TLR9 and Glioma: Friends or Foes?
Abstract
1. Introduction
2. Glioma
3. TLR9 Overview
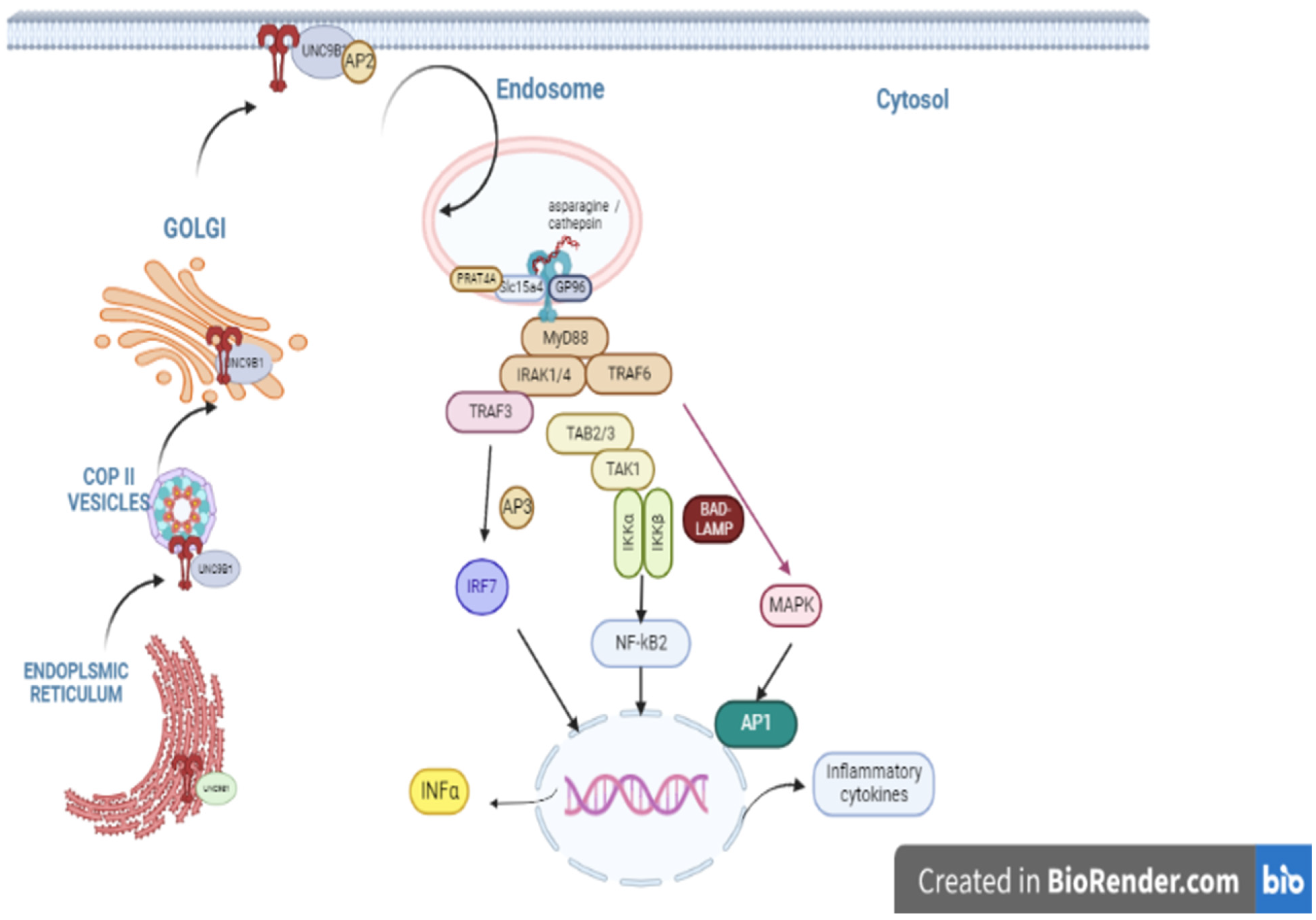
3.1. TLR9 Discovery, Structure, Ligands
3.2. TLR9 Signaling Pathway
3.3. TLR9 Expression on Immune Cells
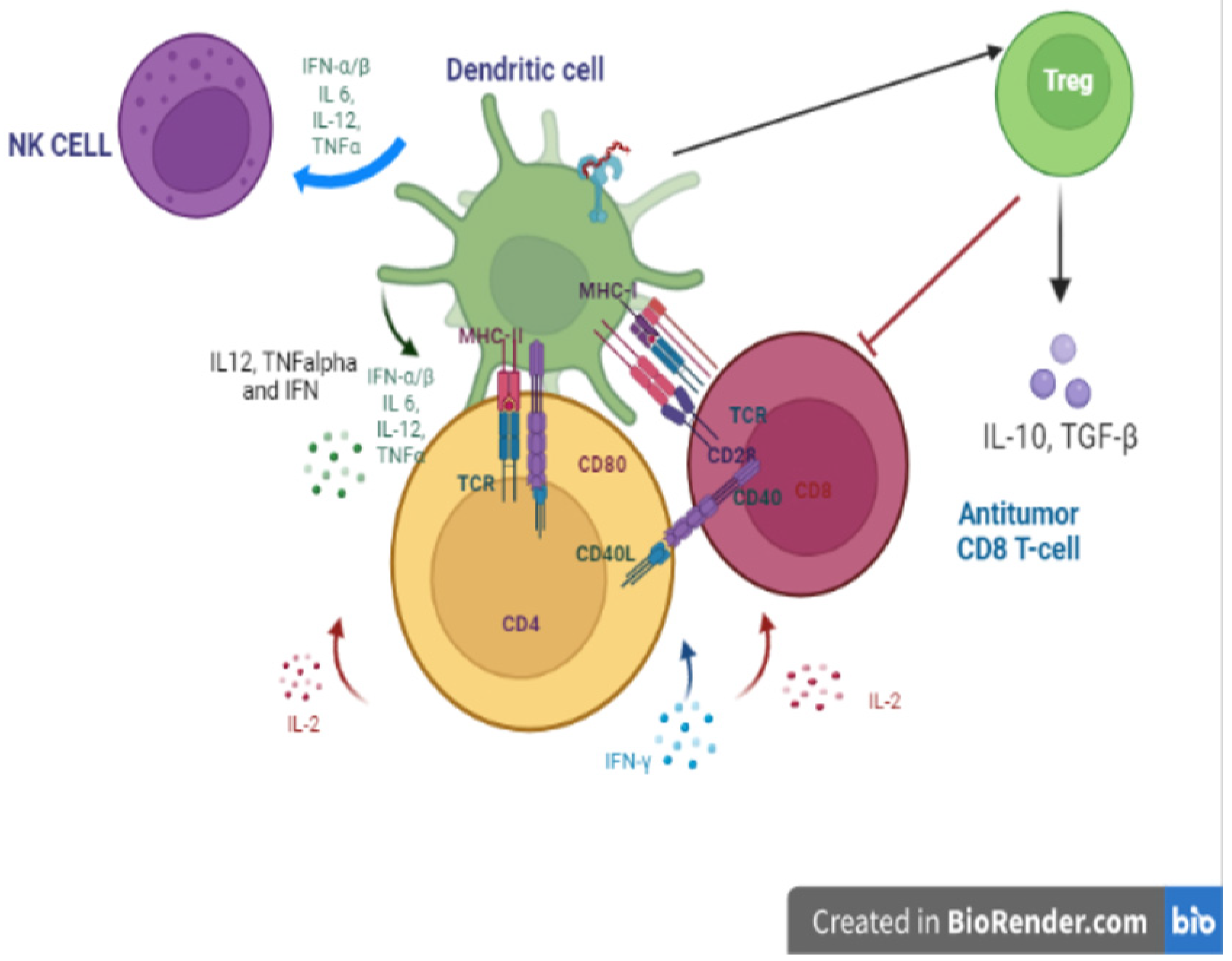
3.4. TLR9 Critically Bridges Innate and Adaptative Immunity: How Does the TLR9-MyD88 Pathway Promote Adaptive Immune Responses?
3.5. The Expression of TLR9 in Cancer Cells Can Corrupt the Process
4. TLR9 Expressions and Function in Gliomas
TLR Expressions in the Glioma Microenvironment
5. Current Status of TLR9 Agonists in Glioma Treatment and Clinical Trial Based on CpG Agonists
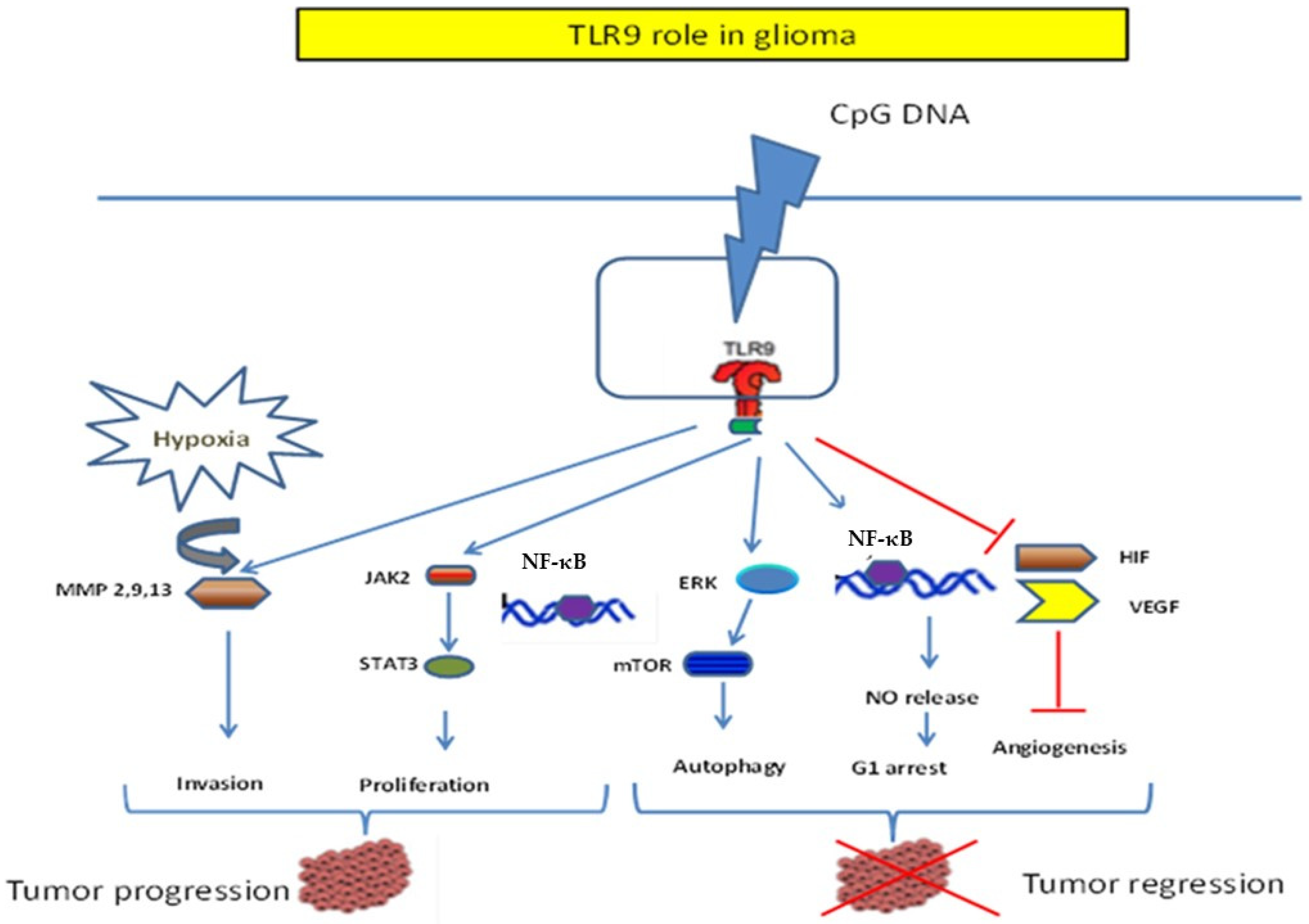
6. TLR9 in Glioma: Dichotomic Role
6.1. TLR9 Can Participate in Immune Responses against Glioma
6.2. TLR9 Promotes Glioma Development
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Behin, A.; Hoang-Xuan, K.; Carpentier, A.F.; Delattre, J.-Y. Primary brain tumours in adults. Lancet 2003, 361, 323–331. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.L.; Schwartzbaum, J.A.; Wrensch, M.; Wiemels, J.L. Epidemiology of Brain Tumors. Neurol. Clin. 2007, 25, 867–890. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H. Epidemiology of Brain Tumors. Methods. Mol Biol. 2009, 472, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.T.; Antonios, J.P.; Soto, H.; Prins, R.M.; Yang, I.; Kasahara, N.; Liau, L.M.; Kruse, C.A. Chronic inflammation drives glioma growth: Cellular and molecular factors responsible for an immunosuppressive microenvironment. Neuroimmunol. Neuroinflamm. 2014, 1, 66–76. [Google Scholar] [CrossRef]
- Nduom, E.; Weller, M.; Heimberger, A.B. Immunosuppressive mechanisms in glioblastoma. Neuro-Oncology 2015, 17, vii9–vii14. [Google Scholar] [CrossRef]
- Ganguly, D.; Fan, M.; Yang, C.H.; Zbytek, B.; Finkelstein, D.; Roussel, M.F.; Pfeffer, L.M. The critical role that STAT3 plays in glioma-initiating cells: STAT3 addiction in glioma. Oncotarget 2018, 9, 22095–22112. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef]
- Sipos, F.; Kiss, A.L.; Constantinovits, M.; Tulassay, Z.; Műzes, G. Modified Genomic Self-DNA Influences In Vitro Survival of HT29 Tumor Cells via TLR9- and Autophagy Signaling. Pathol. Oncol. Res. 2019, 25, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Choi, J.-J.; Seo, E.S.; Kim, M.J.; Kim, W.Y.; Choi, C.H.; Kim, T.-J.; Kim, B.-G.; Song, S.Y.; Bae, D.-S. Increased toll-like receptor 9 expression in cervical neoplasia. Mol. Carcinog. 2007, 46, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Cannella, F.; Pierangeli, A.; Scagnolari, C.; Cacciotti, G.; Tranquilli, G.; Stentella, P.; Recine, N.; Antonelli, G. TLR9 is expressed in human papillomavirus-positive cervical cells and is overexpressed in persistent infections. Immunobiology 2015, 220, 363–368. [Google Scholar] [CrossRef]
- Fehri, E.; Ennaifer, E.; Ardhaoui, M.; Ouerhani, K.; Laassili, T.; Rhouma, R.B.H.; Guizani, I.; Boubaker, S. Expression of Toll-like receptor 9 increases with progression of cervical neoplasia in Tunisian women—A comparative analysis of condyloma, cervical intraepithelial neo-plasia and invasive carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 6145–6150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pradere, J.-P.; Dapito, D.H.; Schwabe, R.F. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2014, 33, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Fehri, E.; Ennaifer, E.; Rhouma, R.B.H.; Guizani-Tabbane, L.; Guizani, I.; Boubaker, S. The role of Toll-like receptor 9 in gynecologic cancer. Curr. Res. Transl. Med. 2016, 64, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Sievert, A.J.; Fisher, M.J. Pediatric Low-Grade Gliomas. J. Child Neurol. 2009, 24, 1397–1408. [Google Scholar] [CrossRef]
