Novel Insights into the Therapeutic Potential of Lung-Targeted Gene Transfer in the Most Common Respiratory Diseases
Abstract
1. Introduction
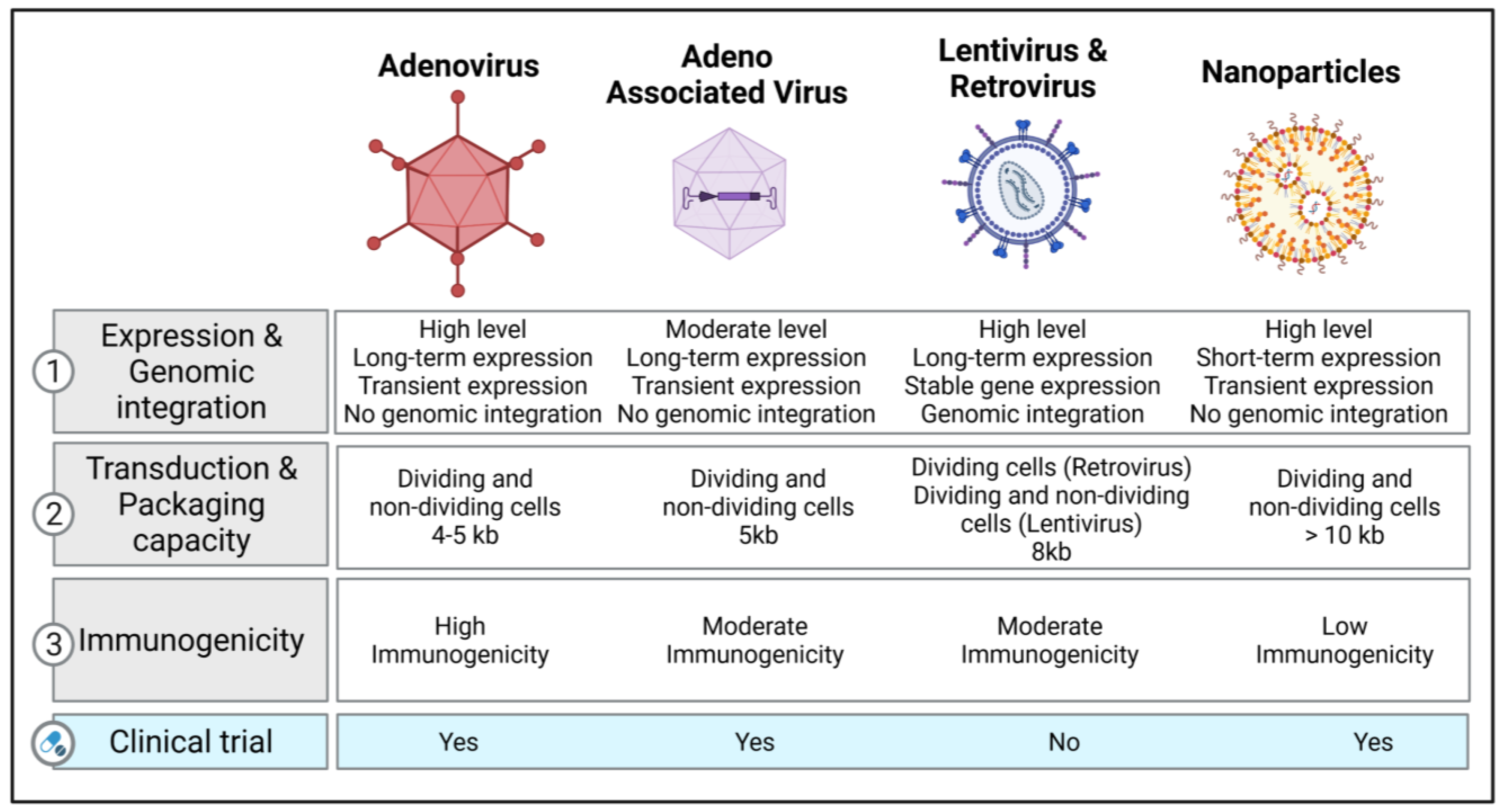
2. Vectors of Gene Therapy in Respiratory Diseases
2.1. Viral Vectors
2.1.1. Retroviral and Lentiviral Vectors
2.1.2. Adenovirus Vectors
2.1.3. Adeno-Associated Viruses (AAV) and Their Vectors
2.1.4. Other Viral Vectors
2.2. Non-Viral Vectors
Nanoparticle-Based Therapeutics
3. Gene-Editing Strategies
3.1. CRISPR-Cas9 as a Genome-Editing Tool
3.2. Base- and Prime-Editing Technologies
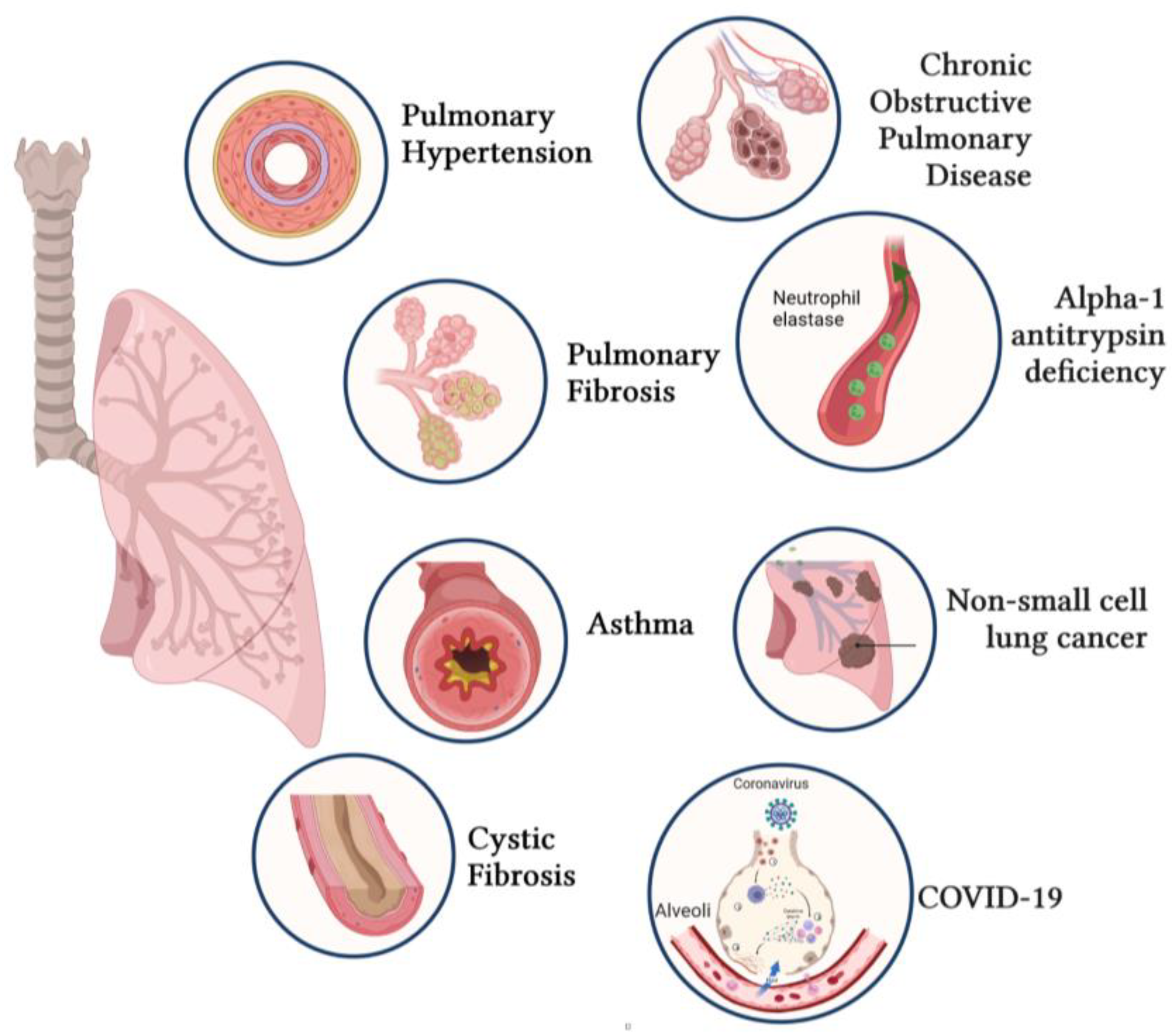
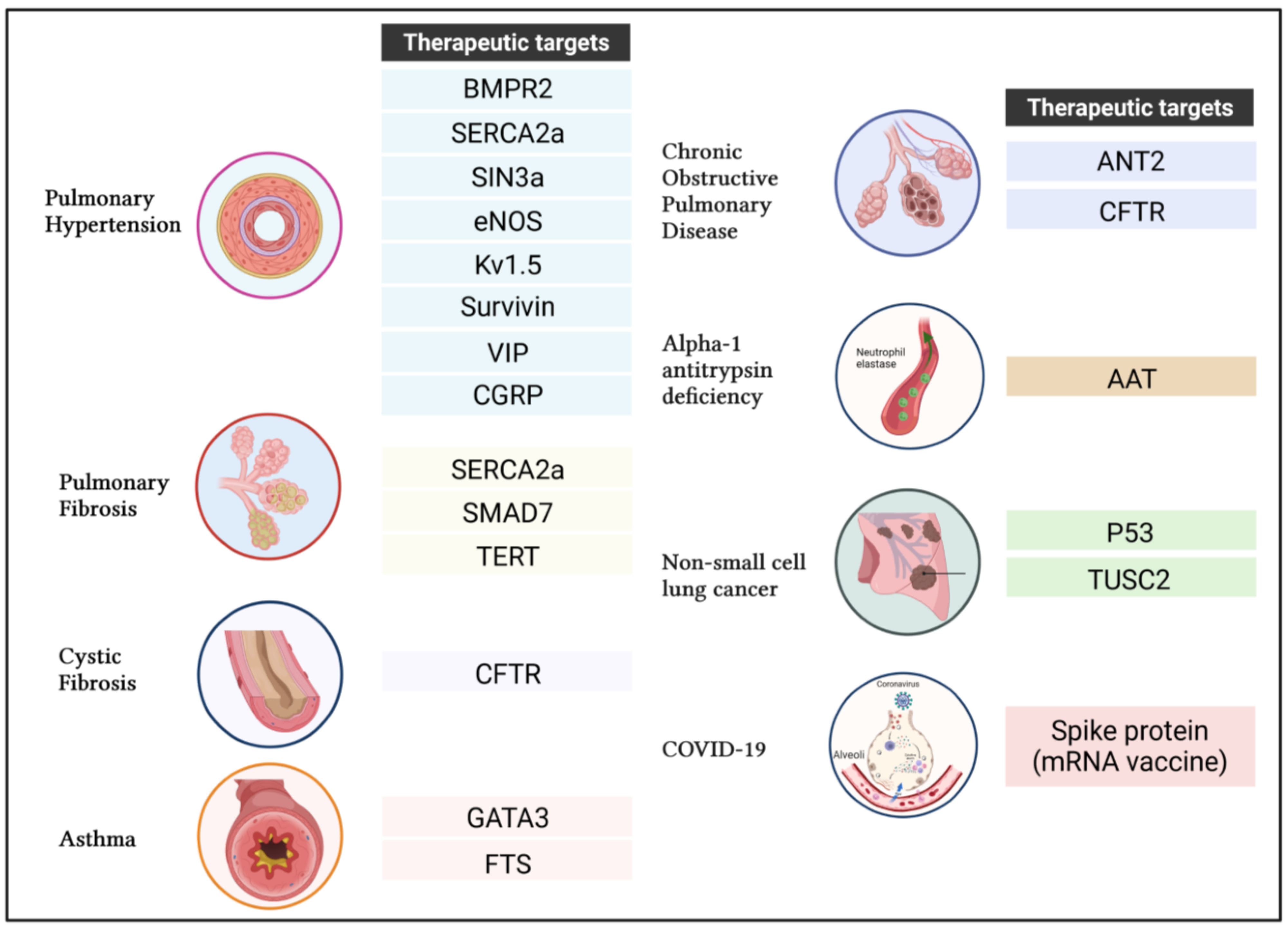
4. Applications of Gene Transfer for Respiratory Diseases
4.1. Pulmonary Arterial Hypertension (PAH)
4.1.1. Bone Morphogenetic Protein Type 2 Receptor (BMPR2)
4.1.2. Sarco-Endoplasmic Reticulum Calcium-ATPase 2a (SERCA2a)
4.1.3. SIN3 Transcription Regulator Family Member A
4.1.4. Endothelial Nitric Oxide Synthase (ENOS)
4.1.5. Voltage-Gated Potassium Channel Kv1.5
4.1.6. Survivin
4.1.7. Vasoactive Intestinal Peptide (VIP)
4.1.8. Calcitonin Gene-Related Peptide (CGRP)
4.2. Idiopathic Pulmonary Fibrosis (IPF)
4.2.1. SERCA2a
4.2.2. Caveolin-1
4.2.3. SMAD Family Member 7 (SMAD7)
4.2.4. Telomerase Reverse Transcriptase (TERT)
4.3. Cystic Fibrosis
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
4.4. Asthma
4.4.1. GATA-Binding Protein 3 (GATA3)
4.4.2. Thymulin (Formerly Called “Serum Thymus Factor” or FTS)
4.5. Chronic Obstructive Pulmonary Disease (COPD)
4.6. Alpha-1 Antitrypsin Deficiency (AATD)
4.7. Non-Small-Cell Lung Cancer (NSCLC)
4.7.1. Tumor Suppressor p53
4.7.2. Tumor Suppressor Candidate 2 Gene (TUSC2)
4.8. Coronavirus Disease (COVID-19)
5. Limitations of Gene Therapy
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Banuls, L.; Pellicer, D.; Castillo, S.; Navarro-Garcia, M.M.; Magallon, M.; Gonzalez, C.; Dasi, F. Gene Therapy in Rare Respiratory Diseases: What Have We Learned So Far? J. Clin. Med. 2020, 9, 2577. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, T.; Roblin, R. Gene therapy for human genetic disease? Science 1972, 175, 949–955. [Google Scholar] [CrossRef] [PubMed]
- International, P.P.H.C.; Lane, K.B.; Machado, R.D.; Pauciulo, M.W.; Thomson, J.R.; Phillips, J.A., 3rd; Loyd, J.E.; Nichols, W.C.; Trembath, R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat. Genet. 2000, 26, 81–84. [Google Scholar] [CrossRef]
- Stoltz, D.A.; Meyerholz, D.K.; Welsh, M.J. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 2015, 372, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; McCray, P.B., Jr.; Sinn, P.L. Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes 2018, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.A.; Engelhardt, J.F. Current status of gene therapy for inherited lung diseases. Annu. Rev. Physiol. 2003, 65, 585–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Corrigan-Curay, J.; Cohen-Haguenauer, O.; O’Reilly, M.; Ross, S.R.; Fan, H.; Rosenberg, N.; Somia, N.; King, N.; Friedmann, T.; Dunbar, C.; et al. Challenges in vector and trial design using retroviral vectors for long-term gene correction in hematopoietic stem cell gene therapy. Mol. Ther. 2012, 20, 1084–1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wall, D.A.; Krueger, J. Chimeric antigen receptor T cell therapy comes to clinical practice. Curr. Oncol. 2020, 27, S115–S123. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Beekman, J.M.; Boyd, A.C.; Brand, J.; Carlon, M.S.; Connolly, M.M.; Chan, M.; Conlon, S.; Davidson, H.E.; Davies, J.C.; et al. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax 2017, 72, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Mitomo, K.; Griesenbach, U.; Inoue, M.; Somerton, L.; Meng, C.; Akiba, E.; Tabata, T.; Ueda, Y.; Frankel, G.M.; Farley, R.; et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol. Ther. 2010, 18, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Aneja, M.K.; Geiger, J.P.; Himmel, A.; Rudolph, C. Targeted gene delivery to the lung. Expert. Opin. Drug Deliv. 2009, 6, 567–583. [Google Scholar] [CrossRef]
- Sinn, P.L.; Cooney, A.L.; Oakland, M.; Dylla, D.E.; Wallen, T.J.; Pezzulo, A.A.; Chang, E.H.; McCray, P.B., Jr. Lentiviral vector gene transfer to porcine airways. Mol. Ther. Nucleic Acids. 2012, 1, e56. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Burnight, E.R.; Hickey, M.A.; Blissard, G.W.; McCray, P.B., Jr. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J. Virol. 2005, 79, 12818–12827. [Google Scholar] [CrossRef]
- Ferrari, S.; Griesenbach, U.; Iida, A.; Farley, R.; Wright, A.M.; Zhu, J.; Munkonge, F.M.; Smith, S.N.; You, J.; Ban, H.; et al. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007, 14, 1371–1379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anson, D.S. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet. Vaccines Ther. 2004, 2, 9. [Google Scholar] [CrossRef]
- Kay, M.A.; Glorioso, J.C.; Naldini, L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Ertl, H.C.; Wilson, J.M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995, 69, 2004–2015. [Google Scholar] [CrossRef]
