Long Noncoding RNA LOC550643 Acts as an Oncogene in the Growth Regulation of Colorectal Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Clinical Samples
2.2. RNA Extraction
2.3. Expression Data from Public Databases
2.4. Gene Ontology and Pathway Enrichment Analysis
2.5. Reverse Transcription and Real-Time Polymerase Chain Reaction
2.6. Cell Proliferation Assay
2.7. Colony Formation Assay
2.8. Cell Migration and Invasion Assay
2.9. Cell Cycle Progression Analysis
2.10. Cell Apoptosis Analysis
2.11. Western Blotting
2.12. miR-29b-2-5p Directly Binding at LOC550643 and Luciferase Activity Assay
2.13. RNA Immunoprecipitation Chip Assay
2.14. Actin Immunofluorescence Staining
2.15. Statistical Analysis
3. Results
3.1. Significant Overexpression of LOC550643 in CRC Cells
3.2. OC550643 Knockdown Suppressed CRC Cell Growth and Motility
3.3. LOC550643 Knockdown Hindered Cell Cycle Progression and Induced Apoptosis
3.4. LOC550643 Knockdown Suppressed Colon Cancer Cell Motility
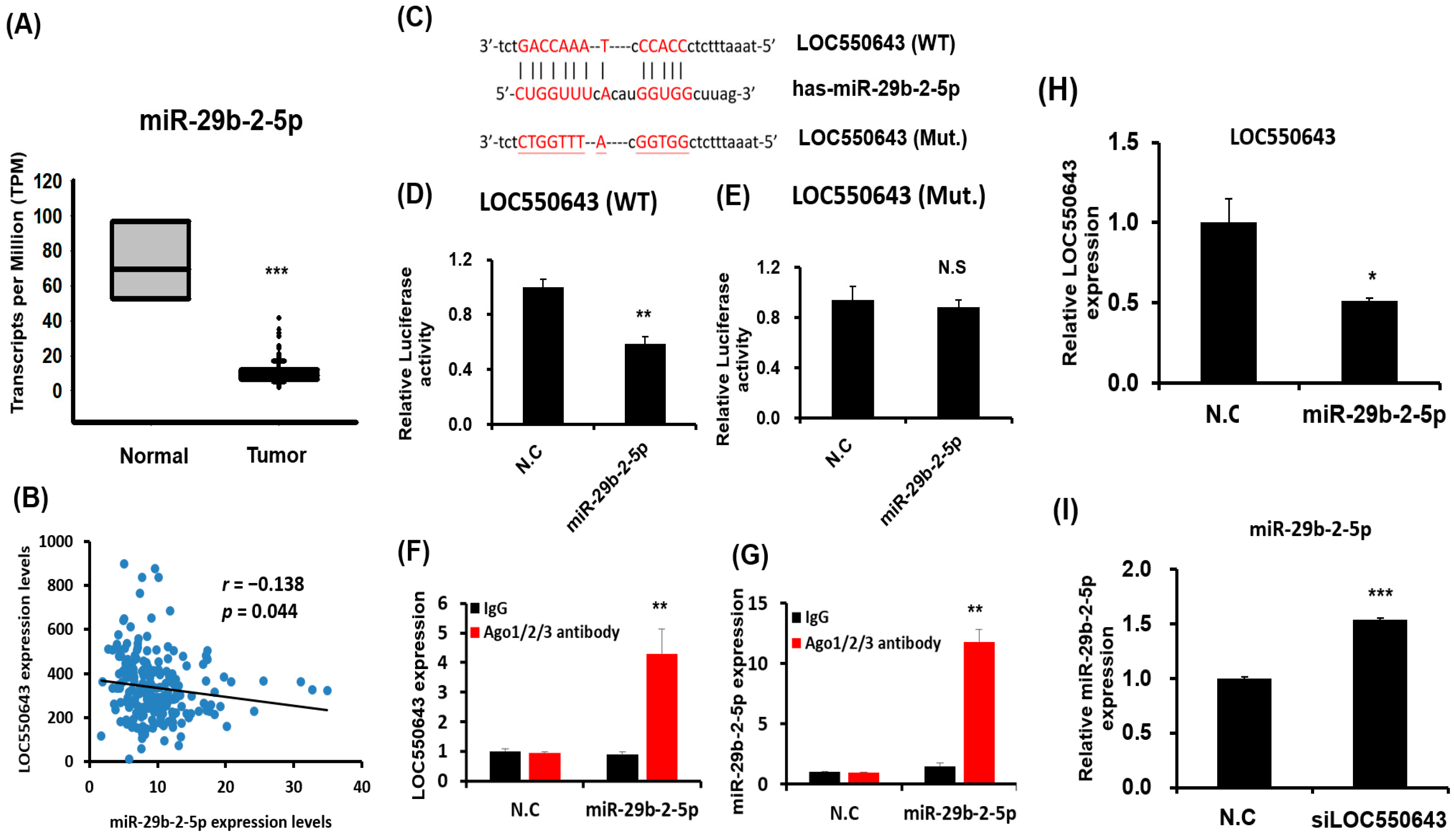
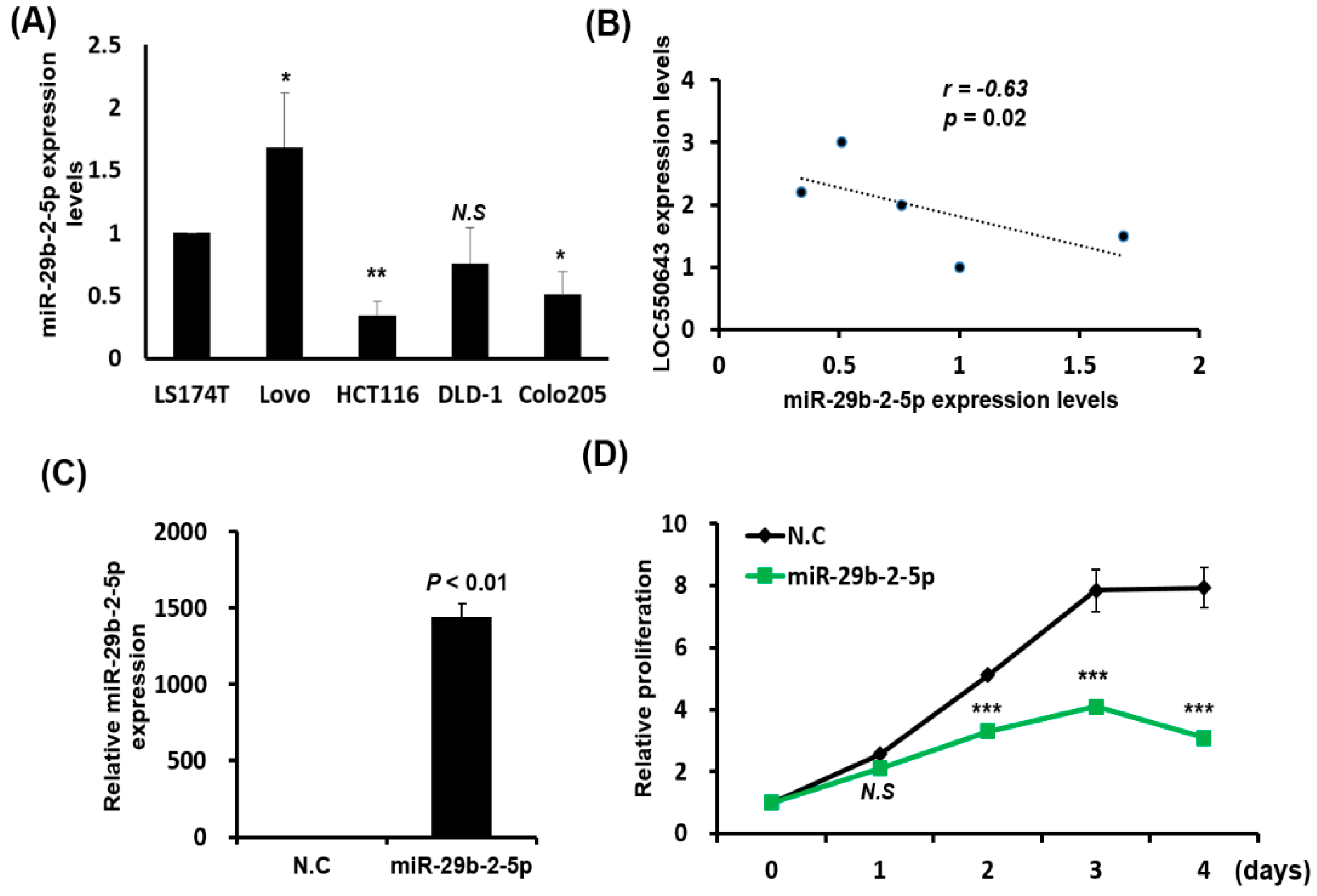
3.5. miR-29b-2-5p Inhibited LOC550643 Expression by Directly Binding at Its Sequence
3.6. MiR-29b-2-5p Expression Suppressed Colon Cancer Cell Growth and Induced Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Chen, X.; Yan, C.C.; Luo, C.; Ji, W.; Zhang, Y.; Dai, Q. Constructing lncRNA functional similarity network based on lncRNA-disease associations and disease semantic similarity. Sci. Rep. 2015, 5, 11338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.H.; Lin, W.C.; Tsai, K.W. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev. Mol. Med. 2014, 16, e1. [Google Scholar] [CrossRef]