- Forst, D.A.; Nahed, B.V.; Loeffler, J.S.; Batchelor, T.T. Low-Grade Gliomas. Oncologist 2014, 19, 403–413. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar] [CrossRef]
- Deng, L.; Shen, L.; Shen, L.; Zhao, Z.; Peng, Y.; Liu, H.; Liu, H.; Zhang, G.; Li, Z.; Li, K.; et al. Prognostic value of magnetic resonance imaging features in low-grade gliomas. Biosci. Rep. 2019, 39, BSR20190544. [Google Scholar] [CrossRef]
- Keles, G.E.; Chang, E.F.; Lamborn, K.R.; Tihan, T.; Chang, C.-J.; Chang, S.M.; Berger, M.S. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J. Neurosurg. 2006, 105, 34–40. [Google Scholar] [CrossRef]
- Komori, T. The 2016 WHO Classification of Tumours of the Central Nervous System: The Major Points of Revision. Neurol. Med.-Chir. 2017, 57, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar-Uniyal, M.; Labagnara, M.; Friedman, M.; Kwasnicki, A.; Murali, R. Glioblastoma: Molecular Pathways, Stem Cells and Therapeutic Targets. Cancers 2015, 7, 538–555. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745, Erratum in Nature 2001, 409, 646. [Google Scholar] [CrossRef]
- Kumagai, Y.; Takeuchi, O.; Akira, S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008, 60, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, J.M.; Sandholm, J.A.; Kaakinen, M.; Hayden, K.L.; Haapasaari, K.M.; Jukkola-Vuorinen, A.; Kauppila, J.H.; Lehenkari, P.P.; Harris, K.W.; Graves, D.E.; et al. Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro. Breast Cancer Res. Treat. 2016, 155, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Rothenfusser, S.; Tuma, E.; Endres, S.; Hartmann, G. Plasmacytoid dendritic cells: The key to CpG. Hum. Immunol. 2002, 63, 1111–1119. [Google Scholar] [CrossRef]
- Hanagata, N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int. J. Nanomed. 2012, 7, 2181–2195. [Google Scholar] [CrossRef]
- Mann, C.C.D.O.; Hornung, V. Molecular mechanisms of nonself nucleic acid recognition by the innate immune system. Eur. J. Immunol. 2021, 51, 1897–1910. [Google Scholar] [CrossRef]
- Tabeta, K.; Hoebe, K.; Janssen, E.M.; Du, X.; Georgel, P.; Crozat, K.; Mudd, S.; Mann, N.; Sovath, S.; Goode, J.; et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 2006, 7, 156–164. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Brinkmann, M.M.; Paquet, M.-E.; Ploegh, H.L. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 2008, 452, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Lind, N.A.; Rael, V.E.; Pestal, K.; Liu, B.; Barton, G.M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 2022, 22, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Linehan, M.M.; Iwasaki, A. Bifurcation of Toll-Like Receptor 9 Signaling by Adaptor Protein 3. Science 2010, 329, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Camosseto, V.; N’Guessan, P.; Argüello, R.J.; Mussard, J.; Caux, C.; Bendriss-Vermare, N.; Pierre, P.; Gatti, E. BAD-LAMP controls TLR9 trafficking and signalling in human plasmacytoid dendritic cells. Nat. Commun. 2017, 8, 913. [Google Scholar] [CrossRef]
- Brooks, J.C.; Sun, W.; Chiosis, G.; Leifer, C.A. Heat shock protein gp96 regulates Toll-like receptor 9 proteolytic processing and conformational stability. Biochem. Biophys. Res. Commun. 2012, 421, 780–784. [Google Scholar] [CrossRef][Green Version]