- Murata, T.; Hori, M.; Lee, S.; Nakamura, A.; Kohama, K.; Karaki, H.; Ozaki, H. Vascular endothelium has a local anti-adenovirus vector system and glucocorticoid optimizes its gene transduction. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccin. Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Cots, D.; Bosch, A.; Chillon, M. Helper dependent adenovirus vectors: Progress and future prospects. Curr. Gene. Ther. 2013, 13, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ouyang, H.; Grasemann, H.; Bartlett, C.; Du, K.; Duan, R.; Shi, F.; Estrada, M.; Seigel, K.E.; Coates, A.L.; et al. Transducing Airway Basal Cells with a Helper-Dependent Adenoviral Vector for Lung Gene Therapy. Hum. Gene Ther. 2018, 29, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, D.; Stiles, K.M.; De, B.P.; Crystal, R.G. Genetic Modification of the Lung Directed Toward Treatment of Human Disease. Hum. Gene Ther. 2017, 28, 3–84. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Thornell, I.M.; Singh, B.K.; Shah, V.S.; Stoltz, D.A.; McCray, P.B., Jr.; Zabner, J.; Sinn, P.L. A Novel AAV-mediated Gene Delivery System Corrects CFTR Function in Pigs. Am. J. Respir. Cell Mol. Biol. 2019, 61, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Halbert, C.L.; Allen, J.M.; Miller, A.D. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat. Biotechnol. 2002, 20, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Fakhiri, J.; Schneider, M.A.; Puschhof, J.; Stanifer, M.; Schildgen, V.; Holderbach, S.; Voss, Y.; El Andari, J.; Schildgen, O.; Boulant, S.; et al. Novel Chimeric Gene Therapy Vectors Based on Adeno-Associated Virus and Four Different Mammalian Bocaviruses. Mol. Ther. Methods Clin. Dev. 2019, 12, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Feng, Z.; Sun, X.; Zhang, Y.; Zou, W.; Wang, Z.; Jensen-Cody, C.; Liang, B.; Park, S.Y.; Qiu, J.; et al. Human Bocavirus Type-1 Capsid Facilitates the Transduction of Ferret Airways by Adeno-Associated Virus Genomes. Hum. Gene Ther. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Keiser, N.W.; Song, Y.; Deng, X.; Cheng, F.; Qiu, J.; Engelhardt, J.F. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol. Ther. 2013, 21, 2181–2194. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, D.V.; Maheshri, N. Directed evolution of AAV mutants for enhanced gene delivery. Conf. Proc. IEEE Eng. Med. Biol Soc. 2004, 2004, 3520–3523. [Google Scholar] [CrossRef]
- Zhong, L.; Li, B.; Mah, C.S.; Govindasamy, L.; Agbandje-McKenna, M.; Cooper, M.; Herzog, R.W.; Zolotukhin, I.; Warrington, K.H., Jr.; Weigel-Van Aken, K.A.; et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. USA 2008, 105, 7827–7832. [Google Scholar] [CrossRef]
- Wu, Z.; Asokan, A.; Samulski, R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006, 14, 316–327. [Google Scholar] [CrossRef]
- Lompre, A.M.; Hadri, L.; Merlet, E.; Keuylian, Z.; Mougenot, N.; Karakikes, I.; Chen, J.; Atassi, F.; Marchand, A.; Blaise, R.; et al. Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: A new perspective for in-stent restenosis gene therapy. Gene Ther. 2013, 20, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Markusic, D.M.; Herzog, R.W.; Aslanidi, G.V.; Hoffman, B.E.; Li, B.; Li, M.; Jayandharan, G.R.; Ling, C.; Zolotukhin, I.; Ma, W.; et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol. Ther. 2010, 18, 2048–2056. [Google Scholar] [CrossRef]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Min, S.H.; Chiodo, V.; Pang, J.J.; Zhong, L.; Zolotukhin, S.; Srivastava, A.; Lewin, A.S.; et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009, 17, 463–471. [Google Scholar] [CrossRef]
- Martini, S.V.; da Silva, A.L.; Ferreira, D.; Gomes, K.; Ornellas, F.M.; Lopes-Pacheco, M.; Zin, E.; Petrs-Silva, H.; Rocco, P.R.; Morales, M.M. Single tyrosine mutation in AAV8 vector capsid enhances gene lung delivery and does not alter lung morphofunction in mice. Cell. Physiol. Biochem. 2014, 34, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M.; Kitoko, J.Z.; Morales, M.M.; Petrs-Silva, H.; Rocco, P.R.M. Self-complementary and tyrosine-mutant rAAV vectors enhance transduction in cystic fibrosis bronchial epithelial cells. Exp. Cell Res. 2018, 372, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.C.; Samulski, R.J. Packaging capacity of adeno-associated virus serotypes: Impact of larger genomes on infectivity and postentry steps. J. Virol. 2005, 79, 9933–9944. [Google Scholar] [CrossRef]
- Gregory, S.M.; Nazir, S.A.; Metcalf, J.P. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol. 2011, 6, 357–374. [Google Scholar] [CrossRef]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Tokusumi, Y.; Ban, H.; Shirakura, M.; Kanaya, T.; Yoshizaki, M.; Hironaka, T.; Nagai, Y.; Iida, A.; Hasegawa, M. Recombinant Sendai virus vectors deleted in both the matrix and the fusion genes: Efficient gene transfer with preferable properties. J. Gene Med. 2004, 6, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.; Reineke, J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent Advances in Nanomaterials for Gene Delivery-A Review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomedicine 2017, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Eltoukhy, A.A.; Anderson, D.G.; Costa, K.D. Lipidoid mRNA Nanoparticles for Myocardial Delivery in Rodents. Methods Mol. Biol. 2017, 1521, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Eltoukhy, A.A.; Fish, K.M.; Nonnenmacher, M.; Ishikawa, K.; Chen, J.; Hajjar, R.J.; Anderson, D.G.; Costa, K.D. Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression. Mol. Ther. 2016, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, P.J.; Cunningham, O.; Greene, C.M.; Cryan, S.A. Targeting miRNA-based medicines to cystic fibrosis airway epithelial cells using nanotechnology. Int. J. Nanomedicine 2013, 8, 3907–3915. [Google Scholar] [CrossRef] [PubMed]