- Wang, J.H.; Lu, T.J.; Kung, M.L.; Yang, Y.F.; Yeh, C.Y.; Tu, Y.T.; Chen, W.S.; Tsai, K.W. The Long Noncoding RNA LOC441461 (STX17-AS1) Modulates Colorectal Cancer Cell Growth and Motility. Cancers 2020, 12, 3171. [Google Scholar] [CrossRef]
- Hong, W.; Ying, H.; Lin, F.; Ding, R.; Wang, W.; Zhang, M. lncRNA LINC00460 Silencing Represses EMT in Colon Cancer through Downregulation of ANXA2 via Upregulating miR-433-3p. Mol. Ther. Nucleic Acids 2020, 19, 1209–1218. [Google Scholar] [CrossRef]
- He, X.; Tan, X.; Wang, X.; Jin, H.; Liu, L.; Ma, L.; Yu, H.; Fan, Z. C-Myc-activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol. 2014, 35, 12181–12188. [Google Scholar] [CrossRef]
- Spizzo, R.; Almeida, M.I.; Colombatti, A.; Calin, G.A. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 2012, 31, 4577–4587. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.W.; Li, S.C.; Tsai, K.W. Metformin Treatment Suppresses Melanoma Cell Growth and Motility Through Modulation of microRNA Expression. Cancers 2019, 11, 209. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Hao, Y.; Dong, X.; Gong, Q.; Chen, J.; Zhang, J.; Tian, W. Molecular mechanisms and function prediction of long noncoding RNA. Sci. World J. 2012, 2012, 541786. [Google Scholar] [CrossRef] [PubMed]
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71, 6320–6326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.H.; Wang, X.L.; Tang, H.M.; Jiang, T.; Chen, J.; Lu, S.; Qiu, G.Q.; Peng, Z.H.; Yan, D.W. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol. Rep. 2014, 32, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Chen, Y.; Liao, X.; Liu, D.; Li, F.; Ruan, H.; Jia, W. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med. Oncol. 2013, 30, 588. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Xu, M.D.; Ni, S.J.; Huang, D.; Wei, P.; Tan, C.; Zhou, X.Y.; Du, X. Low expression of LOC285194 is associated with poor prognosis in colorectal cancer. J. Transl. Med. 2013, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Zheng, H.; Zhuo, C.; Peng, J.; Li, D.; Xu, Y.; Li, X.; Cai, G.; Cai, S. Low expression of novel lncRNA RP11-462C24.1 suggests a biomarker of poor prognosis in colorectal cancer. Med. Oncol. 2014, 31, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, H.; Fesler, A.; Schee, K.; Fodstad, O.; Flatmark, K.; Ju, J. Clinical significance of long intergenic noncoding RNA-p21 in colorectal cancer. Clin. Colorectal Cancer 2013, 12, 261–266. [Google Scholar] [CrossRef]
- Yan, B.; Gu, W.; Yang, Z.; Gu, Z.; Yue, X.; Gu, Q.; Liu, L. Downregulation of a long noncoding RNA-ncRuPAR contributes to tumor inhibition in colorectal cancer. Tumour Biol. 2014, 35, 11329–11335. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sawada, G.; Kurashige, J.; Uchi, R.; Matsumura, T.; Ueo, H.; Takano, Y.; Eguchi, H.; Sudo, T.; Sugimachi, K.; et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br. J. Cancer 2013, 110, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhao, Y.; Chen, J.; Hu, J.; Wang, S.; Zhang, D.; Sun, Y. BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. Oncol. Lett. 2014, 8, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; He, B.; Gao, T.; Pan, Y.; Sun, H.; Xu, Y.; Li, R.; Ying, H.; Wang, F.; Liu, X.; et al. Up-regulation of 91H promotes tumor metastasis and predicts poor prognosis for patients with colorectal cancer. PLoS ONE 2014, 9, e103022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, P.; Diederichs, S.; Wang, W.; Boing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E.; et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031–8041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, D.; He, X.; Zhang, E.; Kong, R.; De, W.; Zhang, Z. Long noncoding RNA GAS5 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Med. Oncol. 