- Blasius, A.L.; Arnold, C.N.; Georgel, P.; Rutschmann, S.; Xia, Y.; Lin, P.; Ross, C.; Li, X.; Smart, N.G.; Beutler, B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2010, 107, 19973–19978. [Google Scholar] [CrossRef]
- Takahashi, K.; Shibata, T.; Akashi-Takamura, S.; Kiyokawa, T.; Wakabayashi, Y.; Tanimura, N.; Kobayashi, T.; Matsumoto, F.; Fukui, R.; Kouro, T.; et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J. Exp. Med. 2007, 204, 2963–2976. [Google Scholar] [CrossRef]
- Ewald, S.E.; Lee, B.L.; Lau, L.; Wickliffe, K.E.; Shi, G.-P.; Chapman, H.A.; Barton, G.M. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 2008, 456, 658–662. [Google Scholar] [CrossRef]
- Park, B.; Brinkmann, M.M.; Spooner, E.; Lee, C.C.; Kim, Y.-M.; Ploegh, H.L. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 2008, 9, 1407–1414. [Google Scholar] [CrossRef]
- Ewald, S.E.; Engel, A.; Lee, J.; Wang, M.; Bogyo, M.; Barton, G.M. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J. Exp. Med. 2011, 208, 643–651. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mohan, C. Toll-Like Receptor Signaling Pathways—Therapeutic Opportunities. Mediat. Inflamm. 2010, 2010, 781235. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.; Beignon, A.-S.; Bhardwaj, N. Plasmacytoid Dendritic Cells: Linking Innate and Adaptive Immunity. J. Virol. 2005, 79, 17–27. [Google Scholar] [CrossRef]
- Juarez, E.; Nuñez, C.; Sada, E.; Ellner, J.J.; Schwander, S.K.; Torres, M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir. Res. 2010, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bourke, E.; Bosisio, D.; Golay, J.; Polentarutti, N.; Mantovani, A. The toll-like receptor repertoire of human B lymphocytes: Inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 2003, 102, 956–963. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG Motifs in Bacterial DNA and Their Immune Effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.J.; Moore, J.M.; Griffith, T.S. Human B Cells Express Functional TRAIL/Apo-2 Ligand after CpG-Containing Oligodeoxynucleotide Stimulation. J. Immunol. 2004, 173, 892–899. [Google Scholar] [CrossRef]
- Vollmer, J.; Jurk, M.; Samulowitz, U.; Lipford, G.; Forsbach, A.; Wüllner, M.; Tluk, S.; Hartmann, H.; Kritzler, A.; Müller, C.; et al. CpG oligodeoxynucleotides stimulate IFN-γ-inducible protein-10 production in human B cells. J. Endotoxin Res. 2004, 10, 431–438. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y. Targeting the TLR9–MyD88 pathway in the regulation of adaptive immune responses. Expert Opin. Ther. Targets 2010, 14, 787–796. [Google Scholar] [CrossRef]
- Ma, Y.; He, B. Recognition of Herpes Simplex Viruses: Toll-Like Receptors and Beyond. J. Mol. Biol. 2014, 426, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.; French, A.R.; Barchet, W.; Fischer, J.A.; Dzionek, A.; Pingel, J.T.; Orihuela, M.M.; Akira, S.; Yokoyama, W.M.; Colonna, M. TLR9-Dependent Recognition of MCMV by IPC and DC Generates Coordinated Cytokine Responses that Activate Antiviral NK Cell Function. Immunity 2004, 21, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Oosting, M.; Joosten, L.A.B.; Netea, M.G.; Van Crevel, R. Innate Immune Recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. 2011, 2011, 405310. [Google Scholar] [CrossRef]
