits the expansion of the injury to surrounding intact tissues [16,17,18]. Astrocytes and oligodendrocytes also express purinergic receptors, and their activity seems to be impacted by high levels of ATP through changes in intracellular Ca2+ concentrations [19,20]. An increase in the extracellular concentration of excitatory amino acid neurotransmitters such as glutamate was also observed. The subsequently repeated overactivation of the receptors for these neurotransmitters induces excitotoxicity, leading to neuronal death and activation of glial cells [21,22]. This leads to increased production of free radicals and related oxidative stress [23], which contributes to the increase in ATP concentration that was described previously [21]. Axotomy of neurons after the initial injury leads to the formation of dysfunctional dystrophic end bulbs. Axonal degeneration is accompanied by secondary demyelination and release of myelin debris at the lesion site, as well as in the perineuronal area—mainly due to Wallerian degeneration—and subsequent oligodendrocyte death [19,21].
All of these extracellular signals lead to the activation and recruitment of immune cells. Resident microglia are the first to be activated and start to release pro-inflammatory cytokines, such as interleukin (IL)-1-β, tumoral necrosis factor α, and IL6 [24,25]. Neutrophils are then recruited to the lesion site and peak around 24 h post-injury [26]. Lymphocytes [27,28], as well as monocytes [27], start to invade the injured spinal cord during the following days, and then monocytes polarize into pro- and anti-inflammatory macrophages [29,30]. Astrocytes exert diverse functions in the healthy CNS, such as the regulation of blood flow, ion, and neurotransmitter levels, energy provision, homeostasis of extracellular fluid, and synapse function and remodeling [31]. Under pathological conditions such as SCIs, they undergo a process called “reactive astrogliosis” and change their phenotype [32]. The recruitment and activation of immune and glial cells—especially of reactive astrocytes and invading fibroblasts—then leads to the formation of a fibro-glial interface, often referred to as a ‘scar’. This process, which is now broadly viewed as a wound-healing response, has been well described in several reviews [19,33,34,35,36]. This fibro-glial ‘scar’ tissue persists and contributes to chronic inflammation, as well to the release of molecules that can inhibit adaptive neuronal plasticity. These inflammatory processes and the diverse molecules released represent targets for SCI repair. For example, these processes occur around the phMN area (C3–5), which is the main topic of this review (Figure 1).
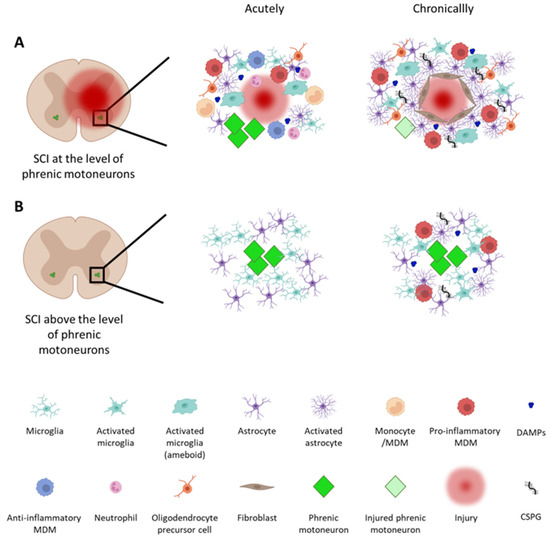
Figure 1.
Immune response and cellular reorganization at the level of phrenic motoneurons following a traumatic cervical spinal cord injury (SCI). SCIs lead to cascades of cellular and molecular events both acutely and chronically after an injury. (A) When an injury occurs at the level of phrenic motoneurons (phMNs), well-known inflammatory processes occur (see the reviews in [19,33,34,35,36]). In this situation, phMNs are not only directly impacted by the injury, but are exposed to secondary inflammation, which can place them at risk of damage and/or death. (B) Even with an injury occurring rostral to phMNs, they are denervated and do not receive input from brainstem neurons within the ventral respiratory column (VRC). When they are only denervated, however, they typically survive and remain recruitable when spontaneous or therapeutically induced repair processes occur. MDM: monocyte-derived macrophage; DAMPs: damage-associated molecular patterns; CSPG: chondroitin sulfate proteoglycan. The figure was created with BioRender.com.
1.2. Plasticity-Supporting and Inhibitory Factors
1.2.1. Plasticity-Supporting Molecules
Neuroinflammatory processes and the various molecules released have an impact on the composition of the perineuronal net after an SCI. This leads first to modifications of extracellular matrix properties [35,36,37]. Several extrinsic molecules—mainly growth factors—are secreted in an effort to acutely “repair” damaged tissue (within hours) following an SCI. Expression of neurotrophins [38,39] that transform growth factor beta and basic fibroblast growth factor [40], for example, has been shown to increase, possibly in an attempt to mimic an ontogenic environment and, therefore, support plasticity-like processes. The most studied neurotrophins are neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF), which have the highest affinity for tyrosine kinase receptors (Trk): TrkA, TrkB, and TrkC, respectively [38,39,41]. The binding to their Trk receptors leads to the intracellular signaling that is involved in neuronal survival, axonal growth, and synaptic plasticity [42]. It has been demonstrated that NGF induces robust axonal sprouting of nociceptive neurons [43]. NT-3 is mainly involved in neuronal survival, as well as sensory and sympathetic neurite outgrowth [38,44]. BDNF has a neuroprotective role and supports axon and dendrite growth after SCIs [45]. In addition, some matrix metalloproteases, such as MMP3 and MMP7, can act to facilitate plasticity processes following SCIs through their function in cleaving pro-neurotrophic molecules in their mature form [38]. Several extracellular matrix molecules, such as collagen, fibronectin, and laminin, also support axonal regrowth [9].
Along with the vast array of extrinsic factors, intrinsic cellular factors supporting axonal growth are also expressed after SCIs. Interestingly, these intrinsic factors differ depending on the neuron subtype and the distance of axon damage from the cell body. For example, rubrospinal and corticospinal neurons upregulate regeneration-associated-gene transcription only when axotomy is near the cell body [46,47]. On the contrary, long descending propriospinal neurons preferentially upregulate these genes when the axotomy occurs far from the cell body [48]. The growth-associated protein-43 (GAP-43) is expressed in the cell body and axon membranes and is involved in neuroplastic processes [49,50]. Following SCIs, GAP-43 mRNA is upregulated in axotomized neurons [51], and its expression is also increased in deafferented phMNs in rats [52]. The transcription factors involved in neuronal plasticity and cell activity also increase in expression/activity after an injury. These include cAMP response element-binding protein (CREB) (whose phosphorylation levels correlate with increased expression of BDNF and its receptor TrkB [53]), as well as c-Jun and its phosphorylated form [54].
1.2.2. Inhibitory Molecules
As described above, some damaged axons initially display a growth response following SCIs, but their regrowth is chronically aborted with the formation of scar tissue. Indeed, some molecules are expressed in the perineuronal net following SCIs and act as chemorepulsive factors for axon growth cones. These molecules, such as DAMPs, Nogo-A, semaphorin 3, Slit, and chondroitin sulfate proteoglycans (CSPGs), therefore, participate in the inhibition of synaptic plasticity after injury [15,33,34], with the latter being one of the most repulsive molecules [36,55].
In normal physiological conditions, CSPGs are involved in regulating plasticity and preventing maladaptive growth in the adult nervous system [55,56]. They are composed of a core glycoprotein along with glycosaminoglycan (GAG) sugar chains that are covalently attached [57]. Fitch and Silver showed in 1997 that reactive astrocytes present in an injured area produced CSPGs, which inhibited neurite outgrowth in vitro [58]. These CSPGs show differential expression depending on the time post-injury [59]. Their GAG chains interact with several high-affinity receptors present on the axonal growth cone membrane: protein tyrosine phosphatase σ (PTPσ), leukocyte common antigen-related phosphatase (LAR), and Nogo 1 and 3 receptors (NgR) [60,61,62]. The pathways activated by these receptors then modulate the activity of a number of signaling molecules and transcription factors, resulting in axonal growth inhibition [19,36,63].
Other types of molecules also inhibit growth and plasticity after SCIs. Some of these molecules are found in myelin and can also be expressed by neurons, including Nogo-A, myelin-associated glycoproteins (MAGs), and myelin oligodendrocyte glycoprotein [19,36]. Nogo-A binds to the NgR1 [64], Nogo-66 [64], and PirB [65] receptors, leading to actin cytoskeleton destabilization through RhoA/ROCK signaling pathway activation and the consequent arrest and collapse of axonal growth cones [64]. The molecules involved in axon guidance during development are also involved in this process. For example, chemorepulsive molecules such as Semaphorin 3A and 7 have an inhibitory effect on axonal growth [66,67,68]. The Slit/Roundabout and ephrin signaling pathways involved in axonal guidance during development also play a role in this inhibition [69,70]. Robo receptors are expressed on the membrane of axonal growth cones and use the same RhoA pathway as NgR [71], and Slit molecules are expressed by monocyte-derived macrophages and activated microglia [72]. Neuronal-intrinsic cellular factors, such as phosphatase and tensin homolog (PTEN), are also involved in this inhibition. Neuronal PTEN expression is increased in the adult CNS and after SCIs [73]. PTEN inhibition of Akt activity (which normally activates signaling axes, such as the mTOR pathway) plays a major role in limiting axonal growth capacity [74].
