Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren’s Syndrome (pSS) Pathogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. pSS and Sicca Patients’ Selection and Enrollment
2.2. MSG Biopsy Collection
2.3. Protein Extraction and WB Analysis
2.4. Immunohistochemistry and Immunofluorescence
2.5. Data Analysis
3. Results
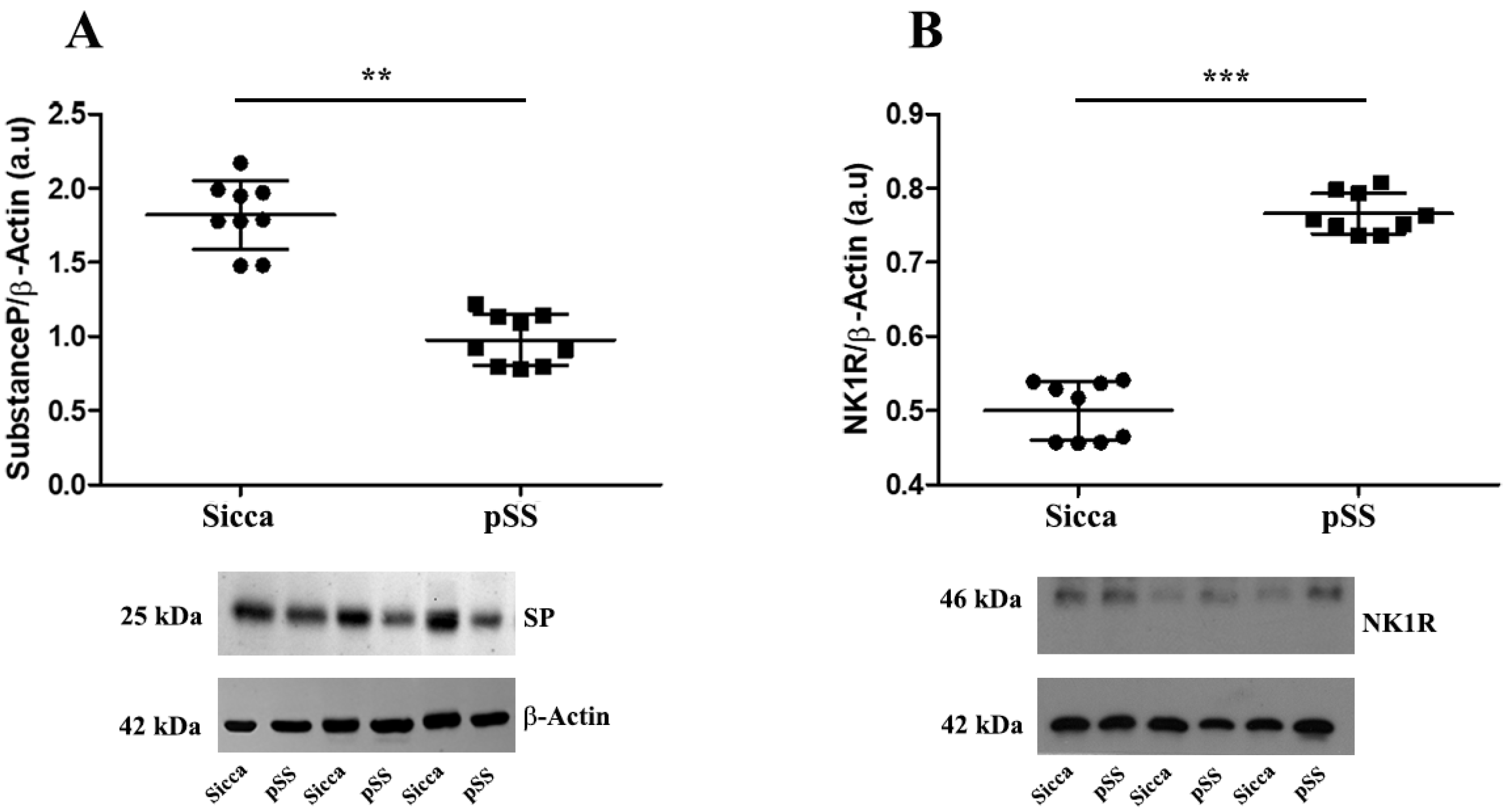
3.1. Lower Expression of SP and Higher Expression of NK1R in MSGs of pSS Patients Compared with Sicca Patients
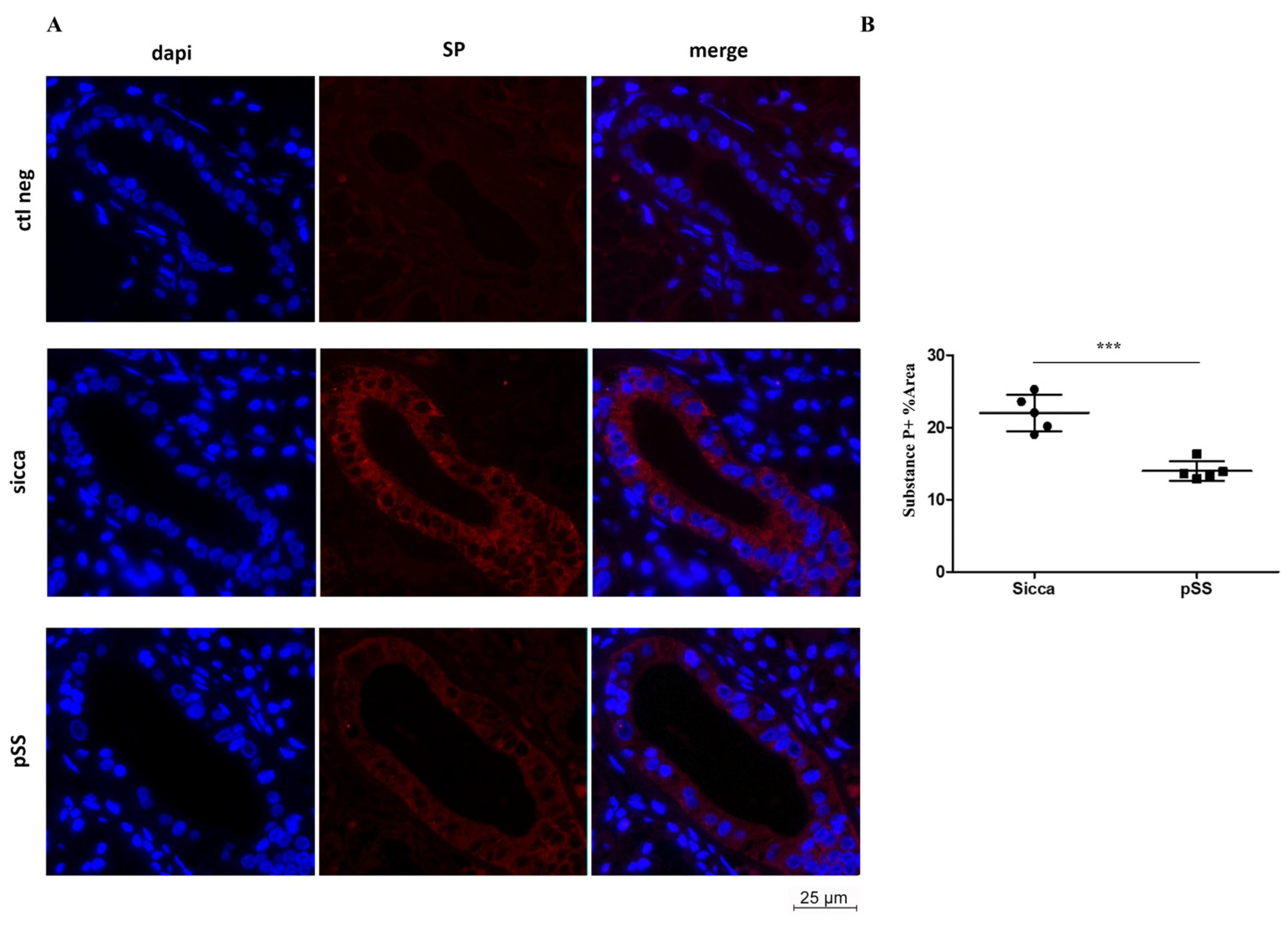
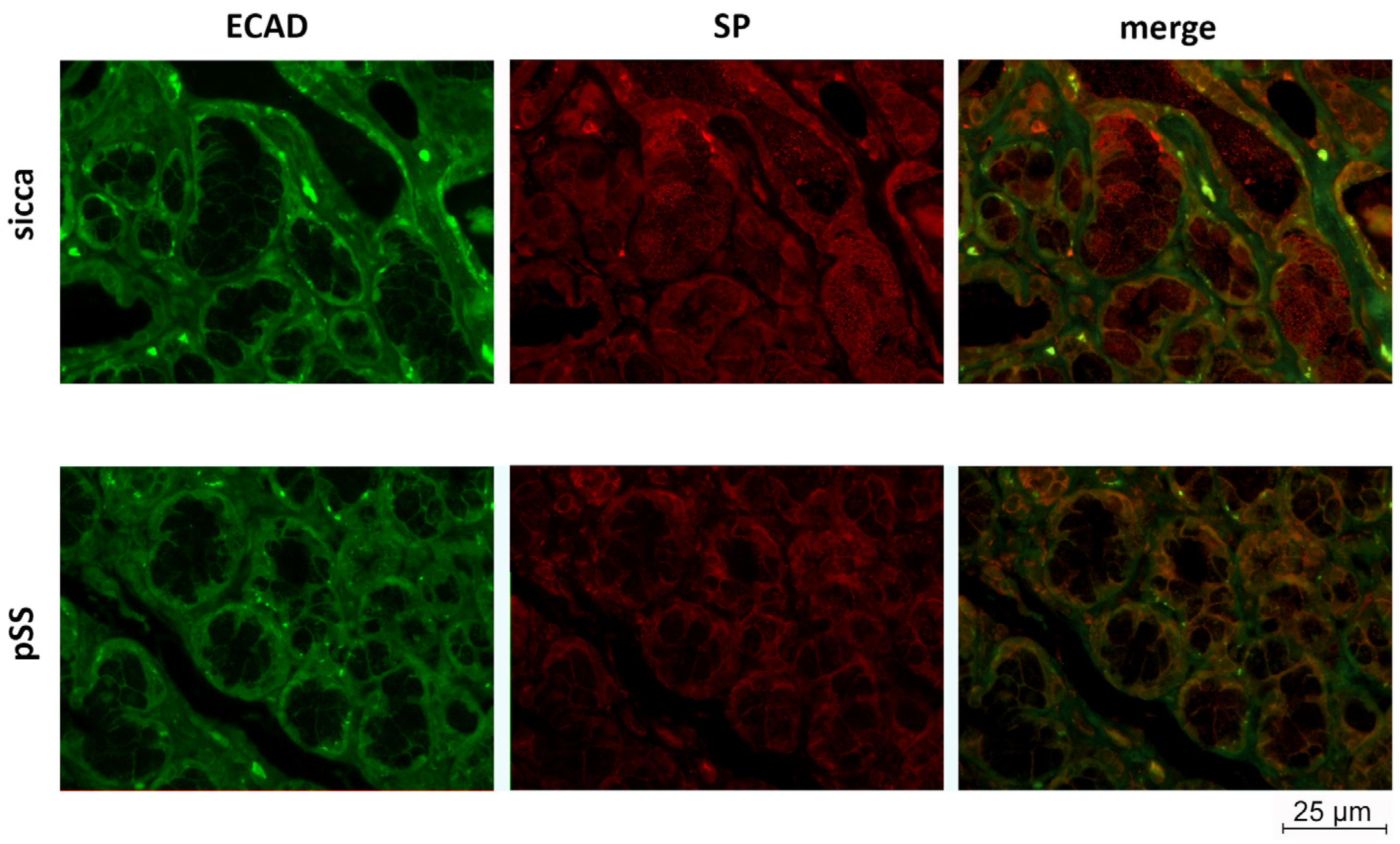
3.2. SP and ECAD Localization in MSG Sections from pSS and Sicca Patients’ Immunofluorescence
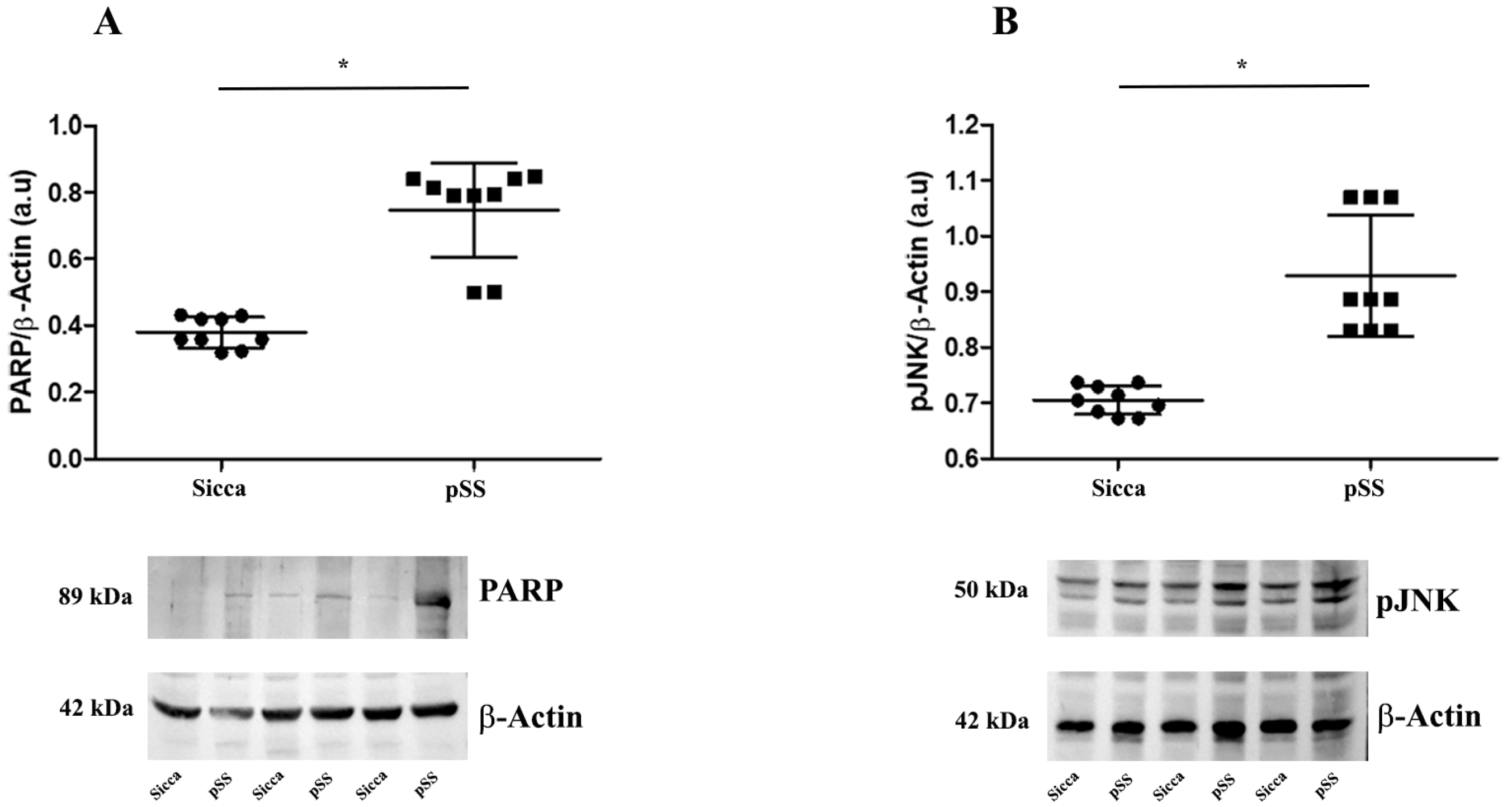
3.3. Western Blot Analysis Reveals Increase in PARP-1 Cleavage and JNK Phosphorylation in MSGs of pSS Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsifis, G.E.; Rekka, S.; Moutsopoulos, N.M.; Pillemer, S.; Wahl, S.M. Systemic and Local Interleukin-17 and Linked Cytokines Associated with Sjögren’s Syndrome Immunopathogenesis. Am. J. Pathol. 2009, 175, 1167. [Google Scholar] [CrossRef]
- Fox, R.I. Sjögren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2022, 22, 9. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T cells in primary Sjögren’s syndrome: Targets for early intervention. Rheumatology 2021, 60, 3088–3098. [Google Scholar] [CrossRef]