- Gomes, M.T.R.; Campos, P.C.; de Almeida, L.A.; Oliveira, F.S.; Costa, M.M.S.; Marim, F.M.; Pereira, G.S.; Oliveira, S.C. The role of innate immune signals in immunity to Brucella abortus. Front. Cell. Infect. Microbiol. 2012, 2, 130. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Oertli, M.; Arnold, I.C. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun. Signal. 2011, 9, 25. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyazato, A.; Xiao, G.; Hatta, M.; Inden, K.; Aoyagi, T.; Shiratori, K.; Takeda, K.; Akira, S.; Saijo, S.; et al. Deoxynucleic Acids from Cryptococcus neoformans Activate Myeloid Dendritic Cells via a TLR9-Dependent Pathway. J. Immunol. 2008, 180, 4067–4074. [Google Scholar] [CrossRef]
- Granucci, F.; Zanoni, I.; Ricciardi-Castagnoli, P. Central role of dendritic cells in the regulation and deregulation of immune responses. Cell. Mol. Life Sci. 2008, 65, 1683–1697. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Sabado, R.L.; Bhardwaj, N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy 2010, 2, 37–56. [Google Scholar] [CrossRef]
- Heit, A.; Maurer, T.; Hochrein, H.; Bauer, S.; Huster, K.M.; Busch, D.H.; Wagner, H. Cutting Edge: Toll-Like Receptor 9 Expression Is Not Required for CpG DNA-Aided Cross-Presentation of DNA-Conjugated Antigens but Essential for Cross-Priming of CD8 T Cells. J. Immunol. 2003, 170, 2802–2805. [Google Scholar] [CrossRef]
- Kabelitz, D.; Medzhitov, R. Innate immunity—Cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr. Opin. Immunol. 2007, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.; Medzhitov, R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009, 227, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Duttagupta, P.A.; Boesteanu, A.C.; Katsikis, P.D. Costimulation signals for memory CD8+ T cells during viral infections. Crit. Rev. Immunol. 2009, 29, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, L.; Zhao, Y. Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clin. Exp. Immunol. 2010, 160, 168–175. [Google Scholar] [CrossRef]
- Parihar, R.; Dierksheide, J.; Hu, Y.; Carson, W.E. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J. Clin. Investig. 2002, 110, 983–992. [Google Scholar] [CrossRef]
- Müller, L.; Aigner, P.; Stoiber, D. Type I Interferons and Natural Killer Cell Regulation in Cancer. Front. Immunol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Temizoz, B.; Ishii, K.J. Type I and II interferons toward ideal vaccine and immunotherapy. Expert Rev. Vaccines 2021, 20, 527–544. [Google Scholar] [CrossRef]
- Maldonado, R.A.; von Andrian, U.H. How Tolerogenic Dendritic Cells Induce Regulatory T Cells. Adv. Immunol. 2010, 108, 111–165. [Google Scholar] [CrossRef]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef]
- Ruan, M.; Li, S.; Yang, W.; Liu, S.; Andrew, O.; Wang, L.; Zhang, C. Toll-like receptor-9 agonists increase cyclin D1 expression partly through activation of activator protein-1 in human oral squamous cell carcinoma cells. Cancer Sci. 2012, 103, 1938–1945. [Google Scholar]
- Ruan, M.; Zhang, Z.; Li, S.; Yan, M.; Liu, S.; Yang, W.; Wang, L.; Zhang, C. Activation of Toll-Like Receptor-9 Promotes Cellular Mi-gration via Up-Regulating MMP-2 Expression in Oral Squamous Cell Carcinoma. PLoS ONE 2014, 9, e92748. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, Y.; Liu, Q.; Luo, J.M.; Qing, M.; Tang, X.Y.; Yao, X.S.; Wang, C.H.; Wen, Z.K. CXCR4/SDF-1 pathway is crucial for TLR9 agonist enhanced metastasis of human lung cancer cell. Biochem. Biophys. Res. Commun. 2009, 382, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Ma, A.; Li, Y.; Li, R.; Wang, Y. Functional expression of TLR9 in esophageal cancer. Oncol. Rep. 2014, 31, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Wu, M.S.; Lin, J.T.; Sheu, B.S.; Muta, T. Induction of Cyclooxygenase-2 Overexpression in Human Gastric Epithelial Cells by Helicobacter pylori Involves TLR2/ TLR9 and c-Src-Dependent Nuclear Factor-_B Activation. Mol. Pharmacol. 2004, 66, 1465–1477. [Google Scholar] [CrossRef]