- McLendon, J.M.; Joshi, S.R.; Sparks, J.; Matar, M.; Fewell, J.G.; Abe, K.; Oka, M.; McMurtry, I.F.; Gerthoffer, W.T. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J. Control. Release 2015, 210, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Satija, S.; Paudel, K.R.; Malyla, V.; Kannaujiya, V.K.; Chellappan, D.K.; Bebawy, M.; Hansbro, P.M.; Wich, P.R.; Dua, K. Targeting respiratory diseases using miRNA inhibitor based nanotherapeutics: Current status and future perspectives. Nanomedicine 2021, 31, 102303. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Paudel, K.R.; Shukla, S.D.; Allam, V.; Kannaujiya, V.K.; Panth, N.; Das, A.; Parihar, V.K.; Chakraborty, A.; Ali, M.K.; et al. Recent trends of NFkappaB decoy oligodeoxynucleotide-based nanotherapeutics in lung diseases. J. Control. Release 2021, 337, 629–644. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, D.; Ungaro, F.; Giovino, C.; Polimeno, A.; Quaglia, F.; Carnuccio, R. Sustained inhibition of IL-6 and IL-8 expression by decoy ODN to NF-kappaB delivered through respirable large porous particles in LPS-stimulated cystic fibrosis bronchial cells. J. Gene Med. 2011, 13, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wardwell, P.R.; Bader, R.A. Immunomodulation of cystic fibrosis epithelial cells via NF-kappaB decoy oligonucleotide-coated polysaccharide nanoparticles. J. Biomed. Mater. Res. A 2015, 103, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Egashira, K.; Chen, L.; Nakano, K.; Iwata, E.; Miyagawa, M.; Tsujimoto, H.; Hara, K.; Morishita, R.; Sueishi, K.; et al. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 2009, 53, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Boyd, A.C.; Porteous, D.J.; Davies, G.; Davies, J.C.; Griesenbach, U.; Higgins, T.E.; Gill, D.R.; Hyde, S.C.; Innes, J.A.; et al. A Phase I/IIa Safety and Efficacy Study of Nebulized Liposome-mediated Gene Therapy for Cystic Fibrosis Supports a Multidose Trial. Am. J. Respir. Crit. Care Med. 2015, 192, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 684–691. [Google Scholar] [CrossRef]
- Konstan, M.W.; Davis, P.B.; Wagener, J.S.; Hilliard, K.A.; Stern, R.C.; Milgram, L.J.; Kowalczyk, T.H.; Hyatt, S.L.; Fink, T.L.; Gedeon, C.R.; et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum. Gene Ther. 2004, 15, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Alapati, D.; Morrisey, E.E. Gene Editing and Genetic Lung Disease. Basic Research Meets Therapeutic Application. Am. J. Respir. Cell Mol. Biol 2017, 56, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Linder, S.J.; Cascio, V.M.; Fu, Y.; Ho, Q.H.; Joung, J.K. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 2013, 10, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Alapati, D.; Zacharias, W.J.; Hartman, H.A.; Rossidis, A.C.; Stratigis, J.D.; Ahn, N.J.; Coons, B.; Zhou, S.; Li, H.; Singh, K.; et al. In utero gene editing for monogenic lung disease. Sci. Transl. Med. 2019, 11, 8375. [Google Scholar] [CrossRef]
- Maule, G.; Arosio, D.; Cereseto, A. Gene Therapy for Cystic Fibrosis: Progress and Challenges of Genome Editing. Int. J. Mol. Sci 2020, 21, 3903. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, I.; Qujeq, D.; Yousefi, T.; Ferns, G.A.; Maniati, M.; Vaghari-Tabari, M. CRISPR/Cas9 gene editing: A new therapeutic approach in the treatment of infection and autoimmunity. IUBMB Life 2020, 72, 1603–1621. [Google Scholar] [CrossRef]
- Nair, J.; Nair, A.; Veerappan, S.; Sen, D. Translatable gene therapy for lung cancer using Crispr CAS9-an exploratory review. Cancer Gene Ther. 2020, 27, 116–124. [Google Scholar] [CrossRef]
- Ferdosi, S.R.; Ewaisha, R.; Moghadam, F.; Krishna, S.; Park, J.G.; Ebrahimkhani, M.R.; Kiani, S.; Anderson, K.S. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat. Commun. 2019, 10, 1842. [Google Scholar] [CrossRef]
- Cyranoski, D. CRISPR gene-editing tested in a person for the first time. Nature 2016, 539, 479. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Ooi, K.H.; Liu, M.M.; Tay, J.W.D.; Teo, S.Y.; Kaewsapsak, P.; Jin, S.; Lee, C.K.; Hou, J.; Maurer-Stroh, S.; Lin, W.; et al. An engineered CRISPR-Cas12a variant and DNA-RNA hybrid guides enable robust and rapid COVID-19 testing. Nat. Commun. 2021, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Li, S.; Luo, W.; Zhang, X.; Wang, C.; Chen, Q.; Yu, S.; Tai, J.; Wang, Y. Rapid, Ultrasensitive, and Highly Specific Diagnosis of COVID-19 by CRISPR-Based Detection. ACS Sens. 2021, 6, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, I.G. Prime Editing: Genome Editing for Rare Genetic Diseases Without Double-Strand Breaks or Donor DNA. Front. Genet. 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; Tu, L.; Le Hiress, M.; Ricard, N.; Sattler, C.; Seferian, A.; Huertas, A.; Humbert, M.; Montani, D. Pathogenesis of pulmonary arterial hypertension: Lessons from cancer. Eur. Respir. Rev. 2013, 22, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Potoka, K.C.; Champion, H.C.; Mora, A.L.; Gladwin, M.T. Pulmonary arterial hypertension: The clinical syndrome. Circ. Res. 2014, 115, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Bisserier, M.; Janostiak, R.; Lezoualc’h, F.; Hadri, L. Targeting epigenetic mechanisms as an emerging therapeutic strategy in pulmonary hypertension disease. Vasc Biol 2020, 2, R17–R34. [Google Scholar] [CrossRef] [PubMed]
- Bisserier, M.; Pradhan, N.; Hadri, L. Current and emerging therapeutic approaches to pulmonary hypertension. Rev. Cardiovasc Med. 2020, 21, 163–179. [Google Scholar] [CrossRef]
- Duarte, J.D.; Hanson, R.L.; Machado, R.F. Pharmacologic treatments for pulmonary hypertension: Exploring pharmacogenomics. Future Cardiol. 2013, 9, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Paulin, R.; Courboulin, A.; Lambert, C.; Barrier, M.; Bonnet, P.; Bisserier, M.; Roy, M.; Sussman, M.A.; Agharazii, M.; et al. RAGE-dependent activation of the oncoprotein Pim1 plays a critical role in systemic vascular remodeling processes. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Meloche, J.; Jacob, M.H.; Bisserier, M.; Courboulin, A.; Bonnet, S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1798–H1809. [Google Scholar] [CrossRef]
- Courboulin, A.; Tremblay, V.L.; Barrier, M.; Meloche, J.; Jacob, M.H.; Chapolard, M.; Bisserier, M.; Paulin, R.; Lambert, C.; Provencher, S.; et al. Kruppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir. Res. 2011, 12, 128. [Google Scholar] [CrossRef]