- Retamozo, S.; Flores-Chavez, A.; Consuegra-Fernández, M.; Lozano, F.; Ramos-Casals, M.; Brito-Zerón, P. Cytokines as therapeutic targets in primary Sjögren syndrome. Pharmacol. Ther. 2018, 184, 81–97. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S.; Hwang, C.S.; Pitcher, J.D.; Pangelinan, S.B.; Rahimy, E.; Chen, W.; Yoon, K.C.; Farley, W.J.; Niederkorn, J.Y.; Stern, M.E.; et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology 2010, 49, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ekström, J.; Ekman, R.; Håkanson, R.; Sjögren, S.; Sundler, F. Calcitonin gene-related peptide in rat salivary glands: Neuronal localization, depletion upon nerve stimulation, and effects on salivation in relation to substance P. Neuroscience 1988, 26, 933–949. [Google Scholar] [CrossRef]
- Batbayar, B.; Nagy, G.; Kövesi, G.; Zelles, T.; Fehér, E. Morphological basis of sensory neuropathy and neuroimmunomodulation in minor salivary glands of patients with Sjögren’s syndrome. Arch. Oral Biol. 2004, 49, 529–538. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Hukkanen, M.; Kemppinen, P.; Segerberg, M.; Sorsa, T.; MalmstrÖM, M.; Rose, S.; Itescu, S.; Polak, J.M. Peptide-containing nerves in labial salivary glands in Sjögren’s syndrome. Arthritis Rheum. 1992, 35, 815–820. [Google Scholar] [CrossRef]
- Manganelli, P.; Fietta, P. Apoptosis and Sjögren syndrome. Semin. Arthritis Rheum. 2003, 33, 49–65. [Google Scholar] [CrossRef]