- Chang, Y.J.; Wu, M.S.; Lin, J.T.; Chen, C.C. Helicobacter pylori-Induced Invasion and Angiogenesis of Gastric Cells Is Mediated by Cyclooxygenase-2 Induction through TLR2/TLR9 and Promoter Regulation. J. Immunol. 2005, 175, 8242–8252. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef]
- Mashayekhi, S.; Saberi, A.; Salehi, Z. Expression of Matrix Metalloproteinase-2 and -9 in Meningioma. Casp. J. Neurol. Sci. 2018, 4, 24–29. [Google Scholar] [CrossRef]
- Merrell, M.A.; Ilvesaro, J.M.; Lehtonen, N.; Sorsa, T.; Gehrs, B.; Rosenthal, E.; Chen, D.; Shackley, B.; Harris, K.W.; Selander, K.S. Toll-Like Receptor 9 Agonists Promote Cellular Invasion by Increasing Matrix Metalloproteinase Activity. Mol. Cancer Res. 2006, 4, 437–447. [Google Scholar] [CrossRef]
- Ilvesaro, J.M.; Merrell, M.A.; Swain, T.M.; Davidson, J.; Zayzafoon, M.; Harris, K.W.; Selander, K.S. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro. Prostate 2007, 67, 774–781. [Google Scholar] [CrossRef]
- Sandholm, J.; Tuomela, J.; Kauppila, J.H.; Harris, K.W.; Graves, D.; Selander, K.S. Hypoxia regulates Toll-like receptor-9 expression and invasive function in human brain cancer cells in vitro. Oncol. Lett. 2014, 8, 266–274. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Zhang, P.; Yang, L.; Yu, X.; Ye, X.; Yang, J.; Qian, C.; Zhang, X.; Cui, Y.-H.; Bian, X.-W. Activation of toll-like receptor 2 promotes invasion by upregulating MMPs in glioma stem cells. Am. J. Transl. Res. 2015, 7, 607–615. [Google Scholar] [PubMed]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad Expression of Toll-Like Receptors in the Human Central Nervous System. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, L.; Luke, J.J.; Davar, D. Toll-Like Receptor 9 Agonists in Cancer. Oncotargets Ther. 2020, 13, 10039–10061. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.; Yang, H.; Kaminska, B.; You, H. Toll-like receptors and toll-like receptor-targeted immunotherapy against glioma. J. Hematol. Oncol. 2021, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.C.; Shah, M.; Choi, S. Toll-like receptor-induced cytokines as immunotherapeutic targets in cancers and autoimmune diseases. Semin. Cancer Biol. 2019, 64, 61–82. [Google Scholar] [CrossRef]
- Leng, L.; Jiang, T.; Zhang, Y. TLR9 expression is associated with prognosis in patients with glioblastoma multiforme. J. Clin. Neurosci. 2012, 19, 75–80. [Google Scholar] [CrossRef]
- Mu, L.; Wang, Y.; Wang, Y.; Zhang, H.; Shang, D.; Tan, F.; Li, Y.; Chen, X. Tumor Location and Survival Outcomes in Adult Patients with Supratentorial Glioblastoma by Levels of Toll-Like Receptor 9 Expression. World Neurosurg. 2017, 97, 279–283. [Google Scholar] [CrossRef]
- Meng, Y.; Kujas, M.; Marie, Y.; Paris, S.; Thillet, J.; Delattre, J.-Y.; Carpentier, A.F. Expression of TLR9 within human glioblastoma. J. Neurooncol. 2008, 88, 19–25. [Google Scholar] [CrossRef]
- Moretti, I.F.; Gil Franco, D.; Galatro, T.; Oba-Shinjo, S.M.; Marie, S. Plasmatic membrane toll-like receptor expressions in human astrocytomas. PLoS ONE 2018, 13, e0199211. [Google Scholar] [CrossRef]