- Meloche, J.; Courchesne, A.; Barrier, M.; Carter, S.; Bisserier, M.; Paulin, R.; Lauzon-Joset, J.F.; Breuils-Bonnet, S.; Tremblay, E.; Biardel, S.; et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J. Am. Heart Assoc. 2013, 2, e005157. [Google Scholar] [CrossRef]
- Jones, C.; Bisserier, M.; Bueno-Beti, C.; Bonnet, G.; Neves-Zaph, S.; Lee, S.Y.; Milara, J.; Dorfmuller, P.; Humbert, M.; Leopold, J.A.; et al. A novel secreted-cAMP pathway inhibits pulmonary hypertension via a feed-forward mechanism. Cardiovasc. Res. 2020, 116, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Fazal, S.; Bisserier, M.; Hadri, L. Molecular and Genetic Profiling for Precision Medicines in Pulmonary Arterial Hypertension. Cells 2021, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.D.; Southgate, L.; Eichstaedt, C.A.; Aldred, M.A.; Austin, E.D.; Best, D.H.; Chung, W.K.; Benjamin, N.; Elliott, C.G.; Eyries, M.; et al. Pulmonary Arterial Hypertension: A Current Perspective on Established and Emerging Molecular Genetic Defects. Hum. Mutat. 2015, 36, 1113–1127. [Google Scholar] [CrossRef]
- Andruska, A.; Spiekerkoetter, E. Consequences of BMPR2 Deficiency in the Pulmonary Vasculature and Beyond: Contributions to Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 2499. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Harper, R.L.; Reynolds, P.N. BMPR2 gene delivery reduces mutation-related PAH and counteracts TGF-beta-mediated pulmonary cell signalling. Respirology 2016, 21, 526–532. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Xia, W.; Holmes, M.D.; Hodge, S.J.; Danilov, S.; Curiel, D.T.; Morrell, N.W.; Reynolds, P.N. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1182–L1192. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, M.S.; Moudgil, R.; Hashimoto, K.; Bonnet, S.; Michelakis, E.D.; Archer, S.L. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L872–L878. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.L.; Reynolds, A.M.; Bonder, C.S.; Reynolds, P.N. BMPR2 gene therapy for PAH acts via Smad and non-Smad signalling. Respirology 2016, 21, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Orriols, M.; Gomez-Puerto, M.C.; Ten Dijke, P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol. Life Sci. 2017, 74, 2979–2995. [Google Scholar] [CrossRef] [PubMed]
- Bisserier, M.; Katz, M.G.; Bueno-Beti, C.; Brojakowska, A.; Zhang, S.; Gubara, S.; Kohlbrenner, E.; Fazal, S.; Fargnoli, A.; Dorfmuller, P.; et al. Combination Therapy with STAT3 Inhibitor Enhances SERCA2a-Induced BMPR2 Expression and Inhibits Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 9105. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.D.; Chan, S.Y. Emerging Metabolic Therapies in Pulmonary Arterial Hypertension. J. Clin. Med. 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA control of cell death and survival. Cell Calcium. 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Lipskaia, L.; del Monte, F.; Capiod, T.; Yacoubi, S.; Hadri, L.; Hours, M.; Hajjar, R.J.; Lompre, A.M. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ. Res. 2005, 97, 488–495. [Google Scholar] [CrossRef]
- Hadri, L.; Kratlian, R.G.; Benard, L.; Maron, B.A.; Dorfmuller, P.; Ladage, D.; Guignabert, C.; Ishikawa, K.; Aguero, J.; Ibanez, B.; et al. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation 2013, 128, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Strauss, B.; Sassi, Y.; Bueno-Beti, C.; Ilkan, Z.; Raad, N.; Cacheux, M.; Bisserier, M.; Turnbull, I.C.; Kohlbrenner, E.; Hajjar, R.J.; et al. Intra-tracheal gene delivery of aerosolized SERCA2a to the lung suppresses ventricular arrhythmias in a model of pulmonary arterial hypertension. J. Mol. Cell Cardiol. 2019, 127, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Strauss, B.; Bisserier, M.; Obus, E.; Katz, M.G.; Fargnoli, A.; Cacheux, M.; Akar, J.G.; Hummel, J.P.; Hadri, L.; Sassi, Y.; et al. Right predominant electrical remodeling in a pure model of pulmonary hypertension promotes reentrant arrhythmias. Heart Rhythm. 2022, 19, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ishikawa, K.; Plataki, M.; Bikou, O.; Kohlbrenner, E.; Aguero, J.; Hadri, L.; Zarragoikoetxea, I.; Fish, K.; Leopold, J.A.; et al. Safety and long-term efficacy of AAV1.SERCA2a using nebulizer delivery in a pig model of pulmonary hypertension. Pulm. Circ. 2018, 8, 2045894018799738. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.G.; Fargnoli, A.S.; Gubara, S.M.; Bisserier, M.; Sassi, Y.; Bridges, C.R.; Hajjar, R.J.; Hadri, L. The Left Pneumonectomy Combined with Monocrotaline or Sugen as a Model of Pulmonary Hypertension in Rats. J. Vis. Exp. 2019, 10, 59050. [Google Scholar] [CrossRef] [PubMed]
- Grzenda, A.; Lomberk, G.; Zhang, J.S.; Urrutia, R. Sin3: Master scaffold and transcriptional corepressor. Biochim. Biophys. Acta 2009, 1789, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Bisserier, M.; Mathiyalagan, P.; Zhang, S.; Elmastour, F.; Dorfmuller, P.; Humbert, M.; David, G.; Tarzami, S.; Weber, T.; Perros, F.; et al. Regulation of the Methylation and Expression Levels of the BMPR2 Gene by SIN3a as a Novel Therapeutic Mechanism in Pulmonary Arterial Hypertension. Circulation 2021, 144, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.G.; Najjar, S.F.; Kapadia, M.R.; Murar, J.; Eng, J.; Lyle, B.; Aalami, O.O.; Jiang, Q.; Hrabie, J.A.; Saavedra, J.E.; et al. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic. Biol. Med. 2008, 44, 73–81. [Google Scholar] [CrossRef]
