Urine-Derived Kidney Progenitor Cells in Cystinosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Estimation of the Number of Undifferentiated Cells in Urine via Quantitative Polymerase Chain Reaction (qPCR)
2.3. Establishment of Cystinosis Urine-Derived Kidney Progenitor Cells (Cys-uKPCs)
2.4. Characterization of Cys-uKPCs
2.5. Differentiation of Cys-uKPCs to Podocytes and Proximal Tubular Epithelial Cells
2.6. Western Blot
2.7. Immunofluorescence Staining and Microscopy
2.8. Functional Assessment of Cys-uKPC-Podo and Cys-uKPC-PTEC
2.8.1. Albumin Endocytosis Assay
2.8.2. Calcium Influx Assay
2.8.3. Transferrin Endocytosis Assay
2.9. Kidney Biopsy Specimen & Immunohistochemistry
2.10. Lentiviral Vector Design and Transduction Experiments
2.11. Cystine Measurements
2.12. Statistical Analysis
3. Results
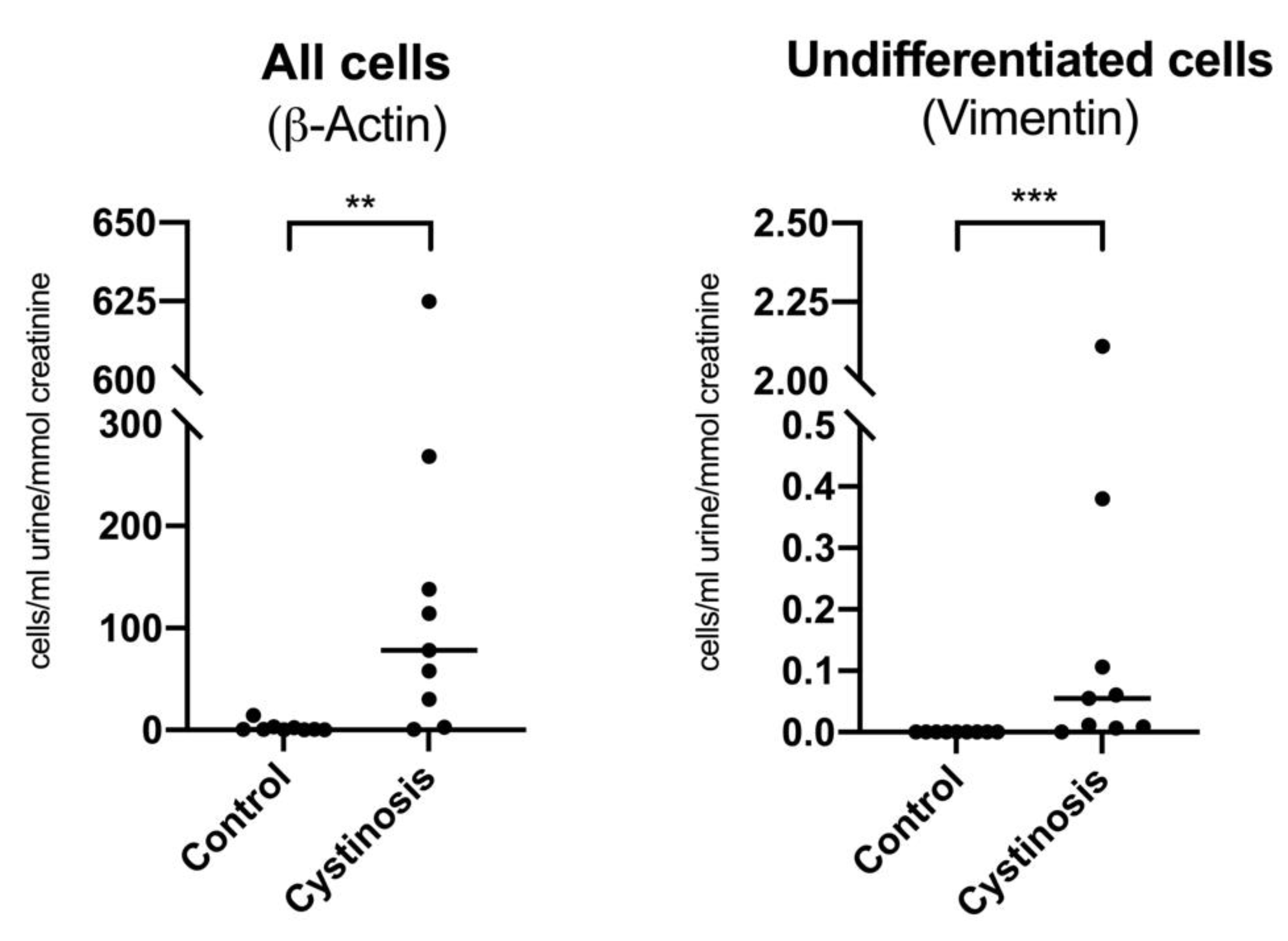
3.1. Nephropathic Cystinosis Is Characterized by a Loss of Undifferentiated Cells in Urine
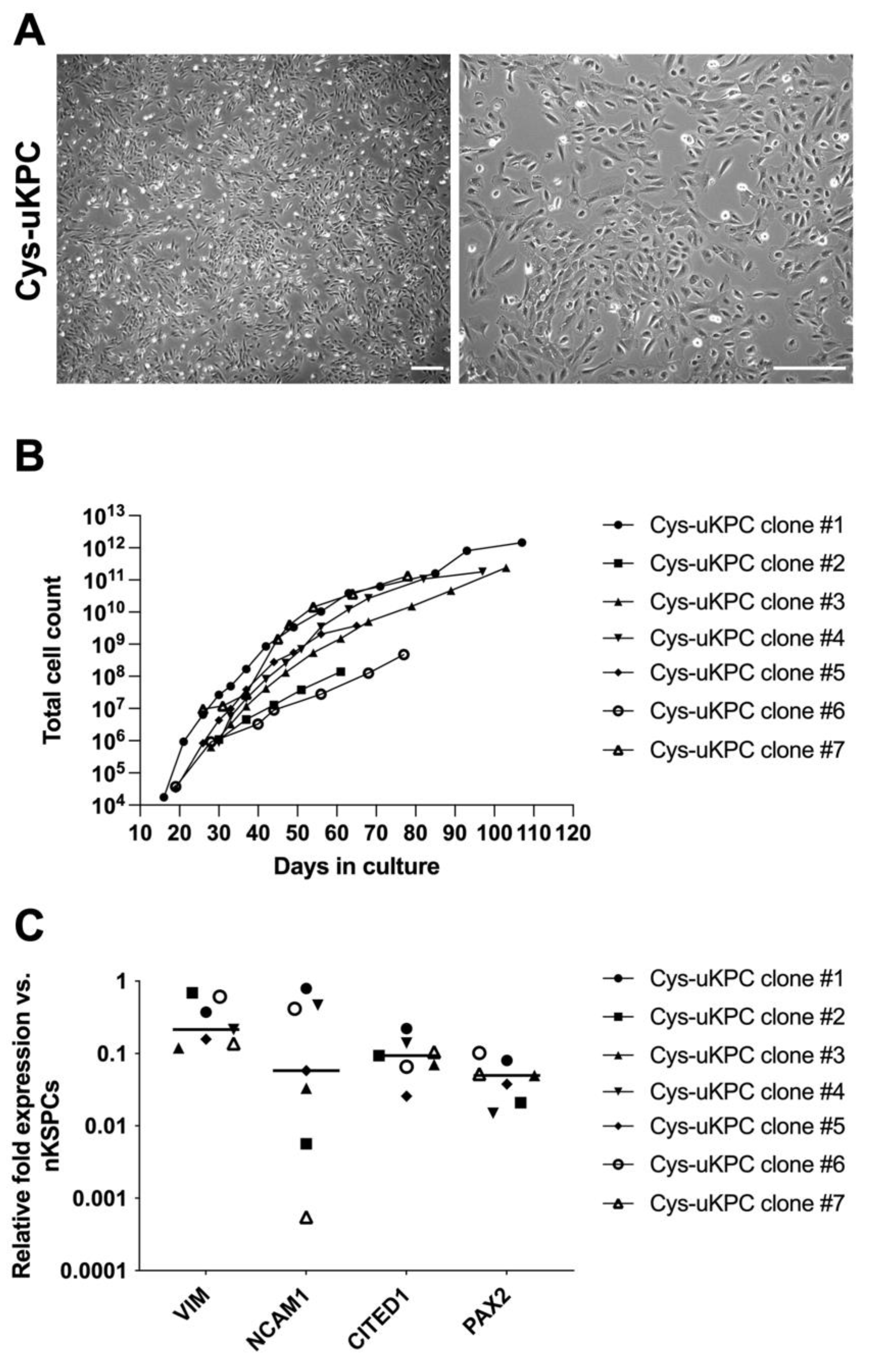
3.2. Undifferentiated Cells in Urine of Cystinosis Patients Comprise Kidney Progenitor Cells (Cys-uKPCs)
3.3. Differentiation of Cys-uKPCs In Vitro towards Functional Podocytes and PTECs
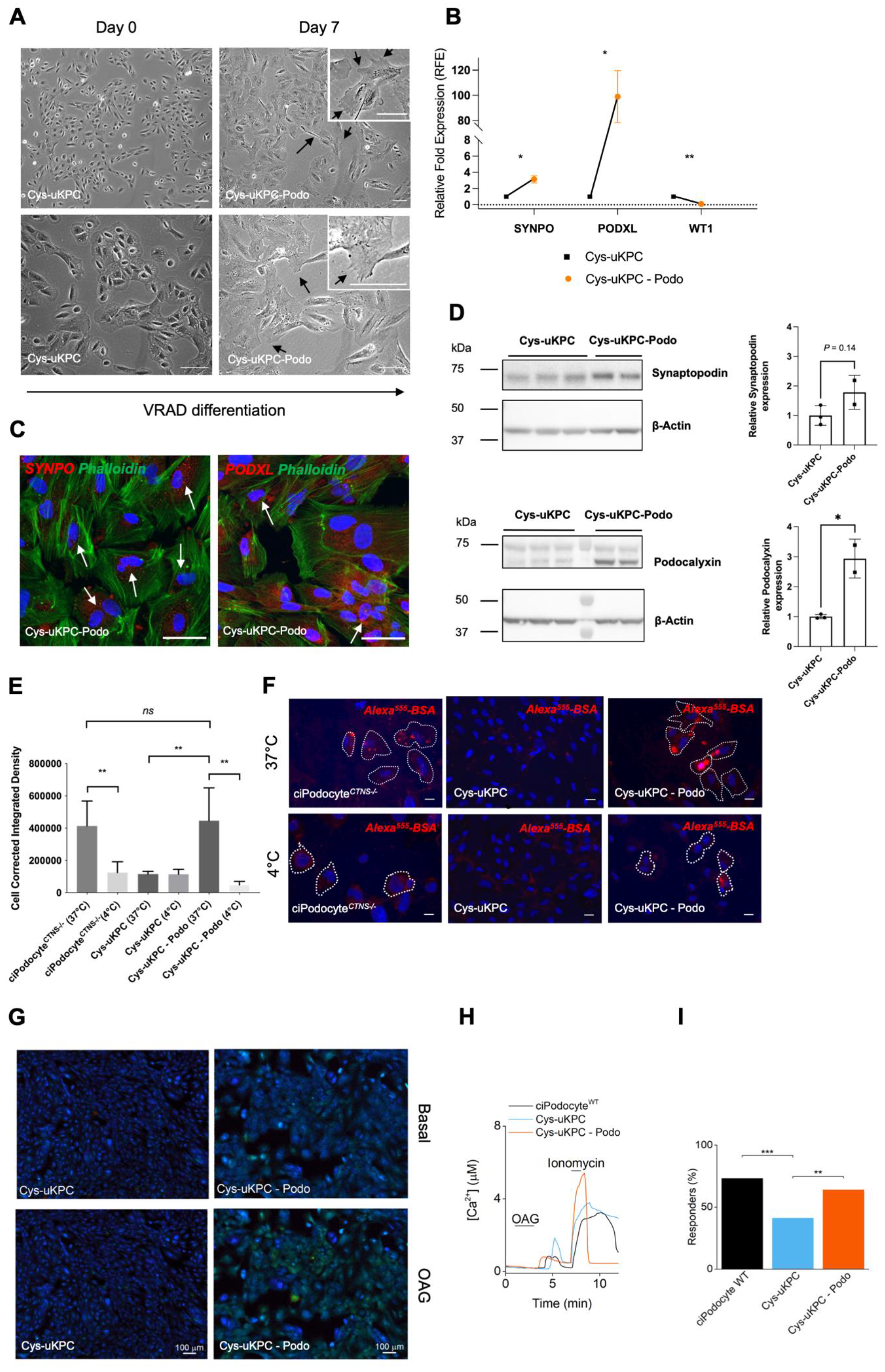
3.3.1. Differentiation of Cys-uKPCs into Functional Podocytes
3.3.2. Differentiation of Cys-uKPCs into Functional PTECs