- Hussain, S.F.; Yang, D.; Suki, D.; Aldape, K.; Grimm, E.; Heimberger, A.B. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology 2006, 8, 261–279. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Lubas, M.; Yuan, Y.; Yalcin, F.; Efe, I.E.; Xia, P.; Motta, E.; Buonfiglioli, A.; Lehnardt, S.; et al. Synergistic Toll-like receptor 3/9 signaling affects properties and impairs glioma-promoting activity of microglia. J. Neurosci. 2020, 40, 6428–6443. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Cherryholmes, G.; Schroeder, A.; Phallen, J.; Alizadeh, D.; Xin, H.; Wang, T.; Lee, H.; Lahtz, C.; Swiderski, P.; et al. TLR9 Is Critical for Glioma Stem Cell Maintenance and Targeting. Cancer Res. 2014, 74, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Chang, A.L.; Miska, J.; Wainwright, D.A.; Ahmed, A.U.; Balyasnikova, I.V.; Pytel, P.; Han, Y.; Tobias, A.; Zhang, L.; et al. Dendritic Cell–Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma. J. Immunol. 2015, 195, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Merlin, D.M.; Maldonado-Bernal, C.; Alvarez-Arellano, L. Toll-Like Receptors as Therapeutic Targets in Central Nervous System Tumors. BioMed Res. Int. 2019, 2019, 5286358. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Laigle-Donadey, F.; Zohar, S.; Capelle, L.; Behin, A.; Tibi, A.; Martin-Duverneuil, N.; Sanson, M.; Lacomblez, L.; Taillibert, S.; et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma1. Neuro-Oncology 2006, 8, 60–66. [Google Scholar] [CrossRef]
- Carpentier, A.F.; Metellus, P.; Ursu, R.; Zohar, S.; Lafitte, F.; Barrié, M.; Meng, Y.; Richard, M.; Parizot, C.; Laigle-Donadey, F.; et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: A phase II study. Neuro-Oncology 2010, 12, 401–408. [Google Scholar] [CrossRef]
- Ursu, R.; Taillibert, S.; Banissi, C.; Vicaut, E.; Bailon, O.; Le Rhun, E.; Guillamo, J.; Psimaras, D.; Tibi, A.; Sacko, A.; et al. Immunotherapy with CpG-ODNin neoplastic meningitis: A phase I trial. Cancer Sci. 2015, 106, 1212–1218. [Google Scholar] [CrossRef]
- Ursu, R.; Carpentier, A.; Metellus, P.; Lubrano, V.; Laigle-Donadey, F.; Capelle, L.; Guyotat, J.; Langlois, O.; Bauchet, L.; Desseaux, K.; et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma—A phase II multicentric, randomised study. Eur. J. Cancer 2017, 73, 30–37. [Google Scholar] [CrossRef]
- Meng, Y.; Carpentier, A.F.; Chen, L.; Boisserie, G.; Simon, J.-M.; Mazeron, J.-J.; Delattre, J.-Y. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int. J. Cancer 2005, 116, 992–997. [Google Scholar] [CrossRef]
- Grauer, O.M.; Molling, J.W.; Bennink, E.; Toonen, L.W.J.; Sutmuller, R.P.M.; Nierkens, S.; Adema, G.J. TLR Ligands in the Local Treatment of Established Intracerebral Murine Gliomas. J. Immunol. 2008, 181, 6720–6729. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Liu, X.; Jiang, W.; Zhou, W.; Yan, W.; Cen, Y.; Li, B.; Cao, G.; Ding, G.; et al. CpG ODN107 potentiates radiosensitivity of human glioma cells via TLR9-mediated NF-κB activation and NO production. Tumor Biol. 2012, 33, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cen, Y.; Cai, Y.; Liu, T.; Liu, H.; Cao, G.; Liu, D.; Li, B.; Peng, W.; Zou, J.; et al. TLR9-ERK-mTOR signaling is critical for autophagic cell death induced by CpG oligodeoxynucleotide 107 combined with irradiation in glioma cells. Sci. Rep. 2016, 6, 27104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, D.; Cao, G.; Cen, Y.; Liu, T.; Peng, W.; Sun, J.; Li, X.; Zhou, H. The radiosensitizing effect of CpG ODN107 on human glioma cells is tightly related to its antiangiogenic activity via suppression of HIF-1α/VEGF pathway. Int. Immunopharmacol. 