2014, 31, 253. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.W.; Chong, K.H.; Li, C.H.; Tu, Y.T.; Chen, Y.R.; Lee, M.C.; Chan, S.H.; Wang, L.H.; Chang, Y.J. LOC550643, a Long Non-coding RNA, Acts as Novel Oncogene in Regulating Breast Cancer Growth and Metastasis. Front. Cell Dev. Biol. 2021, 9, 695632. [Google Scholar] [CrossRef]
- Park, S.J.; Yoon, B.H.; Kim, S.K.; Kim, S.Y. GENT2: An updated gene expression database for normal and tumor tissues. BMC Med. Genom. 2019, 12, 101. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
- Reimand, J.; Kull, M.; Peterson, H.; Hansen, J.; Vilo, J. g:Profiler—A web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007, 35, W193–W200. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Lechuga, S.; Huang, E.H.; Ivanov, A.I. Myosin Motors: Novel Regulators and Therapeutic Targets in Colorectal Cancer. Cancers 2021, 13, 741. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Aghagolzadeh, P.; Radpour, R. New trends in molecular and cellular biomarker discovery for colorectal cancer. World J. Gastroenterol. 2016, 22, 5678–5693. [Google Scholar] [CrossRef]
- Al-Shamsi, H.O.; Alhazzani, W.; Wolff, R.A. Extended RAS testing in metastatic colorectal cancer-Refining the predictive molecular biomarkers. J. Gastrointest. Oncol. 2015, 6, 314–321. [Google Scholar] [CrossRef]
- He, R.Z.; Luo, D.X.; Mo, Y.Y. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019, 6, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Z.; Qin, L.; Zhang, L.J. Expression and function of long non-coding RNA LINC01420 in thyroid cancer. Oncol. Lett. 2020, 19, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Tang, Y.; He, Y.; Wang, Y.; Lian, Y.; Xiong, F.; Shi, L.; Zhang, S.; Gong, Z.; Zhou, Y.; et al. High Expression of LINC01420 indicates an unfavorable prognosis and modulates cell migration and invasion in nasopharyngeal carcinoma. J. Cancer 2017, 8, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Zhang, X.; Sun, X.; Zhang, D.; Ma, S. Long Non-coding RNA LINC01420 Contributes to Pancreatic Cancer Progression Through Targeting KRAS Proto-oncogene. Dig. Dis. Sci. 2020, 65, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Chen, T.W.; Yang, T.H.; Hu, L.Y.; Pan, H.W.; Leung, C.M.; Li, S.C.; Ho, M.R.; Shu, C.W.; Liu, P.F.; et al. Co-modulated behavior and effects of differentially expressed miRNA in colorectal cancer. BMC Genom. 2013, 14 (Suppl. 5), S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Majumdar, A.P.N. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol. Cancer 2017, 16, 155. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X. Long noncoding RNAs: Functions and mechanisms in colon cancer. Mol. Cancer 2020, 19, 167. [Google Scholar] [CrossRef]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef]
- Alizadeh, M.; Safarzadeh, A.; Beyranvand, F.; Ahmadpour, F.; Hajiasgharzadeh, K.; Baghbanzadeh, A.; Baradaran, B. The potential role of miR-29 in health and cancer diagnosis, prognosis, and therapy. J. Cell Physiol. 2019, 234, 19280–19297. [Google Scholar] [CrossRef]
- Inoue, A.; Yamamoto, H.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Ikenaga, M.; Ikeda, M.; Murata, K.; Mizushima, T.; et al. MicroRNA-29b is a Novel Prognostic Marker in Colorectal Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S1410–S1418. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, J.; Huang, G.; Zhang, Y.; Zhao, M.; Zhang, Y.; Xie, J. microRNA-29b inhibits cell growth and promotes sensitivity to oxaliplatin in colon cancer by targeting FOLR1. Biofactors 2020, 46, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, Q.; Che, X.; Xu, L.; Li, Z.; Fan, Y.; Hou, K.; Wang, S.; Qu, J.; Xu, L.; et al. MicroRNA-29b-2-5p inhibits cell proliferation by directly targeting Cbl-b in pancreatic ductal adenocarcinoma. BMC Cancer 2018, 18, 681. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.W.; Lo, Y.H.; Liu, H.; Yeh, C.Y.; Chen, Y.Z.; Hsu, C.W.; Chen, W.S.; Wang, J.H. Linc00659, a long noncoding RNA, acts as novel oncogene in regulating cancer cell growth in colorectal cancer. Mol. Cancer 2018, 17, 72. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.H. MiR-29b reverses oxaliplatin-resistance in colorectal cancer by targeting SIRT1. Oncotarget 2018, 9, 12304–12315. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Cheng, D.; Liu, Y.; Pan, H.; Li, G.; Li, P.; Li, Y.; Sun, W.; Ma, D.; Ni, C. LncRNA-ATB regulates epithelial-mesenchymal transition progression in pulmonary fibrosis via sponging miR-29b-2-5p and miR-34c-3p. J. Cell Mol. Med. 2021, 25, 7294–7306. [Google Scholar] [CrossRef]
- Sunkavalli, U.; Aguilar, C.; Silva, R.J.; Sharan, M.; Cruz, A.R.; Tawk, C.; Maudet, C.; Mano, M.; Eulalio, A. Analysis of host microRNA function uncovers a role for miR-29b-2-5p in Shigella capture by filopodia. PLoS Pathog. 2017, 13, e1006327. [Google Scholar] [CrossRef]
- Shen, K.; Li, R.; Zhang, X.; Qu, G.; Li, R.; Wang, Y.; Liu, B.; Lv, C.; Li, M.; Song, X. Acetyl oxygen benzoate engeletin ester promotes KLF4 degradation leading to the attenuation of pulmonary fibrosis via inhibiting TGFbeta1-smad/p38MAPK-lnc865/lnc556-miR-29b-2-5p-STAT3 signal pathway. Aging 2021, 13, 13807–13821. [Google Scholar] [CrossRef]