- Janssens, S.P.; Bloch, K.D.; Nong, Z.; Gerard, R.D.; Zoldhelyi, P.; Collen, D. Adenoviral-mediated transfer of the human endothelial nitric oxide synthase gene reduces acute hypoxic pulmonary vasoconstriction in rats. J. Clin. Investig. 1996, 98, 317–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Budts, W.; Pokreisz, P.; Nong, Z.; Van Pelt, N.; Gillijns, H.; Gerard, R.; Lyons, R.; Collen, D.; Bloch, K.D.; Janssens, S. Aerosol gene transfer with inducible nitric oxide synthase reduces hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation 2000, 102, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Du, L.; Hu, Y.; Fan, Y.; Xu, Q. Stem/Progenitor Cells and Pulmonary Arterial Hypertension. Arterioscler Thromb. Vasc. Biol. 2021, 41, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, R.; Michelakis, E.D.; Archer, S.L. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: Implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation 2006, 13, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.L.; Platoshyn, O.; Brevnova, E.E.; Burg, E.D.; Powell, F.; Haddad, G.H.; Yuan, J.X. Hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Ann. N. Y. Acad. Sci. 2009, 1177, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Platoshyn, O.; Brevnova, E.E.; Burg, E.D.; Yu, Y.; Remillard, C.V.; Yuan, J.X. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 2006, 290, C907–C916. [Google Scholar] [CrossRef] [PubMed]
- Brevnova, E.E.; Platoshyn, O.; Zhang, S.; Yuan, J.X. Overexpression of human KCNA5 increases IK V and enhances apoptosis. Am. J. Physiol. Cell Physiol. 2004, 287, C715–C722. [Google Scholar] [CrossRef] [PubMed]
- Pozeg, Z.I.; Michelakis, E.D.; McMurtry, M.S.; Thebaud, B.; Wu, X.C.; Dyck, J.R.; Hashimoto, K.; Wang, S.; Moudgil, R.; Harry, G.; et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 2003, 107, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, M.S.; Archer, S.L.; Altieri, D.C.; Bonnet, S.; Haromy, A.; Harry, G.; Bonnet, S.; Puttagunta, L.; Michelakis, E.D. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J. Clin. Investig. 2005, 115, 1479–1491. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Cao, L.; Han, H.; Wang, J.; Yang, B.; Nattel, S.; Wang, Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002, 62, 4843–4848. [Google Scholar]
- Petkov, V.; Mosgoeller, W.; Ziesche, R.; Raderer, M.; Stiebellehner, L.; Vonbank, K.; Funk, G.C.; Hamilton, G.; Novotny, C.; Burian, B.; et al. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J. Clin. Investig. 2003, 111, 1339–1346. [Google Scholar] [CrossRef]
- Haberl, I.; Frei, K.; Ramsebner, R.; Doberer, D.; Petkov, V.; Albinni, S.; Lang, I.; Lucas, T.; Mosgoeller, W. Vasoactive intestinal peptide gene alterations in patients with idiopathic pulmonary arterial hypertension. Eur. J. Hum. Genet. 2007, 15, 18–22. [Google Scholar] [CrossRef]
- Said, S.I.; Hamidi, S.A.; Dickman, K.G.; Szema, A.M.; Lyubsky, S.; Lin, R.Z.; Jiang, Y.P.; Chen, J.J.; Waschek, J.A.; Kort, S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 2007, 115, 1260–1268. [Google Scholar] [CrossRef]
- St Hilaire, R.C.; Kadowitz, P.J.; Jeter, J.R., Jr. Adenoviral transfer of vasoactive intestinal peptide (VIP) gene inhibits rat aortic and pulmonary artery smooth muscle cell proliferation. Peptides 2009, 30, 2323–2329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tjen, A.L.S.; Kraiczi, H.; Ekman, R.; Keith, I.M. Sensory CGRP depletion by capsaicin exacerbates hypoxia-induced pulmonary hypertension in rats. Regul. Pept. 1998, 74, 1–10. [Google Scholar] [CrossRef]
- Chattergoon, N.N.; D’Souza, F.M.; Deng, W.; Chen, H.; Hyman, A.L.; Kadowitz, P.J.; Jeter, J.R., Jr. Antiproliferative effects of calcitonin gene-related peptide in aortic and pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L202–L211. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.C.; Bivalacqua, T.J.; Toyoda, K.; Heistad, D.D.; Hyman, A.L.; Kadowitz, P.J. In vivo gene transfer of prepro-calcitonin gene-related peptide to the lung attenuates chronic hypoxia-induced pulmonary hypertension in the mouse. Circulation 2000, 101, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Bivalacqua, T.J.; Hyman, A.L.; Kadowitz, P.J.; Paolocci, N.; Kass, D.A.; Champion, H.C. Role of calcitonin gene-related peptide (CGRP) in chronic hypoxia-induced pulmonary hypertension in the mouse. Influence of gene transfer in vivo. Regul. Pept. 2002, 108, 129–133. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Wang, Z.; Yang, C.; Liu, J.; Lu, J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann. Thorac. Surg. 2007, 84, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Gunther, A.; Korfei, M.; Mahavadi, P.; von der Beck, D.; Ruppert, C.; Markart, P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2012, 21, 152–160. [Google Scholar] [CrossRef]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Bisserier, M.; Milara, J.; Abdeldjebbar, Y.; Gubara, S.; Jones, C.; Bueno-Beti, C.; Chepurko, E.; Kohlbrenner, E.; Katz, M.G.; Tarzami, S.; et al. AAV1.SERCA2a Gene Therapy Reverses Pulmonary Fibrosis by Blocking the STAT3/FOXM1 Pathway and Promoting the SNON/SKI Axis. Mol. Ther. 2020, 28, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Bisserier, M.; Hadri, L. Lung-targeted SERCA2a Gene Therapy: From Discovery to Therapeutic Application in Bleomycin-Induced Pulmonary Fibrosis. J. Cell Immunol. 2020, 2, 149–156. [Google Scholar] [PubMed]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.S.; Glenney, J.R.; Anderson, R.G. Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Bucci, M.; Gratton, J.P.; Rudic, R.D.; Acevedo, L.; Roviezzo, F.; Cirino, G.; Sessa, W.C. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. 2000, 6, 1362–1367. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, Y.; Kim, H.P.; Zhou, Z.; Feghali-Bostwick, C.A.; Liu, F.; Ifedigbo, E.; Xu, X.; Oury, T.D.; Kaminski, N.; et al. Caveolin-1: A critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. 2006, 203, 2895–2906. [Google Scholar] [CrossRef]