2013, 17, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Waxman, D.J. CpG-1826 immunotherapy potentiates chemotherapeutic and anti-tumor immune responses to metronomic cyclophosphamide in a preclinical glioma model. Cancer Lett. 2016, 373, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, D.; White, E.E.; Sanchez, T.C.; Liu, S.; Zhang, L.; Badie, B.; Berlin, J.M. Immunostimulatory CpG on Carbon Nanotubes Selectively Inhibits Migration of Brain Tumor Cells. Bioconj. Chem. 2018, 29, 1659–1668. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Singh, S.; Gupta, C.L.; Pandey, P.; Singh, V.K.; Sayyed, U.; Shekh, R.; Bajpai, P. Repolarization of glioblastoma macrophage cells using non-agonistic Dectin-1 ligand encapsulating TLR-9 agonist: Plausible role in regenerative medicine against brain tumor. Int. J. Neurosci. 2021, 131, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, P.; Li, D.; Nuñez, F.M.; Altshuler, D.; Doherty, R.; Kuai, R.; Yu, M.; Kamran, N.; Edwards, M.; Moon, J.J.; et al. High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-immunotherapy against Glioblastoma Multiforme. ACS Nano 2019, 13, 1365–1384. [Google Scholar] [CrossRef]
- Zhu, S.; Lv, X.; Zhang, X.; Li, T.; Zang, G.; Yang, N.; Wang, X.; Wu, J.; Chen, W.; Liu, Y.; et al. An effective dendritic cell-based vaccine containing glioma stem-like cell lysate and CpG adjuvant for an orthotopic mouse model of glioma. Int. J. Cancer 2019, 144, 2867–2879. [Google Scholar] [CrossRef]
- Patel, D.M.; Foreman, P.M.; Nabors, L.; Riley, K.; Gillespie, G.Y.; Markert, J.M. Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016, 27, 69–78. [Google Scholar] [CrossRef]
- El Andaloussi, A.; Sonabend, A.M.; Han, Y.; Lesniak, M.S. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia 2006, 54, 526–535. [Google Scholar] [CrossRef]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.J.; Badie, B. Carbon Nanotubes Enhance CpG Uptake and Potentiate Antiglioma Immunity. Clin. Cancer Res. 2011, 17, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Koul, N.; Dixit, D.; Sharma, V.; Sen, E. IGF-1 induced HIF-1α-TLR9 cross talk regulates inflammatory responses in glioma. Cell. Signal. 2011, 23, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Adamus, T.; Hung, C.-Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther. Nucleic Acids 2021, 27, 611–620. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Singh, S.; Gupta, C.L.; Bajpai, P. Microglial TLR9: Plausible Novel Target for Therapeutic Regime Against Glioblastoma Multiforme. Cell. Mol. Neurobiol. 2021, 41, 1391–1393. [Google Scholar] [CrossRef]
- Wei, J.; Wu, D.; Zhao, S.; Shao, Y.; Xia, Y.; Ni, D.; Qiu, X.; Zhang, J.; Chen, J.; Meng, F.; et al. Immunotherapy of Malignant Glioma by Noninvasive Administration of TLR9 Agonist CpG Nano-Immunoadjuvant. Adv. Sci. 2022, 9, e2103689. [Google Scholar] [CrossRef] [PubMed]
- Ginzkey, C.; Eicker, S.O.; Marget, M.; Krause, J.; Brecht, S.; Westphal, M.; Hugo, H.H.; Mehdorn, H.M.; Steinmann, J.; Hamel, W. Increase in tumor size following intratumoral injection of immunostimulatory CpG-containing oligonucleotides in a rat glioma model. Cancer Immunol. Immunother. 2010, 59, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Miyar, A.; Habibi, I.; Ebrahimi, A.; Mansourpour, D.; Mokarizadeh, A.; Rajabi, A.; Farshgar, R.; Eshaghzadeh, M.; Zamani-Ahmadmahmudi, M.; Nodushan, S.M.H.T. Predictive and prognostic value of TLR9 and NFKBIA gene expression as potential biomarkers for human glioma diagnosis. J. Neurol. Sci. 2016, 368, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, S.; Yan, Y.; Ying, Q.; Jiang, T.; Xu, K.; Wu, A. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer 2010, 10, 415. [Google Scholar] [CrossRef]