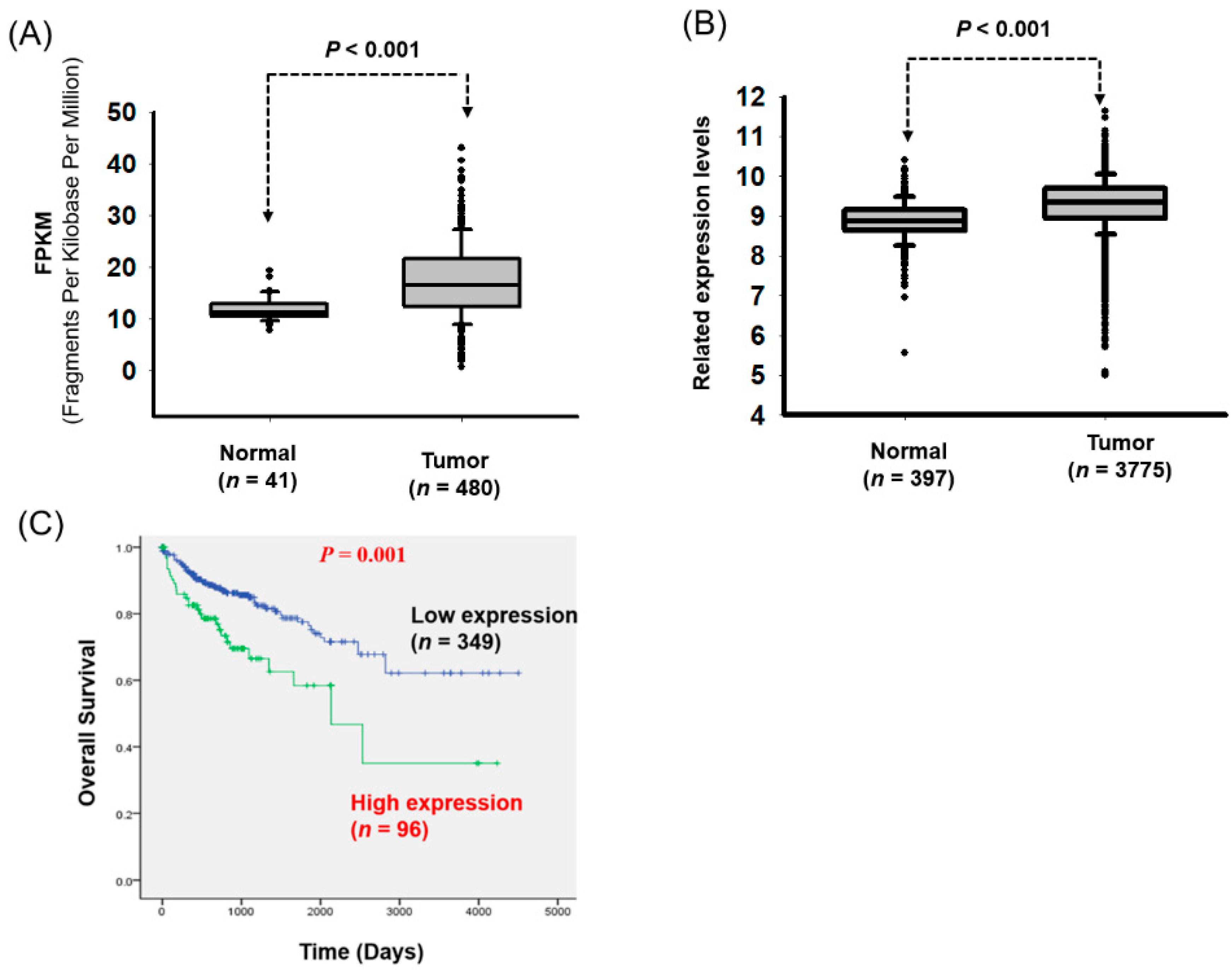
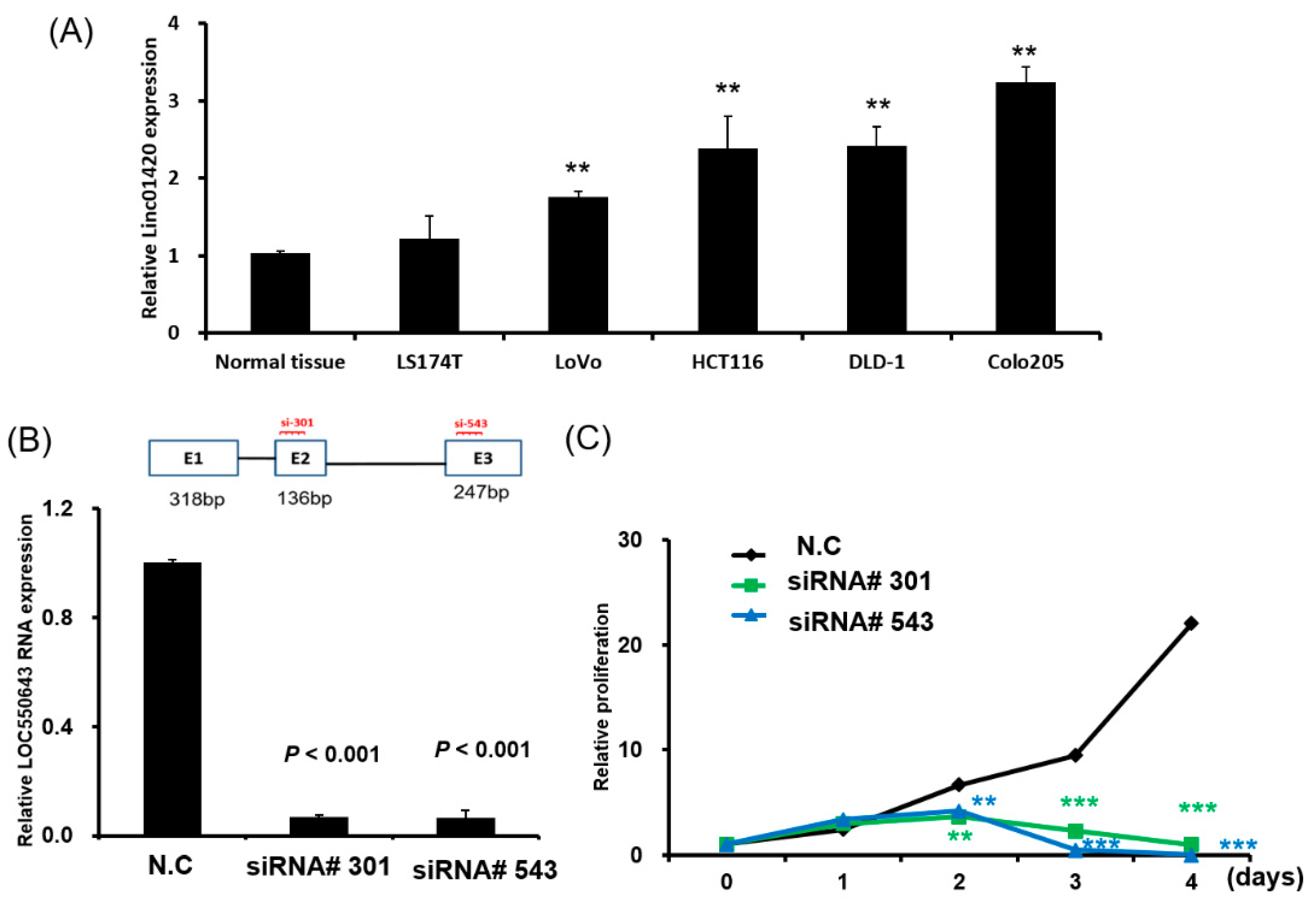
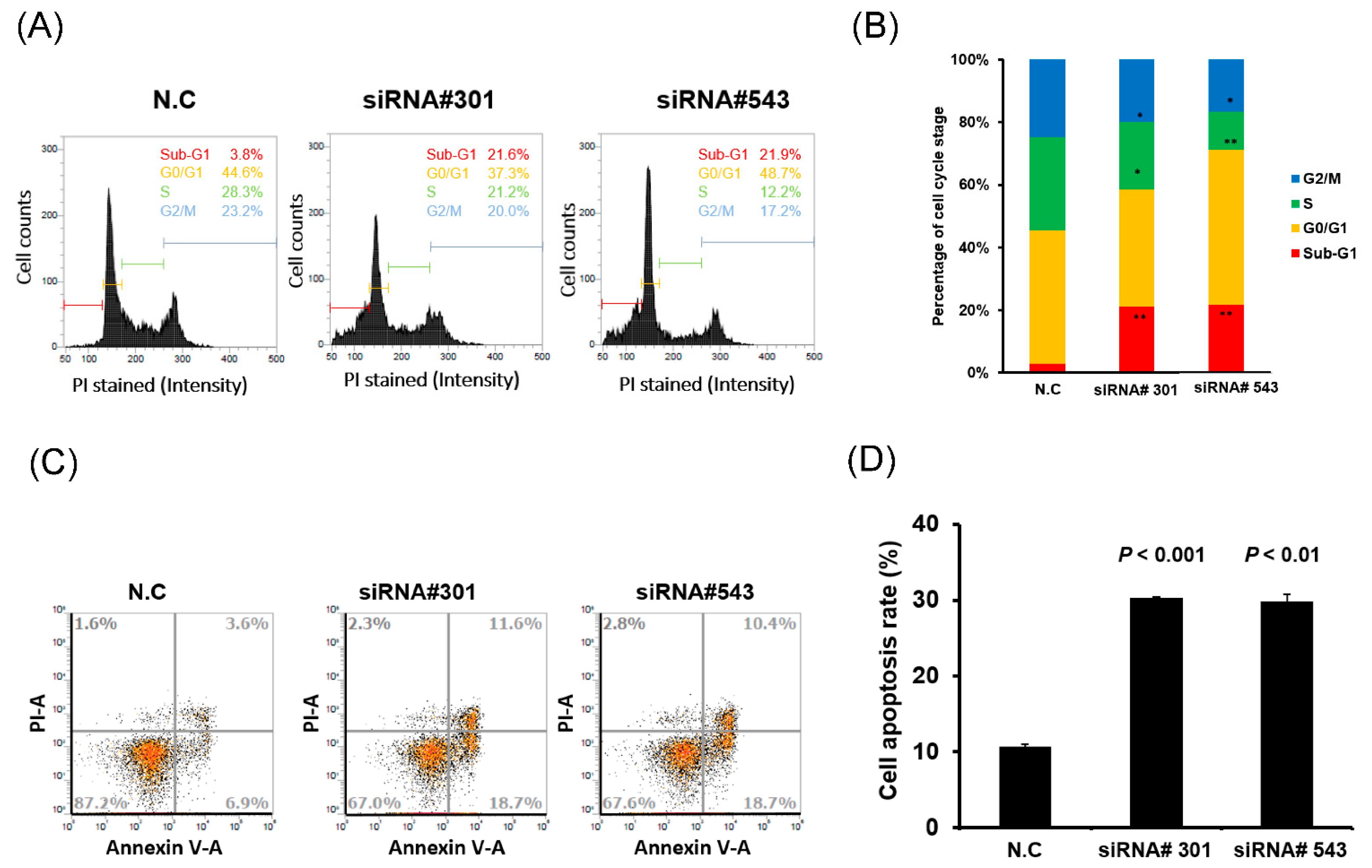


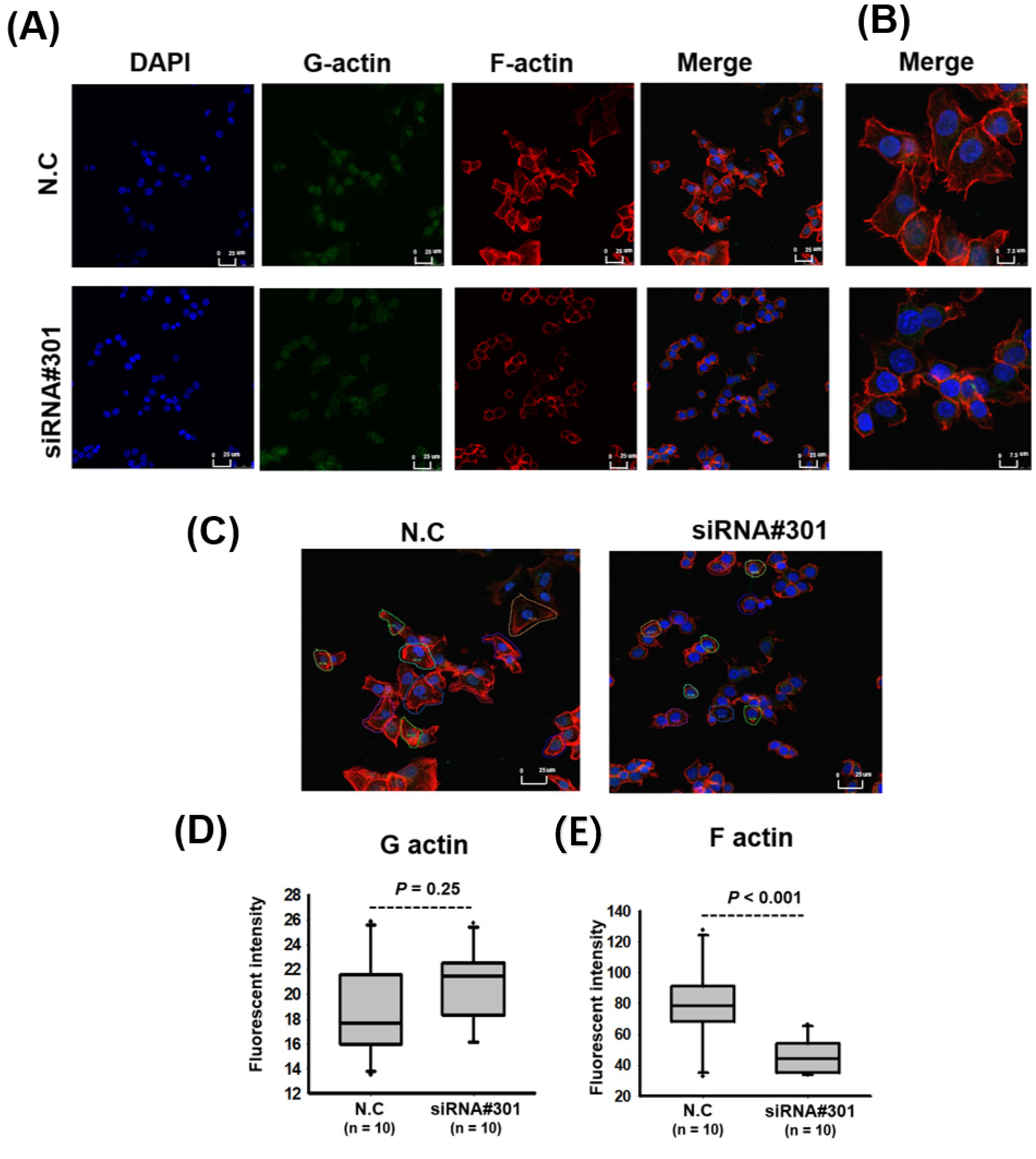


| Variables | LOC550643 (n = 446) | |||
|---|---|---|---|---|
| No. (%) | Mean ± SD | Median | p-Value | |
| Pathology stage | ||||
| I | 71 (15.9) | 16.92 ± 7.27 | 15.97 | 0.029 a |
| II | 197 (44.2) | 17.23 ± 7.60 | 16.15 | |
| III | 121 (27.1) | 16.51 ± 6.81 c | 16.19 | |
| IV | 57 (12.8) | 19.95 ± 7.59 c | 19.14 | |
| pT stage | ||||
| T1 | 10 (2.2) | 21.50 ± 9.18 | 19.10 | 0.146 a |
| T2 | 71 (15.9) | 16.22 ± 6.52 | 15.97 | |
| T3 | 316 (70.9) | 17.32 ± 7.38 | 16.53 | |
| T4 | 49 (11.0) | 18.15 ± 8.04 | 16.32 | |
| pN stage | ||||
| N0 | 275 (61.7) | 17.19 ± 7.49 | 16.15 | 0.778 a |
| N1 | 97 (21.7) | 17.80 ± 6.60 | 17.67 | |
| N2 | 74 (16.6) | 17.24 ± 8.02 | 16.38 | |
| pM stage | ||||
| M0 | 389 (87.2) | 16.95 ± 7.29 | 16.10 | 0.004 b |
| M1 | 57 (12.8) | 19.95 ± 7.59 | 19.14 | |
| Characteristic | No. (%) | OS | |||
|---|---|---|---|---|---|
| CHR (95% CI) | p-Value | AHR (95% CI) | p-Value | ||
| LOC550643 | (n = 445) | ||||
| Low | 349 (78.4) | 1.00 | 0.001 | 1.00 | 0.006 |
| High | 96 (21.6) | 2.10 (1.34–3.28) | 1.90 (1.21–3.00) | ||
| Gene Ontology | Function | p-Value |
|---|---|---|
| Molecular Function | ||
| GO:0005515 | protein binding | 0.000160535 |
| GO:0000146 | microfilament motor activity | 0.005916827 |
| GO:0003774 | cytoskeletal motor activity | 0.008611768 |
| GO:0000166 | nucleotide binding | 0.01087002 |
| GO:1901265 | nucleoside phosphate binding | 0.010988224 |
| GO:0036094 | small molecule binding | 0.014742833 |
| GO:0043168 | anion binding | 0.0247294 |
| Biological Process | ||