- Nakao, A.; Fujii, M.; Matsumura, R.; Kumano, K.; Saito, Y.; Miyazono, K.; Iwamoto, I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Investig. 1999, 104, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, A.J.; Dobrovic, A.; Cooper, W.A. TERT gene: Its function and dysregulation in cancer. J. Clin. Pathol. 2019, 72, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.M.; Morin, G.B.; Chapman, K.B.; Weinrich, S.L.; Andrews, W.H.; Lingner, J.; Harley, C.B.; Cech, T.R. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277, 955–959. [Google Scholar] [CrossRef]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef]
- Povedano, J.M.; Martinez, P.; Serrano, R.; Tejera, A.; Gomez-Lopez, G.; Bobadilla, M.; Flores, J.M.; Bosch, F.; Blasco, M.A. Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres. Elife 2018, 7, 31299. [Google Scholar] [CrossRef]
- Brown, S.D.; White, R.; Tobin, P. Keep them breathing: Cystic fibrosis pathophysiology, diagnosis, and treatment. JAAPA 2017, 30, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, C.; Davies, J.C. Current and future treatment options for cystic fibrosis lung disease: Latest evidence and clinical implications. Ther. Adv. Chronic. Dis. 2016, 7, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Caplan, A.I. Adult mesenchymal stem cells: An innovative therapeutic for lung diseases. Discov. Med. 2010, 9, 337–345. [Google Scholar]
- Cambraia, A.; Junior, M.C.; Zembrzuski, V.M.; Junqueira, R.M.; Cabello, P.H.; de Cabello, G.M.K. Next-Generation Sequencing for Molecular Diagnosis of Cystic Fibrosis in a Brazilian Cohort. Dis. Markers 2021, 2021, 9812074. [Google Scholar] [CrossRef] [PubMed]
- Donnelley, M.; Parsons, D.W. Gene Therapy for Cystic Fibrosis Lung Disease: Overcoming the Barriers to Translation to the Clinic. Front. Pharmacol. 2018, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Couture, L.A.; Gregory, R.J.; Graham, S.M.; Smith, A.E.; Welsh, M.J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993, 75, 207–216. [Google Scholar] [CrossRef]
- Knowles, M.R.; Hohneker, K.W.; Zhou, Z.; Olsen, J.C.; Noah, T.L.; Hu, P.C.; Leigh, M.W.; Engelhardt, J.F.; Edwards, L.J.; Jones, K.R.; et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 1995, 333, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.G.; McElvaney, N.G.; Herena, J.; Crystal, R.G. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum. Gene Ther. 1995, 6, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Ramsey, B.W.; Meeker, D.P.; Aitken, M.L.; Balfour, R.P.; Gibson, R.L.; Launspach, J.; Moscicki, R.A.; Richards, S.M.; Standaert, T.A.; et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Investig. 1996, 97, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Singh, B.K.; Loza, L.M.; Thornell, I.M.; Hippee, C.E.; Powers, L.S.; Ostedgaard, L.S.; Meyerholz, D.K.; Wohlford-Lenane, C.; Stoltz, D.A.; et al. Widespread airway distribution and short-term phenotypic correction of cystic fibrosis pigs following aerosol delivery of piggyBac/adenovirus. Nucleic Acids Res. 2018, 46, 9591–9600. [Google Scholar] [CrossRef] [PubMed]
- Guggino, W.B.; Cebotaru, L. Adeno-Associated Virus (AAV) gene therapy for cystic fibrosis: Current barriers and recent developments. Expert. Opin. Biol. Ther. 2017, 17, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified mRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Salahudeen, A.A.; Sellers, Z.M.; Bravo, D.T.; Choi, S.S.; Batish, A.; Le, W.; Baik, R.; de la, O.S.; Kaushik, M.P.; et al. High-Efficiency, Selection-free Gene Repair in Airway Stem Cells from Cystic Fibrosis Patients Rescues CFTR Function in Differentiated Epithelia. Cell Stem Cell 2020, 26, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Mention, K.; Cavusoglu-Doran, K.; Sanz, D.J.; Bacalhau, M.; Lopes-Pacheco, M.; Harrison, P.T.; Farinha, C.M. Comparison of Cas9 and Cas12a CRISPR editing methods to correct the W1282X-CFTR mutation. J. Cyst. Fibros. 2022, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Oren, Y.S.; Avizur-Barchad, O.; Ozeri-Galai, E.; Elgrabli, R.; Schirelman, M.R.; Blinder, T.; Stampfer, C.D.; Ordan, M.; Laselva, O.; Cohen-Cymberknoh, M.; et al. Antisense oligonucleotide splicing modulation as a novel Cystic Fibrosis therapeutic approach for the W1282X nonsense mutation. J. Cyst. Fibros. 2021, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sivetz, N.; Layne, J.; Voss, D.M.; Yang, L.; Zhang, Q.; Krainer, A.R. Exon-skipping antisense oligonucleotides for cystic fibrosis therapy. Proc. Natl. Acad. Sci. USA 2022, 119, 118. [Google Scholar] [CrossRef] [PubMed]
- Limb, S.L.; Brown, K.C.; Wood, R.A.; Wise, R.A.; Eggleston, P.A.; Tonascia, J.; Adkinson, N.F., Jr. Irreversible lung function deficits in young adults with a history of childhood asthma. J. Allergy Clin. Immunol. 2005, 116, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.; Doberer, D.; Eber, E.; Horak, E.; Pohl, W.; Riedler, J.; Szepfalusi, Z.; Wantke, F.; Zacharasiewicz, A.; Studnicka, M. Diagnosis and management of asthma—Statement on the 2015 GINA Guidelines. Wien. Klin. Wochenschr. 2016, 128, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ghaffar, O.; Olivensstein, R.; Taha, R.A.; Soussi-Gounni, A.; Zhang, D.H.; Ray, A.; Hamid, Q. Gene expression of the GATA-3 transcription factor is increased in atopic asthma. J. Allergy Clin. Immunol. 1999, 103, 215–222. [Google Scholar] [CrossRef]