- Chaudhary, R.; Morris, R.J.; Steinson, E. The multifactorial roles of microglia and macrophages in the maintenance and progression of glioblastoma. J. Neuroimmunol. 2021, 357, 577633. [Google Scholar] [CrossRef]
- Briceño, E.; Calderon, A.; Sotelo, J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Surg. Neurol. 2007, 67, 388–391. [Google Scholar] [CrossRef]
- Briceño, E.; Reyes, S.; Sotelo, J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg. Focus 2003, 14, 3. [Google Scholar] [CrossRef] [PubMed]
| Agostist-TL9 | Combinational Treatment | Target | Featured Outcome | Mechanistic Features | References |
|---|---|---|---|---|---|
| CpG 28 | ns | Fisher rats bearing 9L glioma | complete tumor remission in one-third of the animals | T cells in antitumor effects | [99] |
| radiotherapy | Fisher rats bearing 9L glioma | complete tumor remission in two-thirds | [99] | ||
| CpGODN1668 | ns | glioma-bearing C57BL/6 mice. | inhibit of glioma growth in vivo and cured 80% of animals | diminish Treg and increase CD8 | [100] |
| murine GL261 glioma cells in vitro | inhibit GL261 cell proliferation | [100] | |||
| CpG ODN 107 | radiotherapy | U251 and U87/orthotopic tumor-bearing nude mice | induce autophagy | TLR9-ERK-mTOR signaling pathway, | [102] |
| CpG ODN 107 | radiotherapy | U87/human U87 implanted xenographt in nude mice | not induce apoptosis but induce cell cycle arrest at G1 phase/inhibit angiogenesis | TLR9-mediated NF-κB activation and NO production in the tumor cells/VEGF/HIF inhibition | [101,103] |
| CpG 1826 | metronomic cyclophosphamide | GL261 mouse glioma cells/GL261 tumor-bearing mice | elicit anti-tumor immune response | increased tumor T-cell infiltration | [104] |
| CpG 1826 | Schizophyllan (SPG) nanoparticles | C6 | repolarizing the M2 macrophages to much-desired M1 and apoptosis | [106] | |
| CpG ODN | carbone nanotubes SWNT | K-Luc murine glioma cell line | inhibit cell migration, activate macrophage | decreased NF-κB activation in glioma cells | [105] |
| CpG ODN | DTX-sHDL-CpG nanodiscs | Mouse, GL26-WT, GL26-Cit, GL26-OVA, rat CNS-1, and human HF2303, U251 | tumor regression and anti-tumor CD8+ T-cell responses in the brain TME | long-term survival and immunological memory | [107] |
| CpG ODN | DTX-sHDL-CpG nanodiscs +RT | of GBM-bearing animals | tumor regression and long-term survival in 80% | [107] | |
| CpG ODN | DCs harboring (GSC)-associated antigens | orthotopic mouse model of glioma | improved survival and tumor regression by enhancing anti-tumor immune function | upregulated programmed death 1 (PD-1) and its ligand PD-L1, decreased T cells | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehri, E.; Ennaifer, E.; Bel Haj Rhouma, R.; Ardhaoui, M.; Boubaker, S. TLR9 and Glioma: Friends or Foes? Cells 2023, 12, 152. https://doi.org/10.3390/cells12010152
Fehri E, Ennaifer E, Bel Haj Rhouma R, Ardhaoui M, Boubaker S. TLR9 and Glioma: Friends or Foes? Cells. 2023; 12(1):152. https://doi.org/10.3390/cells12010152
Chicago/Turabian StyleFehri, Emna, Emna Ennaifer, Rahima Bel Haj Rhouma, Monia Ardhaoui, and Samir Boubaker. 2023. "TLR9 and Glioma: Friends or Foes?" Cells 12, no. 1: 152. https://doi.org/10.3390/cells12010152
APA StyleFehri, E., Ennaifer, E., Bel Haj Rhouma, R., Ardhaoui, M., & Boubaker, S. (2023). TLR9 and Glioma: Friends or Foes? Cells, 12(1), 152. https://doi.org/10.3390/cells12010152