3.4. A kidney Progenitor Cell Niche Is Present In Situ in the Kidneys of a Nephropathic Cystinosis Patient
3.5. Reduction in Cystine Levels in Cys-uKPCs via an Ex Vivo Gene Addition Approach
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Town, M.; Jean, G.; Cherqui, S.; Attard, M.; Forestier, L.; Whitmore, S.A.; Callen, D.F.; Gribouval, O.; Broyer, M.; Bates, G.P.; et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat. Genet. 1998, 18, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Elmonem, M.A.; Veys, K.R.; Soliman, N.A.; Van Dyck, M.; Van Den Heuvel, L.P.; Levtchenko, E. Cystinosis: A review. Orphanet J. Rare Dis. 2016, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Markello, T.C.; Bernardini, I.M.; Gahl, W.A. Improved renal function in children with cystinosis treated with cysteamine. N. Engl. J. Med. 1993, 328, 1157–1162. [Google Scholar] [CrossRef]
- Gahl, W.A.; Balog, J.Z.; Kleta, R. Nephropathic cystinosis in adults: Natural history and effects of oral cysteamine therapy. Ann. Intern. Med. 2007, 147, 242–250. [Google Scholar] [CrossRef]
- Brodin-Sartorius, A.; Tête, M.J.; Niaudet, P.; Antignac, C.; Guest, G.; Ottolenghi, C.; Charbit, M.; Moyse, D.; Legendre, C.; Lesavre, P.; et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012, 81, 179–189. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Arcolino, F.O.; Elmonem, M.A.; Rastaldi, M.P.; Giardino, L.; Cornelissen, E.M.; Van Den Heuvel, L.P.; Levtchenko, E.N. Cystinosin deficiency causes podocyte damage and loss associated with increased cell motility. Kidney Int. 2016, 89, 1037–1048. [Google Scholar] [CrossRef]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosali, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef]
- Huling, J.; Yoo, J.J. Comparing adult renal stem cell identification, characterization and applications. J. Biomed. Sci. 2017, 24, 32. [Google Scholar] [CrossRef]
- Marcheque, J.; Bussolati, B.; Csete, M.; Perin, L. Concise reviews: Stem cells and kidney regeneration: An update. Stem Cells Transl. Med. 2019, 8, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, E.; Sagrinati, C.; Angelotti, M.L.; Lazzeri, E.; Mazzinghi, B.; Ballerini, L.; Parente, E.; Becherucci, F.; Gacci, M.; Carini, M.; et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009, 20, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Angelotti, M.L.; Ronconi, E.; Ballerini, L.; Peired, A.; Mazzinghi, B.; Sagrinati, C.; Parente, E.; Gacci, M.; Carini, M.; Rotondi, M.; et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012, 30, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Harari-Steinberg, O.; Metsuyanim, S.; Omer, D.; Gnatek, Y.; Gershon, R.; Pri-Chen, S.; Ozdemir, D.D.; Lerenthal, Y.; Noiman, T.; Ben-Hur, H.; et al. Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol. Med. 2013, 5, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Buzhor, E.; Omer, D.; Harari-Steinberg, O.; Dotan, Z.; Vax, E.; Pri-Chen, S.; Metsuyanim, S.; Pleniceanu, O.; Goldstein, R.S.; Dekel, B. Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am. J. Pathol. 2013, 183, 1621–1633. [Google Scholar] [CrossRef]
- Lazzeri, E.; Ronconi, E.; Angelotti, M.L.; Peired, A.; Mazzinghi, B.; Becherucci, F.; Conti, S.; Sansavini, G.; Sisti, A.; Ravaglia, F.; et al. Human urine-derived renal progenitors for personalized modeling of genetic kidney disorders. J. Am. Soc. Nephrol. 2015, 26, 1961–1974. [Google Scholar] [CrossRef]
- Arcolino, F.O.; Zia, S.; Held, K.; Papadimitriou, E.; Theunis, K.; Bussolati, B.; Raaijmakers, A.; Allegaert, K.; Voet, T.; Deprest, J.; et al. Urine of preterm neonates as a novel source of kidney progenitor cells. J. Am. Soc. Nephrol. 2016, 27, 2762–2770. [Google Scholar] [CrossRef]
- Leuning, D.G.; Reinders, M.E.J.; Li, J.; Peired, A.J.; Lievers, E.; de Boer, H.C.; Fibbe, W.E.; Romagnani, P.; van Kooten, C.; Little, M.H.; et al. Clinical-grade isolated human kidney perivascular stromal cells as an organotypic cell source for kidney regenerative medicine. Stem Cells Transl. Med. 2017, 6, 405–418. [Google Scholar] [CrossRef]