1.3. From Preclinical Models to Humans
Growth-inhibitory molecules limit repair post-SCI even in the presence of plasticity-supporting molecules (e.g., growth factors). While some treatments aim to overcome this by enhancing the expression of plasticity-supporting molecules, the extent of growth that is achievable remains limited. Instead, a key feature of these strategies is that they rely on spared tissue, cells, and axonal pathways. To test these therapeutics for respiratory recovery, two preclinical models of cervical SCIs were used: contusion injury—often at mid-cervical levels [75,76,77,78,79,80,81,82]—and high cervical hemisection—usually at the second cervical segment [83,84,85,86,87,88,89,90,91,92]. Contusion paradigms better model human pathophysiology but are often associated with greater variability in anatomical and functional outcomes. Contusive injuries were performed rostral to C3 to deafferent phMNs (located in the C3–C5 spinal cord in most mammalian species) or at the level of phMNs to directly damage them. Notably, most SCIs occur clinically at mid-cervical (C3–5) levels. Hemisection models are mainly used to deafferent phMNs and allow a high level of reproducibility to study specific plasticity processes or molecular pathways in response to injuries. The C2 hemisection model—which has been used for nearly 150 years—has the ability to reveal crossed phrenic pathways (decussating at the spinal midline) [10,93,94,95] that can be used to support diaphragm activity recovery. These two models of high SCIs are the most commonly used, and the choice of the model should be carefully considered depending on the purpose of the study. These preclinical models and others have been used to test the effects of a range of therapeutics on functional recovery, including respiratory function. Decades of preclinical work and several important discoveries continue to shape the future of promising therapeutics for SCIs and clinical translations.
2. Activity-Based Therapeutics
2.1. Intermittent Hypoxia
Activity-based therapies (e.g., rehabilitation) have been extensively used to increase the extent and rate of recovery after SCIs. These neuroplasticity-inducing treatments can promote some axonal regrowth (albeit limited), but they also largely rely on pathways spared by the injury. After cervical SCIs, these approaches have been used to amplify plasticity and motor recovery in damaged respiratory networks. Activity-based therapies are often favored as a treatment approach, as they are readily translatable and relatively non-invasive. To target respiratory activity, one strategy has been to expose the subject (pre-clinical animals or humans in some clinical studies) to respiratory challenges, such as hypoxia, which consists of a reduction in the percentage of oxygen in inspired air (normally composed of 21% oxygen) (Figure 2). The ventilatory response to hypoxia depends on the protocol’s duration and the application pattern (intermittent or continuous) and severity [96,97,98,99,100].
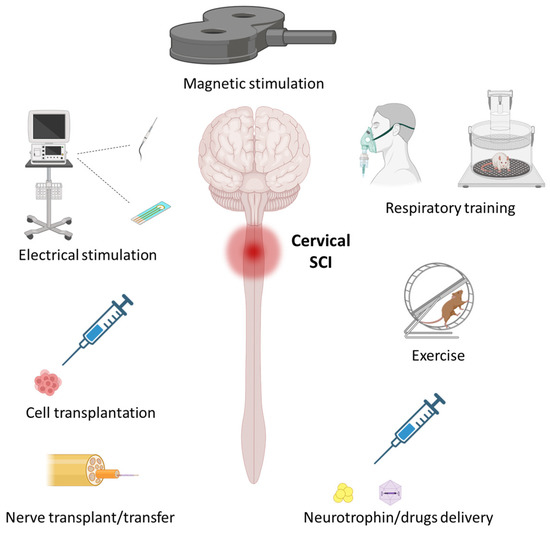
Figure 2.
Therapeutics aimed at promoting respiratory recovery following traumatic cervical spinal cord injury (SCI). Various therapeutics have been developed to induce neuroplasticity in the phrenic motor system to promote respiratory recovery. Some of the most extensively studied are detailed in the present review and involve the following: activity-based therapeutics, such as respiratory training and exercise; stimulation-based therapeutics, such as invasive electrical stimulation and non-invasive magnetic stimulation; therapeutics for promoting axonal growth and neuroprotection, such as nerve transfer, cell transplantation, and neurotrophin/drug delivery. The figure was created with BioRender.com.
The use of intermittent hypoxia (IH) was pioneered by Gordon Mitchell and colleagues, who were originally at the University of Wisconsin and are now at the University of Florida. Following a chronic C2 lateral spinal cord hemisection in rats, a chronic IH protocol (5 min alternating between 11% oxygen and normoxia) was applied for 12 h per night for 7 consecutive days [101]. This protocol led to an increase in phrenic activity on the injured side compared to non-treated animals, suggesting that this protocol strengthened crossed phrenic pathways [101]. Some studies, however, demonstrated that repeated severe/chronic IH protocol application (2–8% oxygen) with a high cycle number could lead to deleterious side effects, such as hypertension [102] or hippocampus apoptosis [103]. It was then found that the use of more moderate protocols (9–16% oxygen) delivered at lower cycle numbers provided beneficial outcomes without adverse side effects [104].
Studies then focused on the application of moderate IH following high SCIs to restore respiratory function. Mitchell and his collaborators were the first to show that acute IH (AIH) enhances respiratory motor output in a rat preclinical model of SCI [105]. Subsequent studies then showed that the application of daily moderate AIH protocols led to persistent respiratory function improvement in C2 hemisected rats [106,107], which was associated with increased expression of BDNF and TrkB [107]. These beneficial effects on respiratory function were confirmed in several studies after C2 hemisection in rats [105,106,108,109,110,111,112] and mice [113], as well as in a cervical contusion model in rats [76,77,114,115]. AIH protocols also impact the expression of several molecules that trigger neuronal plasticity after SCIs, including decreased PTEN expression and increased expression in mTOR, as well as increased S6 and c-Fos expression by phMNs [116]. Recently, it was shown that IH protocols led to increased serotonergic axon density around phMNs, which could support diaphragm activity restoration after SCIs [117].
Daily AIH protocols, therefore, appear to be a safe and non-invasive way to improve respiratory function following high SCIs, and this has been extensively reviewed [8,118,119,120]. These preclinical studies demonstrated the feasibility and safety of repeated IH protocol application at low doses. IH protocols also improve respiratory plasticity and recovery, though the improvement is greater when applied acutely after injury rather than at more chronic time points [8].
Respiratory training approaches are also translatable to the clinic [104]. In individuals with chronic (>1 year) incomplete cervical SCIs, daily AIH protocol application led to increased minute ventilation during the first two days, corresponding to long-term respiratory facilitation. This effect could be maintained through the protocol’s duration, but without any changes over time [121]. In similar cases, AIH protocols seemed to have some global beneficial effects on respiratory output, although complete recovery has not yet been observed [122,123].
Ongoing pre-clinical studies have also begun to show that the observed beneficial effects can be amplified in combination with other therapeutics, such as the delivery of chondroitinase ABC (an enzyme that digests GAGs from CSPGs) [124] or pre-AIH prednisolone (anti-inflammatory corticosteroid) administration [125]. Daily AIH has also been combined with other therapeutics in clinical studies to maximize efficacy, but so far, this was only in trials aimed at improving limb motor function in people with lower SCIs [126,127,128,129]. These effects can be maximized when combined with those of other therapeutics; it is now critical to investigate their impacts on respiratory recovery after SCIs.
2.2. Exercise
Another form of activity-based therapy used to improve respiratory function is non-respiratory training. Locomotor training has been extensively used to improve hindlimb function in preclinical models and clinical cases following SCIs. Interestingly, these approaches can also can be used to “train” the respiratory system, as exercise induces an increased oxygen demand (Figure 2), and there is respiratory adaptation to locomotor exercise through neuronal interconnections between the respiratory and locomotor circuits [130].
Preclinical studies aimed at promoting respiratory recovery through exercise are limited. Nevertheless, it was shown that in C2 hemisected mice, chronic forced training led to increased fatigue resistance, some muscle (limb and diaphragm) plasticity, and enhanced running capacity in the trained SCI group [131].
In patients, respiratory muscle training showed beneficial outcomes after cervical SCIs [132,133], as evidenced, for example, by increased vital capacity and inspiratory reserve volume [134]. The effects of this type of training have been more extensively studied in athletes [132,135,136]. These training protocols allow for an increased maximum volume of inspired and expired air [135,136]. Vocal exercise, such as singing, is also a studied therapeutic strategy. However, the beneficial effects of such methods for SCIs remain to be determined [137]. Locomotor training has also been shown to have beneficial effects on respiratory function. For example, locomotor training with body-weight support in patients with chronic cervical SCIs led to improved respiratory function [138]. However, the beneficial effects of these exercise protocols remain limited, and more investigation is required to determine the best use of these therapeutic strategies.
3. Stimulation-Based Therapeutics
While activity-based therapies can non-invasively drive activity in neural networks, they tend to activate many networks and, therefore, lack precision in some regards. Neural stimulation can also be achieved by providing an exogenous electrical or chemical stimulus to directly activate neural networks (above the stimulation threshold) or modulate neural activity by providing low levels of stimulation. Broadly, these approaches can be divided into invasive or relatively non-invasive strategies.
3.1. Invasive Stimulation
Invasive neural stimulation strategies are exemplified by the implantation of electrodes (individually or in electrode arrays) that interface with neural tissues, nerves, or the muscles that they innervate (e.g., brain–computer interface (BCI)/brain–machine interface (BMI)). They can range from the use of implantable wires in the brain or spinal cord tissue (e.g., intraspinal microwires, Utah array) to the placement of electrodes on the surface of neural tissues (epidural stimulation). While there is an increasing number of reviews on this subject, the following section will highlight the use of epidural stimulation as a potential strategy for enhancing the recovery of breathing post-SCI.
Epidural electric stimulation (EES) directly and repeatedly stimulates spinal networks, usually at sub-threshold levels, to induce neuronal plasticity by modulating neuronal excitability (Figure 2; essentially acting as an amplifier for incoming signals and making spinal networks more likely to be active). This technique was initially used to reduce chronic pain in patients with SCIs by reducing neuronal excitability and related pain signaling [139,140]. EES has now been demonstrated to improve locomotor function in preclinical models of SCIs [141,142,143] and in humans [144,145]. In the respiratory system, EES has been used to induce neuroplasticity processes at the level of deafferented phMNs. Studies show some beneficial effects of EES application after high SCIs in preclinical models [85,146,147,148,149]. Repeated EES application (inspiration declenched; approximately 0.5 Hz; 24 h per day for 4 days) at the cervical level in awake rats following C2 hemisection led to increased BDNF and vascular endothelial growth factor (VEGF) expression in the ventral C3–C5 spinal cord where phMNs reside [149]. EES at the C4 level at 100–300 Hz led to short-term facilitation of the phrenic motor system after a C1 transection in anesthetized ventilated rats [85]. Likewise, EES potentiated ipsilateral phasic inspiratory activity at both 2 and 12 weeks after C2 hemisection when delivered at 300 Hz [147].