- Ferreira, J.N.; Hoffman, M.P. Interactions between developing nerves and salivary glands. Organogenesis 2013, 9, 199. [Google Scholar] [CrossRef]
- Severini, C.; Improta, G.; Falconieri-Erspamer, G.; Salvadori, S.; Erspamer, V. The tachykinin peptide family. Pharmacol. Rev. 2002, 54, 285–322. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Pieri, M.; Ciotti, M.T.; Carunchio, I.; Canu, N.; Calissano, P.; Zona, C.; Severini, C. Substance P provides neuroprotection in cerebellar granule cells through Akt and MAPK/Erk activation: Evidence for the involvement of the delayed rectifier potassium current. Neuropharmacology 2007, 52, 1366–1377. [Google Scholar] [CrossRef]
- Lallemend, F.; Lefebvre, P.P.; Hans, G.; Rigo, J.M.; Van De Water, T.R.; Moonen, G.; Malgrange, B. Substance P protects spiral ganglion neurons from apoptosis via PKC-Ca2+-MAPK/ERK pathways. J. Neurochem. 2003, 87, 508–521. [Google Scholar] [CrossRef]
- Marolda, R.; Ciotti, M.T.; Matrone, C.; Possenti, R.; Calissano, P.; Cavallaro, S.; Severini, C. Substance P activates ADAM9 mRNA expression and induces α-secretase-mediated amyloid precursor protein cleavage. Neuropharmacology 2012, 62, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Salthun-Lassalle, B.; Traver, S.; Hirsch, E.C.; Michel, P.P. Substance P, neurokinins A and B, and synthetic tachykinin peptides protect mesencephalic dopaminergic neurons in culture via an activity-dependent mechanism. Mol. Pharmacol. 2005, 68, 1214–1224. [Google Scholar] [CrossRef]