| GO:0051650 | establishment of vesicle localization | 0.00607308 |
| GO:0006996 | organelle organization | 0.00996091 |
| GO:0051648 | vesicle localization | 0.013922828 |
| GO:0030029 | actin filament-based process | 0.044425752 |
| GO:0099515 | 0.049163434 | |
| Cellular Component | actin filament-based transport | |
| GO:0005829 | cytosol | 2.84495 × 10−6 |
| GO:0005737 | cytoplasm | 1.19769 × 10−5 |
| GO:0015629 | actin cytoskeleton | 0.010115212 |
| GO:0031090 | organelle membrane | 0.038066502 |
| GO:0016459 | myosin complex | 0.042296337 |
| GO:0098588 | bounding membrane of organelle | 0.043776643 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.F.; Lu, T.-J.; Lo, Y.-H.; Tu, Y.-T.; Chen, Y.-R.; Lee, M.-C.; Chiang, Y.-L.; Yeh, C.-Y.; Tsai, K.-W. Long Noncoding RNA LOC550643 Acts as an Oncogene in the Growth Regulation of Colorectal Cancer Cells. Cells 2022, 11, 1065. https://doi.org/10.3390/cells11071065
Wu HF, Lu T-J, Lo Y-H, Tu Y-T, Chen Y-R, Lee M-C, Chiang Y-L, Yeh C-Y, Tsai K-W. Long Noncoding RNA LOC550643 Acts as an Oncogene in the Growth Regulation of Colorectal Cancer Cells. Cells. 2022; 11(7):1065. https://doi.org/10.3390/cells11071065
Chicago/Turabian StyleWu, Hsuan Franziska, Tzung-Ju Lu, Yi-Hao Lo, Ya-Ting Tu, Yi-Ru Chen, Ming-Cheng Lee, Yu-Lun Chiang, Chung-Yu Yeh, and Kuo-Wang Tsai. 2022. "Long Noncoding RNA LOC550643 Acts as an Oncogene in the Growth Regulation of Colorectal Cancer Cells" Cells 11, no. 7: 1065. https://doi.org/10.3390/cells11071065
APA StyleWu, H. F., Lu, T.-J., Lo, Y.-H., Tu, Y.-T., Chen, Y.-R., Lee, M.-C., Chiang, Y.-L., Yeh, C.-Y., & Tsai, K.-W. (2022). Long Noncoding RNA LOC550643 Acts as an Oncogene in the Growth Regulation of Colorectal Cancer Cells. Cells, 11(7), 1065. https://doi.org/10.3390/cells11071065