EES trials have also been shown to improve function in people with SCIs [150], including by improving breathing [150]. However, further investigation is required to better understand its effects and how to apply this therapeutic in the most efficient way, as complete respiratory recovery would likely not be achieved when delivering therapeutics based only on EES.
Despite the demonstration of its therapeutic efficacy, epidural stimulation is among the more invasive approaches, and ongoing work aims to assess whether less invasive approaches can yield similar levels of benefit.
3.2. Non-Invasive Stimulation
Several non-invasive stimulation procedures following cervical SCIs have been tested. For example, trans-spinal direct current stimulation (TDCs) has proven to induce beneficial functional improvements [151]. Cervical trans-spinal direct current stimulation could also address the loss of respiratory function in humans [152].
One of the most well-characterized non-invasive methods of neural activation is the use of magnetic stimulation (MS). MS, and more specifically repetitive MS (rMS), applies a magnetic field above target cells from outside the body (through intact bone, muscle, and skin) to neuromodulate neuronal excitability (Figure 2). Transcranial rMS was first used clinically to treat depression [153,154,155] or post-traumatic brain disorders [156,157]. In the context of SCIs, rMS has been recently used to reduce spasticity and neuropathic pain, as well as to promote functional motor recovery, in patients, mainly through cortical inhibition [158,159,160,161,162]. This approach, however, has not been used to promote respiratory recovery following cervical SCIs in patients, despite the potential efficacy considering its effects on other motor systems.
MS can be used to evaluate respiratory supraspinal plasticity in clinical studies [163,164,165,166,167,168], as well as in preclinical models [169,170,171]. In addition, a single MS burst can be a useful non-invasive tool for evaluating variations in phrenic system excitability. Concerning the effect of rMS on the respiratory system following high SCIs, it was recently shown that the application of chronic high-frequency repetitive transcranial MS in rats led to some respiratory improvement post-SCI. This strategy also elicits neuronal plasticity with a reduction in deleterious post-traumatic inflammatory processes in the cervical spinal cord [172].
Additional work is required to understand the effects of rMS after SCIs and to determine appropriate protocols for achieving optimal recovery. This new technique is promising, since it is non-invasive and broadly used in clinics to treat other central nervous system pathologies.
4. Therapeutics for Inducing Regeneration/Reorganization of Neural Pathways
4.1. Cell Transplantation
Cell therapy for SCI treatment consists of a vast range of strategies that target a number of goals, including neuroprotection, immune modulation, and the construction of new neuronal networks (Figure 2) [173,174]. Depending on the goal, different types of cells have been utilized by using a variety of delivery methods, such as intravenous injection, transplantation directly into the injured spinal cord, or injection into spinal tissues away from the injury (e.g., the level of deafferented motoneurons). Cell therapies have been tested in a host of preclinical SCI models, improving outcome measures in many cases. For example, improvements of locomotor [175,176], autonomic [177], and respiratory [7,173] function were achieved following cell transplantation into the lesioned spinal cord. A number of different donor cell types were tested after high SCIs to target respiratory recovery [7].
Because astrocytes have important and diverse beneficial roles, such as the regulation of extracellular ion and neurotransmitter homeostasis [31] and synaptogenesis [178], there is great interest in astrocyte transplantation following SCIs [179,180,181]. In contrast to the astrocytes that contribute to fibro-glial scars post-injury, donor astrocytes are pro-reparative and promote tissue repair. Astrocytes derived from sources such as human induced pluripotent stem cells injected into rats and mice at the level of SCIs induce beneficial outcomes, such as a reduced lesion area and increased diaphragm activity on the injured side [182]. Similarly, transplantation of glial-restricted precursors (a class of lineage-restricted astrocyte progenitors) into the lesion site acutely after C2 hemisection led to the partial recovery of diaphragm electromyography amplitude on the injured side, as well as the regeneration of bulbospinal respiratory axons [183].
Neuronal transplantation can also be used to repair a damaged spinal cord, for example, by generating new circuits that can relay information from injured neurons rostral to the injury to deafferented neuron populations caudal to the injury [174,184]. Depending on the donor neuron type, they can also provide neurochemical inputs into damaged spinal networks to modulate activity. Transplantation of fetal spinal cord tissue enriched in neuronal and glial precursor cells led to phrenic nerve activity recovery, which was associated with new connectivity between donor neurons and the host phrenic circuitry [185]. This also led to larger inspiratory tidal volumes in transplanted rats during a respiratory challenge (i.e., brief hypoxic exposures) compared to that in non-transplanted rats [186]. Neural precursor transplantation resulted in similarly improved outcomes. Ongoing work has revealed, however, that the preparation of cells for transplantation and their exposure to cytokines can alter the phenotypes available for transplantation. Accordingly, approaches to directing the differentiation of stem/progenitor cells toward specific neuronal phenotypes are being explored. For example, the identification of V2a spinal interneurons (glutamatergic pre-motor neurons) as a key component of the phrenic motor network [187,188] and, more recently, as a key target for improving other forms of motor recovery post-SCIs [189] has led to the development of stem-cell-derived V2a spinal interneurons for transplantation. Transplantation of V2a interneurons enriched from neural precursor cells after cervical contusion led to improved functional diaphragm recovery in rats [190].
Another cell type used to promote SCI repair is olfactory ensheathing cells. These cells have been extensively tested pre-clinically and clinically. An important consideration is that they can be derived from either the olfactory nasal mucosa or directly (more invasively) from the olfactory bulb. These two cell sources yield quite different cells that have unique properties [184]. Olfactory ecto-mesenchymal stem cells derived from the mucosa were transplanted into the cervical spinal cord to promote the repair of respiratory pathways and were reported to improve diaphragmatic and phrenic activity associated with reduced spinal inflammation 4 months after transplantation in an acute (2 days after SCI) model of C2 contusion in rats [191]. In addition, oligodendrocyte progenitor cell transplantation promoted remyelination and restoration of homeostasis in the extracellular space [192,193]. This approach also promoted functional locomotor and sensory recovery, though its effects on the respiratory system remain to be evaluated [174].
Though invasive, cell transplantation remains an appealing therapeutic. One reason for this is that, unlike activity-based therapies and neural stimulation, transplantation is not necessarily reliant on spared tissue, but can rather promote the restoration of damaged tissue. Clinical trials have demonstrated the safety of transplantation with a number of different cell types, including oligodendrocyte progenitor cells [194] and olfactory ensheathing cells [195]. Some respiratory function improvements were also observed in children with complete cervical SCIs after bone marrow nucleated cell transplantation into the spinal cord cavity with intravenous injection of these cells [196]. However, clinical information about respiratory recovery after cell transplantation remains scarce. There is hope on the horizon for cell therapies to improve breathing post-SCI with the development of new clinical trials in coming years.
4.2. Nerve Grafts/Nerve Transfers
The peripheral nervous system exhibits relatively greater regenerative potential than that of the adult central nervous system. This is due in part to the myelinating cells of the periphery (Schwann cells) that facilitate axonal growth, in contrast to central myelin (from oligodendrocytes), which can inhibit axonal growth [197], among other reasons. Building on early work by Tello and Ramon y Cajal, Albert Aguayo and his team showed that grafting a sciatic nerve between the brainstem and the thoracic spinal cord could create a “bridge” between the two areas. CNS axons could, indeed, grow from one side to the other one to connect neurons across the injury [197,198].
The therapeutic potential of peripheral nerve grafting has been explored in various preclinical models of SCIs, such as cervical hemisections (Figure 2). In rats, one end of the common peroneal nerve was inserted into the C2 spinal cord in a way that compromised descending respiratory pathways. The other end was left free under the skin for several months. The activity of this nerve was then recorded, and it presented a similar pattern of electrophysiological activity to that of the phrenic nerve, demonstrating that respiratory axotomized neurons could grow their axons through the graft [199]. This showed the potential of nerve grafting for acutely promoting respiratory recovery after high SCIs. The same group then showed that nerve grafts could be employed until approximately 3 weeks after a C3 hemisection to stimulate respiratory neuron axon regeneration through the grafted nerve. Treatment at later times post-injury showed less axonal growth through the grafted nerve [200].
Translation of the nerve graft technique to humans gave birth to “nerve transfer” from an intact nerve to an injured one [201]. An attempt was made in humans with cervical SCIs that occurred at the level of phMNs (C3–C5) and for which electrical diaphragmatic “pacing” could not be achieved through the phrenic nerve due to phMN death. In these individuals, end-to-end anastomose from the fourth intercostal nerve to the phrenic nerve allowed for diaphragmatic pacing once interconnections were made between the two nerves [202].
Though promising, this therapeutic approach remains highly invasive and requires removing or deriving an intact nerve from the patient to improve another modality, which could represent a drawback.
4.3. Harnessing the Extracellular Environment
A number of studies have aimed to reduce the inhibitory molecules expressed in the injured spinal cord by using various therapeutic agents (Figure 2) [9]. Among these inhibitory molecules, one key target is CSPG overproduction, which is mainly secreted by the glia [55,56,203]. Even if subsets of neurons can extend their axons through the fibro-glial ‘scar’ at the lesion site [34], the accumulation of CSPGs in the perineuronal net surrounding denervated neurons can limit new synaptic input, which may be needed for repair and recovery.