- Severini, C.; Petrella, C.; Calissano, P. Substance P and Alzheimer’s Disease: Emerging Novel Roles. Curr. Alzheimer Res. 2016, 13, 964–972. [Google Scholar] [CrossRef]
- Cuello, A.C.; Ribeiro-da-Silva, A.; Ma, W.; De Koninck, Y.; Henry, J.L. Organization of substance P primary sensory neurons: Ultrastructural and physiological correlates. Regul. Pept. 1993, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Maftei, D.; Grillo, P.; Bovenzi, R.; Bissacco, J.; Simonetta, C.; Mascioli, D.; Zenuni, H.; Cerroni, R.; Vincenzi, M.; et al. Olfactory neuron Substance P is overexpressed in Parkinson’s disease reflecting gut dysfunction. Mov. Disord. 2023. [Google Scholar] [CrossRef]
- Snijdelaar, D.G.; Dirksen, R.; Slappendel, R.; Crul, B.J.P. Substance P. Eur. J. Pain 2000, 4, 121–135. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Kronberger, C.; Albegger, K.; Saria, A.; Hacker, G.W. Neuropeptides in human salivary (submandibular and parotid) glands. Acta Otolaryngol. 1992, 112, 343–348. [Google Scholar] [CrossRef]
- Fisher, B.A.; Jonsson, R.; Daniels, T.; Bombardieri, M.; Brown, R.M.; Morgan, P.; Bombardieri, S.; Ng, W.-F.; Tzioufas, A.G.; Vitali, C.; et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Colafrancesco, S.; Priori, R.; Smith, C.G.; Minniti, A.; Iannizzotto, V.; Pipi, E.; Lucchesi, D.; Pontarini, E.; Nayar, S.; Campos, J.; et al. CXCL13 as biomarker for histological involvement in Sjögren’s syndrome. Rheumatology 2020, 59, 165–170. [Google Scholar] [CrossRef]