- Eymael, J.; Smeets, B. Origin and fate of the regenerating cells of the kidney. Eur. J. Pharmacol. 2016, 790, 62–73. [Google Scholar] [CrossRef]
- Cheung, P.Y.; Harrison, P.T.; Davidson, A.J.; Hollywood, J.A. In Vitro and In Vivo Models to Study Nephropathic Cystinosis. Cells 2021, 11, 6. [Google Scholar] [CrossRef]
- Racusen, L.C.; Fivush, B.A.; Andersson, H.; Gahl, W.A. Culture of renal tubular cells from the urine of patients with nephropathic cystinosis. J. Am. Soc. Nephrol. 1991, 1, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Hollywood, J.A.; Przepiorski, A.; D’Souza, R.F.; Sreebhavan, S.; Wolvetang, E.J.; Harrison, P.T.; Davidson, A.J.; Holm, T.M. Use of human induced pluripotent stem cells and kidney organoids to develop a cysteamine/mtor inhibition combination therapy for cystinosis. J. Am. Soc. Nephrol. 2020, 31, 962–982. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Diep, D.; Gore, A.; Panopoulos, A.D.; Montserrat, N.; Plongthongkum, N.; Kumar, S.; Fung, H.-L.; Giorgetti, A.; Bilic, J.; et al. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16196–16201. [Google Scholar] [CrossRef] [PubMed]
- The World Medical Association. Declaration of Helsinki. Available online: http://www.wma.net/e/policy/b3.htm (accessed on 13 March 2022).