Selective GAG chain disruption by chondroitinase ABC administration reduced the inhibitory effects of CSPGs on active growth cones, leading to greater axonal sprouting from injured and spared neurons after SCIs [204,205,206,207]. It also modulated inflammatory processes that led to neuroprotection in the injured spinal cord, including the promotion of anti-inflammatory macrophage polarization over pro-inflammatory polarization [208]. This resulted in a pro-repair response mediated by IL-10 [209]. Injection of chondroitinase ABC specifically at the level of the perineuronal nets surrounding phMNs at a chronic time point in rats after C2 hemisection led to the sustained recovery of the previously paralyzed hemidiaphragm [124]. This was accompanied by a reduction in CSPG receptor expression and increases in the TrkB receptor expression and the density of serotonergic fibers [124]. CSPG digestion at the lesion or within the perineuronal net was also combined with other therapeutics, such as rehabilitation training and cell transplantation, in an attempt to amplify these beneficial effects [210]. For example, when combined with an IH protocol, certain molecular effects appeared to be amplified, but without further increasing the observed respiratory recovery [124]. In combination with a tibial nerve graft (one end inserted at C2 and one end inserted at C4) in C2 hemisected rats, CPSG digestion led to increased diaphragm activity, which was correlated with bulbospinal fiber regeneration [211].
A complication associated with the use of chondroitinase to enzymatically degrade CSPGs is that the enzyme is highly sensitive to changes in temperature. While ongoing work is investigating novel ways to modify the enzyme to address this issue, alternatives to the enzyme are also being explored. One such alternative is to use a soluble peptide to inhibit the signaling through CSPG receptors such as PTPσ and LAR, which also leads to diaphragm recovery associated with bulbospinal respiratory axonal regrowth [61,87,212]. Ongoing work by NervGen® aims to prepare a product of this nature that can be used to treat injuries in people.
Biomaterials such as hydrogels can also be used to deliver therapeutic factors to the damaged spinal cord to promote the protection and/or plasticity of respiratory circuitry [9]. For example, ongoing work is exploring whether biomaterials can be used to provide chondroitinase stability for prolonged delivery. In addition to offering a substrate that can support tissue growth and repair, biomaterials provide an effective means of drug delivery. When used to deliver minocycline hydrochloride (an antibiotic/anti-inflammatory agent) focally to the lesion in a model of cervical contusion in rats, preservation of diaphragm activity was observed [213]. This showed that targeting inflammatory processes through interventions in the extracellular space can reduce functional loss. However, to date, these therapeutics have not been translated into clinical testing.
4.4. Neurotrophin Delivery
Neurotrophin expression is increased post-SCI, but not in amounts sufficient to lead to significant axonal growth, synaptic reconnectivity, or functional recovery [39]. Their potential for inducing plasticity has, however, been used to induce improvements after SCIs, such as in locomotor function [214] and respiratory function [53,215,216] (Figure 2). In particular, the BDNF/TrkB signaling pathway has been highly targeted, with a number of different delivery approaches having been tested to date.
For example, delivery of BDNF through chronic intrathecal infusion at the C4 spinal cord after C2 hemisection in rats led to diaphragm activity recovery [53]. Local BDNF hydrogel delivery in the intrathecal space after C4/C5 contusion also partially restored diaphragm function [215]. These studies showed that modulating the BDNF/TrkB signaling pathway in and/or around phMNs can sustain diaphragm activity recovery after SCIs.
In addition, viral delivery of BDNF or TrkB also showed promising results. Following C2 hemisection in rats, ipsi-lesional intraspinal (C3–C5) delivery of adeno-associated virus serotype 2 (AAV2)-BDNF vector promoted significant functional diaphragm recovery. This was correlated with the sprouting of descending bulbospinal respiratory axons and enhanced synaptic connectivity between these growing axon populations and their phMN targets [216]. A more targeted way to increase the BDNF/TrkB signaling pathway specifically in phMNs is through retrograde transduction of phMNs by using intrapleural injection of AAV vector to express TrkB receptor [217]. The resulting TrkB receptor overexpression was sufficient to increase the recovery of ipsi-lesional rhythmic phrenic activity after C2 hemisection in rats [217].
Some human studies also evaluated the effects of BDNF via systemic or intrathecal delivery. However, these BDNF administration paradigms seemed to induce several adverse effects [39]. In their review, Sieck and Mantilla suggested specifically targeting phMNs to avoid undesirable side effects [39].
5. Conclusions
Among the host of permanent deficits caused by SCIs, respiratory dysfunction and impaired breathing remain some of the most life-threatening. A diverse array of therapeutics have been developed and preclinically tested to reduce or reverse these deficits by targeting different aspects of the resulting pathology. However, none have been shown to be capable of promoting complete recovery, and when translated to human studies, their efficacy has remained limited. Building on a long history of therapeutic development, future preclinical studies aiming to decipher the biological processes occurring after SCIs will help in finding treatments for promoting functional respiratory recovery. Combinatorial strategies that can each target unique aspects of an injury’s pathology to enhance recovery in an additive or even synergistic manner are an important step toward achieving respiratory restoration after SCIs.
Author Contributions
Conceptualization: P.M.-F. and S.V.; Writing—original draft preparation: P.M.-F.; Writing—review and editing: P.M.-F., S.V., M.A.L. and A.C.L.; Supervision: S.V.; Project administration: S.V.; Funding acquisition: S.V., M.A.L. and A.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chancellerie des Universités de Paris (Legs Poix) (S.V.), the Fondation de France (S.V.), the Fondation Médisite (S.V.), INSERM (S.V.), Université de Versailles Saint-Quentin-en-Yvelines (S.V.), National Institutes of Health: R01-NS104291 (M.A.L.), Wings for Life (M.A.L.), the Lisa Dean Moseley Foundation (M.A.L), National Institutes of Health: R01-NS079702 (A.C.L.), R01-NS110385 (A.C.L.), Craig H. Neilsen Foundation: #733020 (A.C.L.), Pennsylvania Department of Health: SCI Research Grant #4100094298 (A.C.L.), and the Yant Family Spinal Cord Regeneration Fund (A.C.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Hou, S.; Rabchevsky, A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef]
- Burns, S.P. Acute respiratory infections in persons with spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 203–216. [Google Scholar] [CrossRef]
- Raab, A.M.; Mueller, G.; Elsig, S.; Gandevia, S.C.; Zwahlen, M.; Hopman, M.T.E.; Hilfiker, R. Systematic Review of Incidence Studies of Pneumonia in Persons with Spinal Cord Injury. J. Clin. Med. 2021, 11, 211. [Google Scholar] [CrossRef]
- Locke, K.C.; Randelman, M.L.; Hoh, D.J.; Zholudeva, L.V.; Lane, M.A. Respiratory plasticity following spinal cord injury: Perspectives from mouse to man. Neural Regen. Res. 2022, 17, 2141–2148. [Google Scholar] [CrossRef]
- Charsar, B.A.; Urban, M.W.; Lepore, A.C. Harnessing the power of cell transplantation to target respiratory dysfunction following spinal cord injury. Exp. Neurol. 2017, 287, 268–275. [Google Scholar] [CrossRef]
- Vose, A.K.; Welch, J.F.; Nair, J.; Dale, E.A.; Fox, E.J.; Muir, G.D.; Trumbower, R.D.; Mitchell, G.S. Therapeutic acute intermittent hypoxia: A translational roadmap for spinal cord injury and neuromuscular disease. Exp. Neurol. 2022, 347, 113891. [Google Scholar] [CrossRef]
- Haggerty, A.E.; Marlow, M.M.; Oudega, M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci. Lett. 2017, 652, 50–55. [Google Scholar] [CrossRef]
- Ghali, M.G.Z. The crossed phrenic phenomenon. Neural Regen. Res. 2017, 12, 845–864. [Google Scholar] [CrossRef]
- Beattie, M.S.; Hermann, G.E.; Rogers, R.C.; Bresnahan, J.C. Cell death in models of spinal cord injury. Prog. Brain Res. 2002, 137, 37–47. [Google Scholar] [CrossRef]
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular Events after Spinal Cord Injury: Contribution to Secondary Pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [CrossRef]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- James, G.; Butt, A.M. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur. J. Pharm. 2002, 447, 247–260. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Jia, M.; Njapo, S.A.; Rastogi, V.; Hedna, V.S. Taming glutamate excitotoxicity: Strategic pathway modulation for neuroprotection. CNS Drugs 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Kroner, A.; Rosas Almanza, J. Role of microglia in spinal cord injury. Neurosci. Lett. 2019, 709, 134370. [Google Scholar] [CrossRef]
- Zivkovic, S.; Ayazi, M.; Hammel, G.; Ren, Y. For Better or for Worse: A Look Into Neutrophils in Traumatic Spinal Cord Injury. Front. Cell Neurosci. 2021, 15, 648076. [Google Scholar] [CrossRef]