- Jimenez, F.; Aiba-Masago, S.; Al Hashimi, I.; Vela-Roch, N.; Fernandes, G.; Yeh, C.K.; Talal, N.; Dang, H. Activated caspase 3 and cleaved poly(ADP-ribose)polymerase in salivary epithelium suggest a pathogenetic mechanism for Sjögren’s syndrome. Rheumatology 2002, 41, 338–342. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar] [CrossRef]
- Tirassa, P.; Schirinzi, T.; Raspa, M.; Ralli, M.; Greco, A.; Polimeni, A.; Possenti, R.; Mercuri, N.B.; Severini, C. What substance P might tell us about the prognosis and mechanism of Parkinson’s disease? Neurosci. Biobehav. Rev. 2021, 131, 899–911. [Google Scholar] [CrossRef]
- Salum, F.G.; Medella-Junior, F.D.A.C.; Figueiredo, M.A.Z.; Cherubini, K. Salivary hypofunction: An update on therapeutic strategies. Gerodontology 2018, 35, 305–316. [Google Scholar] [CrossRef]
- Sato, Y.; Itoh, H.; Suzuki, Y.; Tatsuta, R.; Takeyama, M. Effect of pilocarpine on substance P and calcitonin gene-related peptide releases correlate with salivary secretion in human saliva and plasma. J. Clin. Pharm. Ther. 2013, 38, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ueha, T.; Nemoto, A.; Kurihara, K. Studies on the salivary secretion induced by substance P in perfused submandibular gland of rat. Proc. Finn. Dent. Soc. 1989, 85, 345–353. [Google Scholar] [PubMed]