- Sel, S.; Wegmann, M.; Dicke, T.; Sel, S.; Henke, W.; Yildirim, A.O.; Renz, H.; Garn, H. Effective prevention and therapy of experimental allergic asthma using a GATA-3-specific DNAzyme. J. Allergy Clin. Immunol. 2008, 121, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Homburg, U.; Renz, H.; Timmer, W.; Hohlfeld, J.M.; Seitz, F.; Luer, K.; Mayer, A.; Wacker, A.; Schmidt, O.; Kuhlmann, J.; et al. Safety and tolerability of a novel inhaled GATA3 mRNA targeting DNAzyme in patients with TH2-driven asthma. J. Allergy Clin. Immunol. 2015, 136, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Renz, H. GATA-3-specific DNAzyme—A novel approach for stratified asthma therapy. Eur. J. Immunol. 2017, 47, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yara, S.; Kawakami, K.; Kudeken, N.; Tohyama, M.; Teruya, K.; Chinen, T.; Awaya, A.; Saito, A. FTS reduces bleomycin-induced cytokine and chemokine production and inhibits pulmonary fibrosis in mice. Clin. Exp. Immunol. 2001, 124, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Savino, W.; Dardenne, M. Neuroendocrine control of thymus physiology. Endocr. Rev. 2000, 21, 412–443. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.L.; Martini, S.V.; Abreu, S.C.; Samary Cdos, S.; Diaz, B.L.; Fernezlian, S.; de Sa, V.K.; Capelozzi, V.L.; Boylan, N.J.; Goya, R.G.; et al. DNA nanoparticle-mediated thymulin gene therapy prevents airway remodeling in experimental allergic asthma. J. Control. Release 2014, 180, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.R.; Mannino, D.M.; Soriano, J.B.; Vermeire, P.A.; Buist, A.S.; Thun, M.J.; Connell, C.; Jemal, A.; Lee, T.A.; Miravitlles, M.; et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur. Respir. J. 2006, 27, 188–207. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P. Pharmacological treatment of chronic obstructive pulmonary disease. Int J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Phougat, N.; Ruhil, S.; Dhankhar, S.; Balhara, M.; Chhillar, A.K. Genomics of Chronic Obstructive Pulmonary Disease (COPD); Exploring the SNPs of Protease-Antiprotease Pathway. Curr. Genomics 2013, 14, 204–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rab, A.; Rowe, S.M.; Raju, S.V.; Bebok, Z.; Matalon, S.; Collawn, J.F. Cigarette smoke and CFTR: Implications in the pathogenesis of COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L530–L541. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duran, M.; Lopez-Campos, J.L.; Barrecheguren, M.; Miravitlles, M.; Martinez-Delgado, B.; Castillo, S.; Escribano, A.; Baloira, A.; Navarro-Garcia, M.M.; Pellicer, D.; et al. Alpha-1 antitrypsin deficiency: Outstanding questions and future directions. Orphanet J. Rare Dis. 2018, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Dunlea, D.M.; Fee, L.T.; McEnery, T.; McElvaney, N.G.; Reeves, E.P. The impact of alpha-1 antitrypsin augmentation therapy on neutrophil-driven respiratory disease in deficient individuals. J. Inflamm Res. 2018, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Seixas, S.; Marques, P.I. Known Mutations at the Cause of Alpha-1 Antitrypsin Deficiency an Updated Overview of SERPINA1 Variation Spectrum. Appl. Clin. Genet. 2021, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Siegfried, W.; Yoshimura, K.; Yoneyama, K.; Fukayama, M.; Stier, L.E.; Paakko, P.K.; Gilardi, P.; Stratford-Perricaudet, L.D.; Perricaudet, M.; et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 1991, 252, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Brantly, M.L.; Chulay, J.D.; Wang, L.; Mueller, C.; Humphries, M.; Spencer, L.T.; Rouhani, F.; Conlon, T.J.; Calcedo, R.; Betts, M.R.; et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 16363–16368. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Choi, Y.K.; Campbell-Thompson, M.; Li, C.; Tang, Q.; Crawford, J.M.; Flotte, T.R.; Song, S. Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J. Gene Med. 2006, 8, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Brantly, M.L.; Spencer, L.T.; Humphries, M.; Conlon, T.J.; Spencer, C.T.; Poirier, A.; Garlington, W.; Baker, D.; Song, S.; Berns, K.I.; et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006, 17, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Trapnell, B.C.; Humphries, M.; Carey, B.; Calcedo, R.; Rouhani, F.; Campbell-Thompson, M.; Yachnis, A.T.; Sandhaus, R.A.; McElvaney, N.G.; et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: Interim results. Hum. Gene Ther. 2011, 22, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Chiuchiolo, M.J.; Kaminsky, S.M.; Sondhi, D.; Mancenido, D.; Hollmann, C.; Crystal, R.G. Phase I/II study of intrapleural administration of a serotype rh.10 replication-deficient adeno-associated virus gene transfer vector expressing the human alpha1-antitrypsin cDNA to individuals with alpha1-antitrypsin deficiency. Hum. Gene Ther. Clin. Dev. 2014, 25, 112–133. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Lara-Guerra, H.; Roth, J.A. Gene Therapy for Lung Cancer. Crit. Rev. Oncog. 2016, 21, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Cai, D.W.; Georges, R.N.; Mukhopadhyay, T.; Grimm, E.A.; Roth, J.A. Therapeutic effect of a retroviral wild-type p53 expression vector in an orthotopic lung cancer model. J. Natl. Cancer Inst. 1994, 86, 1458–1462. [Google Scholar] [CrossRef]
- Zhang, W.W.; Fang, X.; Mazur, W.; French, B.A.; Georges, R.N.; Roth, J.A. High-efficiency gene transfer and high-level expression of wild-type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther. 1994, 1, 5–13. [Google Scholar] [PubMed]
- Bouvet, M.; Fang, B.; Ekmekcioglu, S.; Ji, L.; Bucana, C.D.; Hamada, K.; Grimm, E.A.; Roth, J.A. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low-dose etoposide. Gene Ther. 1998, 5, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Weill, D.; Mack, M.; Roth, J.; Swisher, S.; Proksch, S.; Merritt, J.; Nemunaitis, J. Adenoviral-mediated p53 gene transfer to non-small cell lung cancer through endobronchial injection. Chest 2000, 118, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Nguyen, D.; Lawrence, D.D.; Kemp, B.L.; Carrasco, C.H.; Ferson, D.Z.; Hong, W.K.; Komaki, R.; Lee, J.J.; Nesbitt, J.C.; et al. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat. Med. 1996, 2, 985–991. [Google Scholar] [CrossRef]