- Popovich, P.G.; Stokes, B.T.; Whitacre, C.C. Concept of autoimmunity following spinal cord injury: Possible roles for T lymphocytes in the traumatized central nervous system. J. Neurosci. Res. 1996, 45, 349–363. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Guan, Z.; Popovich, P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Investig. 2009, 119, 2990–2999. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Sieck, G.C.; Mantilla, C.B. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 218–225. [Google Scholar] [CrossRef]
- Logan, A.; Berry, M. Transforming growth factor-beta 1 and basic fibroblast growth factor in the injured CNS. Trends Pharm. Sci. 1993, 14, 337–342. [Google Scholar] [CrossRef]
- Ip, N.Y.; Stitt, T.N.; Tapley, P.; Klein, R.; Glass, D.J.; Fandl, J.; Greene, L.A.; Barbacid, M.; Yancopoulos, G.D. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron 1993, 10, 137–149. [Google Scholar] [CrossRef]
- Blum, R.; Konnerth, A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology 2005, 20, 70–78. [Google Scholar] [CrossRef]
- Romero, M.I.; Rangappa, N.; Li, L.; Lightfoot, E.; Garry, M.G.; Smith, G.M. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 4435–4445. [Google Scholar] [CrossRef]
- Rosenthal, A.; Goeddel, D.V.; Nguyen, T.; Lewis, M.; Shih, A.; Laramee, G.R.; Nikolics, K.; Winslow, J.W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron 1990, 4, 767–773. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Fernandes, K.J.; Fan, D.P.; Tsui, B.J.; Cassar, S.L.; Tetzlaff, W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of GAP-43, tubulins, and neurofilament-M. J. Comp. Neurol. 1999, 414, 495–510. [Google Scholar] [CrossRef]
- Mason, M.R.; Lieberman, A.R.; Anderson, P.N. Corticospinal neurons up-regulate a range of growth-associated genes following intracortical, but not spinal, axotomy. Eur. J. Neurosci. 2003, 18, 789–802. [Google Scholar] [CrossRef]
- Swieck, K.; Conta-Steencken, A.; Middleton, F.A.; Siebert, J.R.; Osterhout, D.J.; Stelzner, D.J. Effect of lesion proximity on the regenerative response of long descending propriospinal neurons after spinal transection injury. BMC Neurosci. 2019, 20, 10. [Google Scholar] [CrossRef]
- Skene, J.H.; Willard, M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J. Cell Biol. 1981, 89, 86–95. [Google Scholar] [CrossRef]
- Oestreicher, A.B.; De Graan, P.N.; Gispen, W.H.; Verhaagen, J.; Schrama, L.H. B-50, the growth associated protein-43: Modulation of cell morphology and communication in the nervous system. Prog. Neurobiol. 1997, 53, 627–686. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Alexander, S.W.; Miller, F.D.; Bisby, M.A. Response of facial and rubrospinal neurons to axotomy: Changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 2528–2544. [Google Scholar] [CrossRef]
- Vinit, S.; Darlot, F.; Stamegna, J.C.; Gauthier, P.; Kastner, A. Effect of cervical spinal cord hemisection on the expression of axon growth markers. Neurosci. Lett. 2009, 462, 276–280. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Gransee, H.M.; Zhan, W.Z.; Sieck, G.C. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp. Neurol. 2013, 247, 101–109. [Google Scholar] [CrossRef]
- Vinit, S.; Darlot, F.; Aoulaïche, H.; Boulenguez, P.; Kastner, A. Distinct expression of c-Jun and HSP27 in axotomized and spared bulbospinal neurons after cervical spinal cord injury. J. Mol. Neurosci. 2011, 45, 119–133. [Google Scholar] [CrossRef]
- Bartus, K.; James, N.D.; Bosch, K.D.; Bradbury, E.J. Chondroitin sulphate proteoglycans: Key modulators of spinal cord and brain plasticity. Exp. Neurol. 2012, 235, 5–17. [Google Scholar] [CrossRef]
- Sánchez-Ventura, J.; Lane, M.A.; Udina, E. The Role and Modulation of Spinal Perineuronal Nets in the Healthy and Injured Spinal Cord. Front. Cell. Neurosci. 2022, 16, 893857. [Google Scholar] [CrossRef]
- Rhodes, K.E.; Fawcett, J.W. Chondroitin sulphate proteoglycans: Preventing plasticity or protecting the CNS? J. Anat. 2004, 204, 33–48. [Google Scholar] [CrossRef]
- Fitch, M.T.; Silver, J. Activated macrophages and the blood-brain barrier: Inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol. 1997, 148, 587–603. [Google Scholar] [CrossRef]
- Jones, L.L.; Margolis, R.U.; Tuszynski, M.H. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003, 182, 399–411. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef]
- Smedfors, G.; Olson, L.; Karlsson, T.E. A Nogo-Like Signaling Perspective from Birth to Adulthood and in Old Age: Brain Expression Patterns of Ligands, Receptors and Modulators. Front. Mol. Neurosci. 2018, 11, 42. [Google Scholar] [CrossRef]
- Ohtake, Y.; Wong, D.; Abdul-Muneer, P.M.; Selzer, M.E.; Li, S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci. Rep. 2016, 6, 37152. [Google Scholar] [CrossRef]
- Niederöst, B.; Oertle, T.; Fritsche, J.; McKinney, R.A.; Bandtlow, C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 10368–10376. [Google Scholar] [CrossRef]
- Cafferty, W.B.J.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp Synergize with Nogo-A to Restrict Axonal Growth and Neurological Recovery after Spinal Cord Trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef]
- De Winter, F.; Oudega, M.; Lankhorst, A.J.; Hamers, F.P.; Blits, B.; Ruitenberg, M.J.; Pasterkamp, R.J.; Gispen, W.H.; Verhaagen, J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 2002, 175, 61–75. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ino, H.; Koda, M.; Murakami, M.; Yoshinaga, K.; Yamazaki, M.; Moriya, H. Regulation of semaphorin 3A expression in neurons of the rat spinal cord and cerebral cortex after transection injury. Acta Neuropathol. 2004, 107, 250–256. [Google Scholar] [CrossRef]
- Loy, K.; Fourneau, J.; Meng, N.; Denecke, C.; Locatelli, G.; Bareyre, F.M. Semaphorin 7A restricts serotonergic innervation and ensures recovery after spinal cord injury. Cell Mol. Life Sci. 2021, 78, 2911–2927. [Google Scholar] [CrossRef]
- Kidd, T.; Brose, K.; Mitchell, K.J.; Fetter, R.D.; Tessier-Lavigne, M.; Goodman, C.S.; Tear, G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92, 205–215. [Google Scholar] [CrossRef]
- Hollis, E.R. Axon Guidance Molecules and Neural Circuit Remodeling after Spinal Cord Injury. Neurotherapeutics 2016, 13, 360–369. [Google Scholar] [CrossRef]
- Russell, S.A.; Bashaw, G.J. Axon guidance pathways and the control of gene expression. Dev. Dyn. 2018, 247, 571–580. [Google Scholar] [CrossRef]
- Wehrle, R.; Camand, E.; Chedotal, A.; Sotelo, C.; Dusart, I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur. J. Neurosci. 2005, 22, 2134–2144. [Google Scholar] [CrossRef]
- Keniry, M.; Parsons, R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 2008, 27, 5477–5485. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Kanter, J.L.; He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010, 223, 45–50. [Google Scholar] [CrossRef]
- Allen, L.L.; Seven, Y.B.; Baker, T.L.; Mitchell, G.S. Cervical spinal contusion alters Na(+)-K(+)-2Cl- and K(+)-Cl- cation-chloride cotransporter expression in phrenic motor neurons. Respir. Physiol. Neurobiol. 2019, 261, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Vinit, S.; Chen, C.L.; Lee, K.Z. 5-HT7 Receptor Inhibition Transiently Improves Respiratory Function Following Daily Acute Intermittent Hypercapnic-Hypoxia in Rats with Chronic Midcervical Spinal Cord Contusion. Neurorehabilit. Neural Repair. 2020, 34, 333–343. [Google Scholar] [CrossRef]
- Wen, M.-H.; Wu, M.-J.; Vinit, S.; Lee, K.-Z. Modulation of Serotonin and Adenosine 2A Receptors on Intermittent Hypoxia-Induced Respiratory Recovery following Mid-Cervical Contusion in the Rat. J. Neurotrauma 2019, 36, 2991–3004. [Google Scholar] [CrossRef]
- Nicaise, C.; Putatunda, R.; Hala, T.J.; Regan, K.A.; Frank, D.M.; Brion, J.-P.; Leroy, K.; Pochet, R.; Wright, M.C.; Lepore, A.C. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J. Neurotrauma 2012, 29, 2748–2760. [Google Scholar] [CrossRef]
- Baussart, B.; Stamegna, J.C.; Polentes, J.; Tadié, M.; Gauthier, P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol. Dis. 2006, 22, 562–574. [Google Scholar] [CrossRef]
- Golder, F.J.; Fuller, D.D.; Lovett-Barr, M.R.; Vinit, S.; Resnick, D.K.; Mitchell, G.S. Breathing patterns after mid-cervical spinal contusion in rats. Exp. Neurol. 2011, 231, 97–103. [Google Scholar] [CrossRef]
- Lee, K.Z. Neuropathology of distinct diaphragm areas following mid-cervical spinal cord contusion in the rat. Spine J. 2022, 22, 1726–1741. [Google Scholar] [CrossRef] [PubMed]
- Bajjig, A.; Michel-Flutot, P.; Migevent, T.; Cayetanot, F.; Bodineau, L.; Vinit, S.; Vivodtzev, I. Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice. Biology 2022, 11, 558. [Google Scholar] [CrossRef]
- Vinit, S.; Gauthier, P.; Stamegna, J.C.; Kastner, A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J. Neurotrauma 2006, 23, 1137–1146. [Google Scholar] [CrossRef]
- Lee, K.Z.; Huang, Y.J.; Tsai, I.L. Respiratory motor outputs following unilateral midcervical spinal cord injury in the adult rat. J. Appl. Physiol. 2014, 116, 395–405. [Google Scholar] [CrossRef]
- Bezdudnaya, T.; Lane, M.A.; Marchenko, V. Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats. J. Appl. Physiol. 2018, 125, 687–696. [Google Scholar] [CrossRef]