- De Caro, G.; Cordella, M.; Miani, P. The treatment of sjoegren’s syndrome with physalaemin. Ophthalmologica 1969, 158, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, W. Treatment of severe courses of keratoconjunctivitis sicca with Eledoisin. Klin. Monbl. Augenheilkd. 1988, 192, 163–166. [Google Scholar] [CrossRef]
- Spinelli, D.; Trabucchi, P.; Formenti, F. [Eledoisin eyedrops in the therapy of keratoconjunctivitis sicca]. Ophtalmologie 1988, 2, 145–147. [Google Scholar] [PubMed]
- Robinson, C.P.; Yamachika, S.; Bounous, D.I.; Brayer, J.; Jonsson, R.; Holmdahl, R.; Peck, A.B.; Humphreys-Beher, M.G. A novel NOD-derived murine model of primary Sjögren’s syndrome. Arthritis Rheum. 1998, 41, 150–156. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Humphreys-Beher, M.G.; Yamachika, S.; Yamamoto, H.; Maeda, N.; Nakagawa, Y.; Peck, A.B.; Robinson, C.P. Salivary gland changes in the NOD mouse model for Sjögren’s syndrome: Is there a non-immune genetic trigger? Eur. J. Morphol. 1998, 36, 247–251. [Google Scholar]
- Fu, J.; Shi, H.; Cao, N.; Wu, S.; Zhan, T.; Xie, L.; Wang, Z.; Ye, L.; Li, C.; Shen, Y.; et al. Toll-like receptor 9 signaling promotes autophagy and apoptosis via divergent functions of the p38/JNK pathway in human salivary gland cells. Exp. Cell Res. 2019, 375, 51–59. [Google Scholar] [CrossRef]