- Ye, Y.; Wang, B.; Jiang, X.; Hu, W.; Feng, J.; Li, H.; Jin, M.; Ying, Y.; Wang, W.; Mao, X.; et al. Proliferative capacity of stem/progenitor-like cells in the kidney may associate with the outcome of patients with acute tubular necrosis. Hum. Pathol. 2011, 42, 1132–1141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindgren, D.; Boström, A.K.; Nilsson, K.; Hansson, J.; Sjölund, J.; Möller, C.; Jirström, K.; Nilsson, E.; Landberg, G.; Axelson, H.; et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am. J. Pathol. 2011, 178, 828–837. [Google Scholar] [CrossRef]
- Smeets, B.; Boor, P.; Dijkman, H.; Sharma, S.; Jirak, P.; Mooren, F.; Berger, K.; Borneman, J.; Gelman, H.; Floege, J.; et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J. Pathol. 2013, 229, 645–659. [Google Scholar] [CrossRef]
- Picelli, S.; Faridani, O.R.; Björklund, Å.K.; Winberg, G.; Sagasser, S.; Sandberg, R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014, 9, 171. [Google Scholar] [CrossRef]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef]
- DeKoninck, P.; Toelen, J.; Zia, S.; Albersen, M.; Lories, R.; De Coppi, P.; Deprest, J. Routine isolation and expansion late mid trimester amniotic fluid derived mesenchymal stem cells in a cohort of fetuses with congenital diaphragmatic hernia. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 178, 157–162. [Google Scholar] [CrossRef]
- V, R. Doubling Time Computing. Available online: doubling-time.com/compute.php (accessed on 14 March 2022).
- Gianesello, L.; Priante, G.; Ceol, M.; Radu, C.M.; Saleem, M.A.; Simioni, P.; Terrin, L.; Anglani, F.; Prete, D. Del Albumin uptake in human podocytes: A possible role for the cubilin-amnionless (CUBAM) complex. Sci. Rep. 2017, 7, 13705. [Google Scholar] [CrossRef]
- Chan, P.M.; Tan, Y.S.; Chua, K.H.; Sabaratnam, V.; Kuppusamy, U.R. Attenuation of Inflammatory Mediators (TNF-α and Nitric Oxide) and Up-Regulation of IL-10 by Wild and Domesticated Basidiocarps of Amauroderma rugosum (Blume & T. Nees) Torrend in LPS-Stimulated RAW264.7 Cells. PLoS ONE 2015, 10, e0139593. [Google Scholar] [CrossRef]
- Greka, A.; Mundel, P. Regulation of podocyte actin dynamics by calium. Semin. Nephrol. 2012, 32, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wieder, N.; Greka, A. Calcium, TRPC channels, and regulation of the actin cytoskeleton in podocytes: Towards a future of targeted therapies. Pediatr. Nephrol. 2016, 31, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Kasztan, M.; Angielski, S.; Jankowski, M. Intracellular calcium signaling regulates glomerular filtration barrier permeability: The role of the PKGIα-dependent pathway. FEBS Lett. 2016, 590, 1739–1748. [Google Scholar] [CrossRef]
- Khayyat, N.H.; Tomilin, V.N.; Zaika, O.; Pochynyuk, O. Polymodal roles of TRPC3 channel in the kidney. Channels 2020, 14, 257–267. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Staruschenko, A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am. J. Physiol. Physiol. 2015, 309, F393–F397. [Google Scholar] [CrossRef]