- Cheng, L.; Sami, A.; Ghosh, B.; Goudsward, H.J.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. Respiratory axon regeneration in the chronically injured spinal cord. Neurobiol. Dis. 2021, 155, 105389. [Google Scholar] [CrossRef]
- Cheng, L.; Sami, A.; Ghosh, B.; Urban, M.W.; Heinsinger, N.M.; Liang, S.S.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. LAR inhibitory peptide promotes recovery of diaphragm function and multiple forms of respiratory neural circuit plasticity after cervical spinal cord injury. Neurobiol. Dis. 2021, 147, 105153. [Google Scholar] [CrossRef]
- Michel-Flutot, P.; Mansart, A.; Deramaudt, B.T.; Jesus, I.; Lee, K.; Bonay, M.; Vinit, S. Permanent diaphragmatic deficits and spontaneous respiratory plasticity in a mouse model of incomplete cervical spinal cord injury. Respir. Physiol. Neurobiol. 2021, 284, 103568. [Google Scholar] [CrossRef]
- Michel-Flutot, P.; Zholudeva, L.V.; Randelman, M.L.; Deramaudt, T.B.; Mansart, A.; Alvarez, J.-C.; Lee, K.-Z.; Petitjean, M.; Bonay, M.; Lane, M.A.; et al. High frequency repetitive Transcranial Magnetic Stimulation promotes long lasting phrenic motoneuron excitability via GABAergic networks. Respir. Physiol. Neurobiol. 2021, 284, 103704. [Google Scholar] [CrossRef]
- Rana, S.; Zhan, W.-Z.; Sieck, G.C.; Mantilla, C.B. Cervical spinal hemisection alters phrenic motor neuron glutamatergic mRNA receptor expression. Exp. Neurol. 2022, 353, 114030. [Google Scholar] [CrossRef]
- Allen, L.L.; Nichols, N.L.; Asa, Z.A.; Emery, A.T.; Ciesla, M.C.; Santiago, J.V.; Holland, A.E.; Mitchell, G.S.; Gonzalez-Rothi, E.J. Phrenic motor neuron survival below cervical spinal cord hemisection. Exp. Neurol. 2021, 346, 113832. [Google Scholar] [CrossRef] [PubMed]
- Ghali, M.G.; Marchenko, V. Dynamic changes in phrenic motor output following high cervical hemisection in the decerebrate rat. Exp. Neurol. 2015, 271, 379–389. [Google Scholar] [CrossRef]
- Minor, K.H.; Akison, L.K.; Goshgarian, H.G.; Seeds, N.W. Spinal cord injury-induced plasticity in the mouse—The crossed phrenic phenomenon. Exp. Neurol. 2006, 200, 486–495. [Google Scholar] [CrossRef]
- Goshgarian, H.G. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 85–93. [Google Scholar] [CrossRef]
- Porter, W.T. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J. Physiol. 1895, 17, 455–485. [Google Scholar] [CrossRef]
- Bach, K.B.; Mitchell, G.S. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J. Appl. Physiol. 1998, 84, 2099–2105. [Google Scholar] [CrossRef]
- Nichols, N.L.; Mitchell, G.S. Mechanisms of severe acute intermittent hypoxia-induced phrenic long-term facilitation. J. Neurophysiol. 2021, 125, 1146–1156. [Google Scholar] [CrossRef]
- Powell, F.L.; Milsom, W.K.; Mitchell, G.S. Time domains of the hypoxic ventilatory response. Respir. Physiol. 1998, 112, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.D.; Mitchell, G.S. Respiratory neuroplasticity—Overview, significance and future directions. Exp. Neurol. 2017, 287, 144–152. [Google Scholar] [CrossRef]
- Mitchell, G.S.; Baker, T.L.; Nanda, S.A.; Fuller, D.D.; Zabka, A.G.; Hodgeman, B.A.; Bavis, R.W.; Mack, K.J.; Olson, E.B., Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001, 90, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.D.; Johnson, S.M.; Olson, E.B., Jr.; Mitchell, G.S. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J. Neurosci. 2003, 23, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C.; Lesske, J.; Qian, W.; Miller, C.C., 3rd; Unger, T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 1992, 19, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Daniel, J.M.; Dohanich, G.P. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2442–2450. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef]
- Golder, F.J.; Mitchell, G.S. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, B.J.; Terada, J.; Springborn, S.R.; Vinit, S.; MacFarlane, P.M.; Mitchell, G.S. Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir. Physiol. Neurobiol. 2018, 256, 50–57. [Google Scholar] [CrossRef]
- Lovett-Barr, M.R.; Satriotomo, I.; Muir, G.D.; Wilkerson, J.E.; Hoffman, M.S.; Vinit, S.; Mitchell, G.S. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci. 2012, 32, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Doperalski, N.J.; Fuller, D.D. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp. Neurol. 2006, 200, 74–81. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Vinit, S.; Dougherty, B.J.; Mitchell, G.S. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp. Neurol. 2015, 266, 1–10. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.A.; Vinit, S.; Mitchell, G.S. Adenosine 2A Receptor Inhibition Enhances Intermittent Hypoxia-Induced Diaphragm but Not Intercostal Long-Term Facilitation. J. Neurotrauma 2014, 31, 1975–1984. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Tadjalli, A.; Allen, L.L.; Ciesla, M.C.; Chami, M.E.; Mitchell, G.S. Protocol-Specific Effects of Intermittent Hypoxia Pre-Conditioning on Phrenic Motor Plasticity in Rats with Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2021, 38, 1292–1305. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Dougherty, B.J.; Mitchell, G.S. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp. Neurol. 2017, 287, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Komnenov, D.; Solarewicz, J.Z.; Afzal, F.; Nantwi, K.D.; Kuhn, D.M.; Mateika, J.H. Intermittent hypoxia promotes recovery of respiratory motor function in spinal cord-injured mice depleted of serotonin in the central nervous system. J. Appl. Physiol. 2016, 121, 545–557. [Google Scholar] [CrossRef]
- Lee, K.Z.; Chiang, S.C.; Li, Y.J. Mild Acute Intermittent Hypoxia Improves Respiratory Function in Unanesthetized Rats with Midcervical Contusion. Neurorehabilit. Neural Repair. 2017, 31, 364–375. [Google Scholar] [CrossRef]
- Lin, M.-T.; Vinit, S.; Lee, K.-Z. Functional role of carbon dioxide on intermittent hypoxia induced respiratory response following mid-cervical contusion in the rat. Exp. Neurol. 2021, 339, 113610. [Google Scholar] [CrossRef]
- Gutierrez, D.V.; Clark, M.; Nwanna, O.; Alilain, W.J. Intermittent hypoxia training after C2 hemisection modifies the expression of PTEN and mTOR. Exp. Neurol. 2013, 248, 45–52. [Google Scholar] [CrossRef]
- Ciesla, M.C.; Seven, Y.B.; Allen, L.L.; Smith, K.N.; Asa, Z.A.; Simon, A.K.; Holland, A.E.; Santiago, J.V.; Stefan, K.; Ross, A.; et al. Serotonergic innervation of respiratory motor nuclei after cervical spinal injury: Impact of intermittent hypoxia. Exp. Neurol. 2021, 338, 113609. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Lee, K.-Z.; Dale, E.A.; Reier, P.J.; Mitchell, G.S.; Fuller, D.D. Intermittent hypoxia and neurorehabilitation. J. Appl. Physiol. 2015, 119, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.A.; Mabrouk, F.B.; Mitchell, G.S. Unexpected Benefits of Intermittent Hypoxia: Enhanced Respiratory and Nonrespiratory Motor Function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Lee, K.Z. Intermittent hypoxia and respiratory recovery in pre-clinical rodent models of incomplete cervical spinal cord injury. Exp. Neurol. 2021, 342, 113751. [Google Scholar] [CrossRef]
- Tester, N.J.; Fuller, D.D.; Fromm, J.S.; Spiess, M.R.; Behrman, A.L.; Mateika, J.H. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am. J. Respir. Crit. Care Med. 2014, 189, 57–65. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Tester, N.J.; Davenport, P.W. Effect of acute intermittent hypoxia treatment on ventilatory load compensation and magnitude estimation of inspiratory resistive loads in an individual with chronic incomplete cervical spinal cord injury. J. Spinal Cord Med. 2016, 39, 103–110. [Google Scholar] [CrossRef]
- Sutor, T.; Cavka, K.; Vose, A.K.; Welch, J.F.; Davenport, P.; Fuller, D.D.; Mitchell, G.S.; Fox, E.J. Single-session effects of acute intermittent hypoxia on breathing function after human spinal cord injury. Exp. Neurol. 2021, 342, 113735. [Google Scholar] [CrossRef]
- Warren, P.M.; Steiger, S.C.; Dick, T.E.; MacFarlane, P.M.; Alilain, W.J.; Silver, J. Rapid and robust restoration of breathing long after spinal cord injury. Nat. Commun. 2018, 9, 4843. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Gray, E.; Kocherginsky, M.; Jayaraman, A.; Mitchell, G.S.; Rymer, W.Z. Prednisolone Pretreatment Enhances Intermittent Hypoxia-Induced Plasticity in Persons with Chronic Incomplete Spinal Cord Injury. Neurorehabilit. Neural Repair. 2019, 33, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Alcayaga, J.; Sepúlveda, O.; Rojas, E.; Astudillo, C. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Neurotrauma 2017, 34, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.Q.; Sohn, W.J.; Naidu, A.; Trumbower, R.D. Daily acute intermittent hypoxia combined with walking practice enhances walking performance but not intralimb motor coordination in persons with chronic incomplete spinal cord injury. Exp. Neurol. 2021, 340, 113669. [Google Scholar] [CrossRef]