- Jensen, S.B.; Vissink, A. Salivary gland dysfunction and xerostomia in Sjögren’s syndrome. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 35–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, P.; Fico, E.; Colafrancesco, S.; Bellizzi, M.G.; Priori, R.; Cerbelli, B.; Leopizzi, M.; Giordano, C.; Greco, A.; Tirassa, P.; et al. Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren’s Syndrome (pSS) Pathogenesis. Cells 2023, 12, 1347. https://doi.org/10.3390/cells12101347
Rosso P, Fico E, Colafrancesco S, Bellizzi MG, Priori R, Cerbelli B, Leopizzi M, Giordano C, Greco A, Tirassa P, et al. Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren’s Syndrome (pSS) Pathogenesis. Cells. 2023; 12(10):1347. https://doi.org/10.3390/cells12101347
Chicago/Turabian StyleRosso, Pamela, Elena Fico, Serena Colafrancesco, Mario Giuseppe Bellizzi, Roberta Priori, Bruna Cerbelli, Martina Leopizzi, Carla Giordano, Antonio Greco, Paola Tirassa, and et al. 2023. "Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren’s Syndrome (pSS) Pathogenesis" Cells 12, no. 10: 1347. https://doi.org/10.3390/cells12101347
APA StyleRosso, P., Fico, E., Colafrancesco, S., Bellizzi, M. G., Priori, R., Cerbelli, B., Leopizzi, M., Giordano, C., Greco, A., Tirassa, P., Severini, C., & Fusconi, M. (2023). Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren’s Syndrome (pSS) Pathogenesis. Cells, 12(10), 1347. https://doi.org/10.3390/cells12101347