- Swisher, S.G.; Roth, J.A.; Nemunaitis, J.; Lawrence, D.D.; Kemp, B.L.; Carrasco, C.H.; Connors, D.G.; El-Naggar, A.K.; Fossella, F.; Glisson, B.S.; et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 1999, 91, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Mhashilkar, A.; Swanson, X.; Zou-Yang, X.H.; Sieger, K.; Kawabe, S.; Branch, C.D.; Zumstein, L.; Meyn, R.E.; Roth, J.A.; et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene 2002, 21, 4558–4566. [Google Scholar] [CrossRef] [PubMed]
- Lerman, M.I.; Minna, J.D. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: Identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000, 60, 6116–6133. [Google Scholar]
- Ito, I.; Ji, L.; Tanaka, F.; Saito, Y.; Gopalan, B.; Branch, C.D.; Xu, K.; Atkinson, E.N.; Bekele, B.N.; Stephens, L.C.; et al. Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Ther. 2004, 11, 733–739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, C.; Stewart, D.J.; Lee, J.J.; Ji, L.; Ramesh, R.; Jayachandran, G.; Nunez, M.I.; Wistuba, I.; Erasmus, J.J.; Hicks, M.E.; et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE 2012, 7, e34833. [Google Scholar] [CrossRef]
- Hamouche, W.; Bisserier, M.; Brojakowska, A.; Eskandari, A.; Fish, K.; Goukassian, D.A.; Hadri, L. Pathophysiology and pharmacological management of pulmonary and cardiovascular features of COVID-19. J. Mol. Cell Cardiol. 2021, 153, 72–85. [Google Scholar] [CrossRef]
- Brojakowska, A.; Eskandari, A.; Bisserier, M.; Bander, J.; Garikipati, V.N.S.; Hadri, L.; Goukassian, D.A.; Fish, K.M. Comorbidities, sequelae, blood biomarkers and their associated clinical outcomes in the Mount Sinai Health System COVID-19 patients. PLoS ONE 2021, 16, e0253660. [Google Scholar] [CrossRef]
- Eskandari, A.; Brojakowska, A.; Bisserier, M.; Bander, J.; Garikipati, V.N.S.; Hadri, L.; Goukassian, D.; Fish, K. Retrospective analysis of demographic factors in COVID-19 patients entering the Mount Sinai Health System. PLoS ONE 2021, 16, e0254707. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Costa Verdera, H.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J., Jr.; et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13, 3438. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Hadas, Y.; Sharkar, M.T.K.; Kaur, K.; Magadum, A.; Kurian, A.A.; Hossain, N.; Alburquerque, B.; Ahmed, S.; Chepurko, E.; et al. Optimization of 5’ Untranslated Region of Modified mRNA for Use in Cardiac or Hepatic Ischemic Injury. Mol. Ther. Methods Clin. Dev. 2020, 17, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, M.; Kherad, N. Advance genome editing technologies in the treatment of human diseases: CRISPR therapy (Review). Int J. Mol. Med. 2020, 46, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Cardenes, N.; Sembrat, J.; Noda, K.; Lovelace, T.; Alvarez, D.; Bittar, H.E.T.; Philips, B.J.; Nouraie, M.; Benos, P.V.; Sanchez, P.G.; et al. Human ex vivo lung perfusion: A novel model to study human lung diseases. Sci. Rep. 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Alysandratos, K.D.; Russo, S.J.; Petcherski, A.; Taddeo, E.P.; Acin-Perez, R.; Villacorta-Martin, C.; Jean, J.C.; Mulugeta, S.; Rodriguez, L.R.; Blum, B.C.; et al. Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep. 2021, 36, 109636. [Google Scholar] [CrossRef] [PubMed]
| Gene | PH Models | Vector | Delivery Methods | Results | References |
|---|---|---|---|---|---|
| BMPR2 | Chronic hypoxia Monocrotaline Sugen/hypoxia Pneumonectomy/monocrotaline | Adenovirus AAV1 | Intravenous Intratracheal | ↓ RVSP ↓ mPAP ↓ RV hypertrophy ↓ Vascular remodeling | [90,91,92,93,94,95] |
| SERCA2A | Sugen/hypoxia Monocrotaline Pneumonectomy/monocrotaline Pulmonary vein banding | AAV1 | Intratracheal | ↓ RVSP ↓ mPAP ↓ RV hypertrophy ↓ Vascular remodeling ↑ Cardiac function ↓ PVR | [95,99,100,101,102,103] |
| SIN3A | Sugen/hypoxia Monocrotaline | AAV1 | Intratracheal | ↓ RVSP ↓ mPAP ↓ RV hypertrophy ↓ Vascular remodeling | [105] |
| ENOS | Chronic hypoxia | Adenovirus | Intratracheal | ↓ mPAP ↓ RV hypertrophy ↓ PVR | [107] |
| KV1.5 | Chronic hypoxia | Adenovirus | Intratracheal | ↑ Cardiac function ↓ PVR ↓ RV hypertrophy ↓ Vasoconstriction | [114] |
| SURVIVIN | Monocrotaline | Adenovirus | Intratracheal | ↓Vascular remodeling ↓ PVR ↓ RV hypertrophy | [115] |
| CGRP | Chronic hypoxia | Adenovirus | Intratracheal | ↓RV hypertrophy ↓Vascular remodeling ↓ PVR | [123,124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisserier, M.; Sun, X.-Q.; Fazal, S.; Turnbull, I.C.; Bonnet, S.; Hadri, L. Novel Insights into the Therapeutic Potential of Lung-Targeted Gene Transfer in the Most Common Respiratory Diseases. Cells 2022, 11, 984. https://doi.org/10.3390/cells11060984
Bisserier M, Sun X-Q, Fazal S, Turnbull IC, Bonnet S, Hadri L. Novel Insights into the Therapeutic Potential of Lung-Targeted Gene Transfer in the Most Common Respiratory Diseases. Cells. 2022; 11(6):984. https://doi.org/10.3390/cells11060984
Chicago/Turabian StyleBisserier, Malik, Xiao-Qing Sun, Shahood Fazal, Irene C. Turnbull, Sébastien Bonnet, and Lahouaria Hadri. 2022. "Novel Insights into the Therapeutic Potential of Lung-Targeted Gene Transfer in the Most Common Respiratory Diseases" Cells 11, no. 6: 984. https://doi.org/10.3390/cells11060984
APA StyleBisserier, M., Sun, X.-Q., Fazal, S., Turnbull, I. C., Bonnet, S., & Hadri, L. (2022). Novel Insights into the Therapeutic Potential of Lung-Targeted Gene Transfer in the Most Common Respiratory Diseases. Cells, 11(6), 984. https://doi.org/10.3390/cells11060984