- Tan, A.Q.; Barth, S.; Trumbower, R.D. Acute intermittent hypoxia as a potential adjuvant to improve walking following spinal cord injury: Evidence, challenges, and future directions. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Trumbower, R.D.; Hayes, H.B.; Mitchell, G.S.; Wolf, S.L.; Stahl, V.A. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology 2017, 89, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Hérent, C.; Diem, S.; Fortin, G.; Bouvier, J. Upregulation of breathing rate during running exercise by central locomotor circuits. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jesus, I.; Michel-Flutot, P.; Deramaudt, T.B.; Paucard, A.; Vanhee, V.; Vinit, S.; Bonay, M. Effects of aerobic exercise training on muscle plasticity in a mouse model of cervical spinal cord injury. Sci. Rep. 2021, 11, 112. [Google Scholar] [CrossRef]
- Lemos, J.R.; da Cunha, F.A.; Lopes, A.J.; Guimarães, F.S.; do Amaral Vasconcellos, F.V.; Dos Santos Vigário, P. Respiratory muscle training in non-athletes and athletes with spinal cord injury: A systematic review of the effects on pulmonary function, respiratory muscle strength and endurance, and cardiorespiratory fitness based on the FITT principle of exercise prescription. J. Back Musculoskelet. Rehabil. 2020, 33, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Ruys, C.L.; Lewis, C.R.H.; Wijeysuriya, N.S.; McBain, R.A.; Lee, B.B.; McKenzie, D.K.; Gandevia, S.C.; Butler, J.E. Impact of respiratory muscle training on respiratory muscle strength, respiratory function and quality of life in individuals with tetraplegia: A randomised clinical trial. Thorax 2020, 75, 279–288. [Google Scholar] [CrossRef]
- Kang, D.; Park, J.; Eun, S.D. A preliminary study on the feasibility of community game-based respiratory muscle training for individuals with high cervical spinal cord injury levels: A novel approach. BMC Sport Sci. Med. Rehabil. 2022, 14, 137. [Google Scholar] [CrossRef]
- Gee, C.M.; Williams, A.M.; Sheel, A.W.; Eves, N.D.; West, C.R. Respiratory muscle training in athletes with cervical spinal cord injury: Effects on cardiopulmonary function and exercise capacity. J. Physiol. 2019, 597, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.A.; Chadwick, M.R.; Davies, M.J. Mechanisms of improved exercise capacity following respiratory muscle training in athletes with cervical spinal cord injury. J. Physiol. 2019, 597, 5531–5532. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hori, S.; Momosaki, R. Effect of Vocal Exercise on Respiratory Function and Voice Quality in Patients with Cervical Spinal Cord Injury: A Mini-review. Prog. Rehabil. Med. 2022, 7, 20220041. [Google Scholar] [CrossRef]
- Terson de Paleville, D.; McKay, W.; Aslan, S.; Folz, R.; Sayenko, D.; Ovechkin, A. Locomotor step training with body weight support improves respiratory motor function in individuals with chronic spinal cord injury. Respir. Physiol. Neurobiol. 2013, 189, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Nath, R.; Wyant, G.M. Treatment of chronic pain by epidural spinal cord stimulation: A 10-year experience. J. Neurosurg. 1991, 75, 402–407. [Google Scholar] [CrossRef]
- Moreno-Duarte, I.; Morse, L.R.; Alam, M.; Bikson, M.; Zafonte, R.; Fregni, F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 2014, 85, 1003–1013. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Avelev, V.D.; Nikitin, O.A.; Lavrov, I.A. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2003, 33, 247–254. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Lavrov, I.A.; Courtine, G.; Ichiyama, R.M.; Dy, C.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J. Neurosci. Methods 2006, 157, 253–263. [Google Scholar] [CrossRef]
- Ichiyama, R.M.; Gerasimenko, Y.P.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005, 383, 339–344. [Google Scholar] [CrossRef]
- Herman, R.; He, J.; D’Luzansky, S.; Willis, W.; Dilli, S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 2002, 40, 65–68. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Kowalski, K.E.; Hsieh, Y.H.; Dick, T.E.; DiMarco, A.F. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp. Neurol. 2013, 247, 689–693. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Streeter, K.A.; Hanna, M.H.; Stamas, A.C.; Reier, P.J.; Baekey, D.M.; Fuller, D.D. High-frequency epidural stimulation across the respiratory cycle evokes phrenic short-term potentiation after incomplete cervical spinal cord injury. J. Neurophysiol. 2017, 118, 2344–2357. [Google Scholar] [CrossRef]
- Dale, E.A.; Zhong, H.; Edgerton, V.R. Cervical Spinal Stimulation and Respiratory Recovery after Upper Cervical Spinal Cord Injury. FASEB J. 2016, 30, 1294–1297. [Google Scholar]
- Dale, E.A.; Sunshine, M.D.; Kelly, M.N.; Mitchell, G.S.; Fuller, D.D.; Reier, P.J. Chronic, closed-loop, cervical epidural stimulation elicits plasticity in diaphragm motor output and upregulates spinal neurotrophic factor gene expression. FASEB J. 2019, 33, 843.10. [Google Scholar] [CrossRef]
- Lin, A.; Shaaya, E.; Calvert, J.S.; Parker, S.R.; Borton, D.A.; Fridley, J.S. A Review of Functional Restoration From Spinal Cord Stimulation in Patients with Spinal Cord Injury. Neurospine 2022, 19, 703–734. [Google Scholar] [CrossRef]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Pereira, M.; Salvador, R.; Miranda, P.C.; de Carvalho, M. Cervical trans-spinal direct current stimulation: A modelling-experimental approach. J. Neuroeng. Rehabil. 2019, 16, 123. [Google Scholar] [CrossRef]
- Martin, D.M.; McClintock, S.M.; Forster, J.J.; Lo, T.Y.; Loo, C.K. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress. Anxiety 2017, 34, 1029–1039. [Google Scholar] [CrossRef]
- Jassova, K.; Albrecht, J.; Ceresnakova, S.; Papezova, H.; Anders, M. Repetitive transcranial magnetic stimulation significantly influences the eating behavior in depressive patients. Neuropsychiatr. Dis. Treat. 2019, 15, 2579–2586. [Google Scholar] [CrossRef]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.F.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 3651. [Google Scholar] [CrossRef]
- Yan, T.; Xie, Q.; Zheng, Z.; Zou, K.; Wang, L. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): A systematic review and meta-analysis. J. Psychiatr. Res. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Kozel, F.A. Clinical Repetitive Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder, Generalized Anxiety Disorder, and Bipolar Disorder. Psychiatr. Clin. N. Am. 2018, 41, 433–446. [Google Scholar] [CrossRef]
- Wincek, A.; Huber, J.; Leszczyńska, K.; Fortuna, W.; Okurowski, S.; Chmielak, K.; Tabakow, P. The Long-Term Effect of Treatment Using the Transcranial Magnetic Stimulation rTMS in Patients after Incomplete Cervical or Thoracic Spinal Cord Injury. J. Clin. Med. 2021, 10, 2975. [Google Scholar] [CrossRef]
- Tazoe, T.; Perez, M.A. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. Arch. Phys. Med. Rehabil. 2015, 96, S145–S155. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Wincek, A.; Fortuna, W.; Huber, J.; Łukaszek, J.; Okurowski, S.; Chmielak, K.; Tabakow, P. Treatment of patients with cervical and upper thoracic incomplete spinal cord injury using repetitive transcranial magnetic stimulation. Int. J. Artif. Organs 2020, 43, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, T.; Brito, R.; Luna, P.; Campêlo, M.; Shirahige, L.; Fontes, L.; Dias, R.; Piscitelli, D.; Monte-Silva, K. Repetitive transcranial magnetic stimulation on the modulation of cortical and spinal cord excitability in individuals with spinal cord injury. Restor. Neurol. Neurosci. 2021, 39, 291–301. [Google Scholar] [CrossRef]
- Belci, M.; Catley, M.; Husain, M.; Frankel, H.L.; Davey, N.J. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004, 42, 417–419. [Google Scholar] [CrossRef]
- Similowski, T.; Straus, C.; Coïc, L.; Derenne, J.P. Facilitation-independent response of the diaphragm to cortical magnetic stimulation. Am. J. Respir. Crit. Care Med. 1996, 154, 1771–1777. [Google Scholar] [CrossRef]
- Similowski, T.; Catala, M.; Rancurel, G.; Derenne, J.P. Impairment of central motor conduction to the diaphragm in stroke. Am. J. Respir. Crit. Care Med. 1996, 154, 436–441. [Google Scholar] [CrossRef]
- Sharshar, T.; Ross, E.; Hopkinson, N.S.; Dayer, M.; Nickol, A.; Lofaso, F.; Moxham, J.; Similowski, T.; Polkey, M.I. Effect of voluntary facilitation on the diaphragmatic response to transcranial magnetic stimulation. J. Appl. Physiol. 2003, 95, 26–34. [Google Scholar] [CrossRef]
- Demoule, A.; Verin, E.; Locher, C.; Derenne, J.P.; Similowski, T. Validation of surface recordings of the diaphragm response to transcranial magnetic stimulation in humans. J. Appl. Physiol. 2003, 94, 453–461. [Google Scholar] [CrossRef]
- Welch, J.F.; Argento, P.J.; Mitchell, G.S.; Fox, E.J. Reliability of diaphragmatic motor-evoked potentials induced by transcranial magnetic stimulation. J. Appl. Physiol. 2020, 129, 1393–1404. [Google Scholar] [CrossRef]
- Ren, M.Y.; Liou, L.M.; Vinit, S.; Lee, K.Z. Position effect of trans-spinal magnetic stimulation on diaphragmatic motor evoked potential in healthy humans. J. Appl. Physiol. 2022, 133, 1042–1054. [Google Scholar] [CrossRef]
- Vinit, S.; Keomani, E.; Deramaudt, T.B.; Spruance, V.M.; Bezdudnaya, T.; Lane, M.A.; Bonay, M.; Petitjean, M. Interdisciplinary approaches of transcranial magnetic stimulation applied to a respiratory neuronal circuitry model. PLoS ONE 2014, 9, e113251. [Google Scholar] [CrossRef]
- Vinit, S.; Keomani, E.; Deramaudt, T.B.; Bonay, M.; Petitjean, M. Reorganization of Respiratory Descending Pathways following Cervical Spinal Partial Section Investigated by Transcranial Magnetic Stimulation in the Rat. PLoS ONE 2016, 11, e0148180. [Google Scholar] [CrossRef]