- Reiser, J.; Polu, K.R.; Möller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-casado, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005, 37, 739–744. [Google Scholar] [CrossRef]
- van Veen, S.; Martin, S.; Van den Haute, C.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.P.; Gelders, G.; Lambie, E.; et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef]
- Levtchenko, E.N.; de Graaf-Hess, A.; Wilmer, M.; van den Heuvel, L.; Monnens, L.; Blom, H. Altered status of glutathione and its metabolites in cystinotic cells. Nephrol. Dial. Transplant. 2005, 20, 1828–1832. [Google Scholar] [CrossRef]
- Fehse, B.; Kustikova, O.S.; Bubenheim, M.; Baum, C. Pois(s)on—It’s a question of dose. Gene Ther. 2004, 11, 879–881. [Google Scholar] [CrossRef]
- Fanni, D.; Fanos, V.; Monga, G.; Gerosa, C.; Locci, A.; Nemolato, S.; Van Eyken, P.; Faa, G. Expression of WT1 during normal human kidney development. J. Matern. Neonatal Med. 2011, 24, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ishibe, S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am. J. Physiol. Physiol. 2015, 309, F398–F405. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Conti, S.; Benigni, A.; Remuzzi, G. Podocyte-actin dynamics in health and disease. Nat. Rev. Nephrol. 2016, 12, 692–710. [Google Scholar] [CrossRef]
- Huber, T.B.; Köttgen, M.; Schilling, B.; Walz, G.; Benzing, T. Interaction with podocin facilitates nephrin signaling. J. Biol. Chem. 2001, 276, 41543–41546. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.B.; Schermer, B.; Muller, R.U.; Hohne, M.; Bartram, M.; Calixto, A.; Hagmann, H.; Reinhardt, C.; Koos, F.; Kunzelmann, K.; et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl. Acad. Sci. USA 2006, 103, 17079–17086. [Google Scholar] [CrossRef] [PubMed]
- Plisov, S.; Tsang, M.; Shi, G.; Boyle, S.; Yoshino, K.; Dunwoodie, S.L.; Dawid, I.B.; Shioda, T.; Perantoni, A.O.; de Caestecker, M.P. Cited1 Is a bifunctional transcriptional cofactor that regulates early nephronic patterning. J. Am. Soc. Nephrol. 2005, 16, 1632–1644. [Google Scholar] [CrossRef]
- Gaide Chevronnay, H.P.; Janssens, V.; Van Der Smissen, P.; Rocca, C.J.; Liao, X.H.; Refetoff, S.; Pierreux, C.E.; Cherqui, S.; Courtoy, P.J. Hematopoietic stem cells transplantation can normalize thyroid function in a cystinosis mouse model. Endocrinology 2016, 157, 1363–1371. [Google Scholar] [CrossRef]
- Gaide Chevronnay, H.P.; Janssens, V.; Van Der Smissen, P.; N’Kuli, F.; Nevo, N.; Guiot, Y.; Levtchenko, E.; Marbaix, E.; Pierreux, C.E.; Cherqui, S.; et al. Time course of pathogenic and adaptation mechanisms in cystinotic mouse kidneys. J. Am. Soc. Nephrol. 2014, 25, 1256–1269. [Google Scholar] [CrossRef]
- Festa, B.P.; Chen, Z.; Berquez, M.; Debaix, H.; Tokonami, N.; Prange, J.A.; Van De Hoek, G.; Alessio, C.; Raimondi, A.; Nevo, N.; et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef]
- Pavathuparambil Abdul Manaph, N.; Al-Hawaas, M.; Bobrovskaya, L.; Coates, P.T.; Zhou, X.F. Urine-derived cells for human cell therapy. Stem Cell Res. Ther. 2018, 9, 189. [Google Scholar] [CrossRef]