- Lee, K.Z.; Liou, L.M.; Vinit, S.; Ren, M.Y. Rostral-Caudal Effect of Cervical Magnetic Stimulation on the Diaphragm Motor Evoked Potential after Cervical Spinal Cord Contusion in the Rat. J. Neurotrauma 2022, 39, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Michel-Flutot, P.; Jesus, I.; Vanhee, V.; Bourcier, C.H.; Emam, L.; Ouguerroudj, A.; Lee, K.-Z.; Zholudeva, L.V.; Lane, M.A.; Mansart, A.; et al. Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology 2022, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Zholudeva, L.V.; Lane, M.A. Transplanting Cells for Spinal Cord Repair: Who, What, When, Where and Why? Cell Transpl. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Stokes, B.T.; Reier, P.J. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp. Neurol. 1992, 116, 1–12. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Hou, S.; Tom, V.J.; Graham, L.; Lu, P.; Blesch, A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17138–17149. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural. Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Chu, T.; Zhou, H.; Li, F.; Wang, T.; Lu, L.; Feng, S. Astrocyte transplantation for spinal cord injury: Current status and perspective. Brain Res. Bull. 2014, 107, 18–30. [Google Scholar] [CrossRef]
- Li, K.; Javed, E.; Hala, T.J.; Sannie, D.; Regan, K.A.; Maragakis, N.J.; Wright, M.C.; Poulsen, D.J.; Lepore, A.C. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Mol. Ther. 2015, 23, 533–548. [Google Scholar] [CrossRef]
- Falnikar, A.; Li, K.; Lepore, A.C. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 2015, 1619, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Javed, E.; Scura, D.; Hala, T.J.; Seetharam, S.; Falnikar, A.; Richard, J.P.; Chorath, A.; Maragakis, N.J.; Wright, M.C.; et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 2015, 271, 479–492. [Google Scholar] [CrossRef]
- Goulão, M.; Ghosh, B.; Urban, M.W.; Sahu, M.; Mercogliano, C.; Charsar, B.A.; Komaravolu, S.; Block, C.G.; Smith, G.M.; Wright, M.C.; et al. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia 2019, 67, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Fortino, T.; Spruance, V.; Niceforo, A.; Harrop, J.S.; Phelps, P.E.; Priest, C.A.; Zholudeva, L.V.; Lane, M.A. Chapter Three—Cell transplantation to repair the injured spinal cord. In International Review of Neurobiology; Lane, E.L., Drew, C.J.G., Lelos, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 166, pp. 79–158. [Google Scholar]
- White, T.E.; Lane, M.A.; Sandhu, M.S.; O’Steen, B.E.; Fuller, D.D.; Reier, P.J. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp. Neurol. 2010, 225, 231–236. [Google Scholar] [CrossRef]
- Lee, K.Z.; Lane, M.A.; Dougherty, B.J.; Mercier, L.M.; Sandhu, M.S.; Sanchez, J.C.; Reier, P.J.; Fuller, D.D. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp. Neurol. 2014, 251, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zholudeva, L.V.; Qiang, L.; Marchenko, V.; Dougherty, K.J.; Sakiyama-Elbert, S.E.; Lane, M.A. The Neuroplastic and Therapeutic Potential of Spinal Interneurons in the Injured Spinal Cord. Trends. Neurosci. 2018, 41, 625–639. [Google Scholar] [CrossRef]
- Romer, S.H.; Seedle, K.; Turner, S.M.; Li, J.; Baccei, M.L.; Crone, S.A. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp. Neurol. 2017, 287, 192–204. [Google Scholar] [CrossRef]
- Kathe, C.; Skinnider, M.A.; Hutson, T.H.; Regazzi, N.; Gautier, M.; Demesmaeker, R.; Komi, S.; Ceto, S.; James, N.D.; Cho, N.; et al. The neurons that restore walking after paralysis. Nature 2022, 611, 540–547. [Google Scholar] [CrossRef]
- Zholudeva, L.V.; Iyer, N.; Qiang, L.; Spruance, V.M.; Randelman, M.L.; White, N.W.; Bezdudnaya, T.; Fischer, I.; Sakiyama-Elbert, S.E.; Lane, M.A. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2883–2903. [Google Scholar] [CrossRef]
- Stamegna, J.C.; Sadelli, K.; Escoffier, G.; Girard, S.D.; Veron, A.D.; Bonnet, A.; Khrestchatisky, M.; Gauthier, P.; Roman, F.S. Grafts of Olfactory Stem Cells Restore Breathing and Motor Functions after Rat Spinal Cord Injury. J. Neurotrauma 2018, 35, 1765–1780. [Google Scholar] [CrossRef]
- Watson, R.A.; Yeung, T.M. What is the potential of oligodendrocyte progenitor cells to successfully treat human spinal cord injury? BMC Neurol. 2011, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Priest, C.A.; Manley, N.C.; Denham, J.; Wirth, E.D., 3rd; Lebkowski, J.S. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen. Med. 2015, 10, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Manley, N.C.; Priest, C.A.; Denham, J.; Wirth, E.D., 3rd; Lebkowski, J.S. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury. Stem Cells Transl. Med. 2017, 6, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Watzlawick, R.; Rind, J.; Sena, E.S.; Brommer, B.; Zhang, T.; Kopp, M.A.; Dirnagl, U.; Macleod, M.R.; Howells, D.W.; Schwab, J.M. Olfactory Ensheathing Cell Transplantation in Experimental Spinal Cord Injury: Effect size and Reporting Bias of 62 Experimental Treatments: A Systematic Review and Meta-Analysis. PLoS Biol. 2016, 14, e1002468. [Google Scholar] [CrossRef]
- Jarocha, D.; Milczarek, O.; Kawecki, Z.; Wendrychowicz, A.; Kwiatkowski, S.; Majka, M. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Transl. Med. 2014, 3, 395–404. [Google Scholar] [CrossRef]
- Richardson, P.M.; McGuinness, U.M.; Aguayo, A.J. Axons from CNS neurons regenerate into PNS grafts. Nature 1980, 284, 264–265. [Google Scholar] [CrossRef]
- David, S.; Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981, 214, 931–933. [Google Scholar] [CrossRef]
- Decherchi, P.; Lammari-Barreault, N.; Gauthier, P. Regeneration of respiratory pathways within spinal peripheral nerve grafts. Exp. Neurol. 1996, 137, 1–14. [Google Scholar] [CrossRef]
- Decherchi, P.; Gauthier, P. Regeneration of acutely and chronically injured descending respiratory pathways within post-traumatic nerve grafts. Neuroscience 2002, 112, 141–152. [Google Scholar] [CrossRef]
- Senjaya, F.; Midha, R. Nerve transfer strategies for spinal cord injury. World Neurosurg. 2013, 80, e319–e326. [Google Scholar] [CrossRef]
- Krieger, L.M.; Krieger, A.J. The Intercostal to Phrenic Nerve Transfer: An Effective Means of Reanimating the Diaphragm in Patients with High Cervical Spine Injury. Plast. Reconstr. Surg. 2000, 105, 1255–1261. [Google Scholar]
- Kwok, J.C.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011, 71, 1073–1089. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef]
- James, N.D.; Shea, J.; Muir, E.M.; Verhaagen, J.; Schneider, B.L.; Bradbury, E.J. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp. Neurol. 2015, 271, 131–135. [Google Scholar] [CrossRef]
- García-Alías, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef]
- Kwok, J.C.F.; Heller, J.P.; Zhao, R.-R.; Fawcett, J.W. Targeting Inhibitory Chondroitin Sulphate Proteoglycans to Promote Plasticity after Injury. In Axon Growth and Regeneration: Methods and Protocols; Murray, A.J., Ed.; Springer: New York, NY, USA, 2014; pp. 127–138. [Google Scholar]
- Bartus, K.; James, N.D.; Didangelos, A.; Bosch, K.D.; Verhaagen, J.; Yanez-Munoz, R.J.; Rogers, J.H.; Schneider, B.L.; Muir, E.M.; Bradbury, E.J. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 4822–4836. [Google Scholar] [CrossRef]
- Didangelos, A.; Iberl, M.; Vinsland, E.; Bartus, K.; Bradbury, E.J. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 16424–16432. [Google Scholar] [CrossRef]
- Muir, E.; De Winter, F.; Verhaagen, J.; Fawcett, J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019, 321, 113032. [Google Scholar] [CrossRef]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef]
- Urban, M.W.; Ghosh, B.; Block, C.G.; Charsar, B.A.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. Protein Tyrosine Phosphatase σ Inhibitory Peptide Promotes Recovery of Diaphragm Function and Sprouting of Bulbospinal Respiratory Axons after Cervical Spinal Cord Injury. J. Neurotrauma 2019, 37, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Nong, J.; Wang, Z.; Urban, M.W.; Heinsinger, N.M.; Trovillion, V.A.; Wright, M.C.; Lepore, A.C.; Zhong, Y. A hydrogel engineered to deliver minocycline locally to the injured cervical spinal cord protects respiratory neural circuitry and preserves diaphragm function. Neurobiol. Dis. 2019, 127, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Ordaz, J.D.; Liu, N.K.; Richardson, Z.; Wu, W.; Xia, Y.; Qu, W.; Wang, Y.; Dai, H.; Zhang, Y.P.; et al. Descending motor circuitry required for NT-3 mediated locomotor recovery after spinal cord injury in mice. Nat. Commun. 2019, 10, 5815. [Google Scholar] [CrossRef]
- Ghosh, B.; Wang, Z.; Nong, J.; Urban, M.W.; Zhang, Z.; Trovillion, V.A.; Wright, M.C.; Zhong, Y.; Lepore, A.C. Local BDNF delivery to the injured cervical spinal cord using an engineered hydrogel enhances diaphragmatic respiratory function. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 5982–5995. [Google Scholar] [CrossRef] [PubMed]
- Charsar, B.A.; Brinton, M.A.; Locke, K.; Chen, A.Y.; Ghosh, B.; Urban, M.W.; Komaravolu, S.; Krishnamurthy, K.; Smit, R.; Pasinelli, P.; et al. AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury. FASEB J. 2019, 33, 13775–13793. [Google Scholar] [CrossRef] [PubMed]
- Gransee, H.M.; Zhan, W.Z.; Sieck, G.C.; Mantilla, C.B. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS ONE 2013, 8, e64755. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).