- Oliveira Arcolino, F.; Tort Piella, A.; Papadimitriou, E.; Bussolati, B.; Antonie, D.J.; Murray, P.; van den Heuvel, L.; Levtchenko, E. Human Urine as a Noninvasive Source of Kidney Cells. Stem Cells Int. 2015, 2015, 362562. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.; Arcolino, F.O.; Schoor, P.; Kok, R.J.; Mastrobattista, E. Gene Based Therapies for Kidney Regeneration. Eur. J. Pharmacol. 2016, 790, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Asico, L.D.; Cuevas, S.; Ma, X.; Jose, P.A.; Armando, I.; Konkalmatt, P.R. Nephron segment-specific gene expression using AAV vectors. Biochem. Biophys. Res. Commun. 2018, 497, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Sun, Z.; Humphreys, B.D.; Ru, X.; Vandenberghe, L.H. Efficient gene transfer to kidney mesenchymal cells using a synthetic adeno-associated viral vector. J. Am. Soc. Nephrol. 2018, 29, 2287–2297. [Google Scholar] [CrossRef]
- Gentner, B.; Tucci, F.; Galimberti, S.; Fumagalli, F.; De Pellegrin, M.; Silvani, P.; Camesasca, C.; Pontesilli, S.; Darin, S.; Ciotti, F.; et al. Hematopoietic Stem- and Progenitor-Cell Gene Therapy for Hurler Syndrome. N. Engl. J. Med. 2021, 385, 1929–1940. [Google Scholar] [CrossRef]


| Patient | Age | Sex | Cystinosis Genotype | eGFR | Urine Protein/Creatinine | WBC Cystine | # Clonal Colonies | Cys-uKPC Clones |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | F | 57kb del + c.926dupG | 78 | 1.97 | 0.5 | 0 | |
| 2 | 4 | F | Hom 57kb del | >90 | 2.85 | 4.26 | 2 | |
| 3 | 16 | M | Hom 57kb del | 89 | 1.82 | 0.3 | 9 | |
| 4 | 13 | F | c.198_218del + c.926dupG | 48 | 3.04 | 0.2 | 16 | |
| 5 | 6 | M | 57kb del + c.926dupG | >90 | 4.93 | 0.32 | 5 | |
| 6 * | 13 | F | 57kb del + c.198_218del | 88 | 1.76 | 2.45 | 51 | #1–5 |
| 7 | 14 | F | Hom 57kb del | 53 | 0.65 | 2.52 | 3 | |
| 8 | 4 | M | 57kb del + IVS 10-7G>A | >90 | 0.41 | 1.8 | 0 | |
| 9 * | 16 | M | Hom 57kb del | 46 | 2.94 | 3.14 | 20 | #6, #7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veys, K.; Berlingerio, S.P.; David, D.; Bondue, T.; Held, K.; Reda, A.; Broek, M.v.d.; Theunis, K.; Janssen, M.; Cornelissen, E.; et al. Urine-Derived Kidney Progenitor Cells in Cystinosis. Cells 2022, 11, 1245. https://doi.org/10.3390/cells11071245
Veys K, Berlingerio SP, David D, Bondue T, Held K, Reda A, Broek Mvd, Theunis K, Janssen M, Cornelissen E, et al. Urine-Derived Kidney Progenitor Cells in Cystinosis. Cells. 2022; 11(7):1245. https://doi.org/10.3390/cells11071245
Chicago/Turabian StyleVeys, Koenraad, Sante Princiero Berlingerio, Dries David, Tjessa Bondue, Katharina Held, Ahmed Reda, Martijn van den Broek, Koen Theunis, Mirian Janssen, Elisabeth Cornelissen, and et al. 2022. "Urine-Derived Kidney Progenitor Cells in Cystinosis" Cells 11, no. 7: 1245. https://doi.org/10.3390/cells11071245
APA StyleVeys, K., Berlingerio, S. P., David, D., Bondue, T., Held, K., Reda, A., Broek, M. v. d., Theunis, K., Janssen, M., Cornelissen, E., Vriens, J., Diomedi-Camassei, F., Gijsbers, R., Heuvel, L. v. d., Arcolino, F. O., & Levtchenko, E. (2022). Urine-Derived Kidney Progenitor Cells in Cystinosis. Cells, 11(7), 1245. https://doi.org/10.3390/cells11071245







