Iron Regulatory Protein 1 Inhibits Ferritin Translation Responding to OsHV-1 Infection in Ark Clams, Scapharca Broughtonii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Challenge Experiment
2.2. Total RNA Extraction and cDNA Synthesis
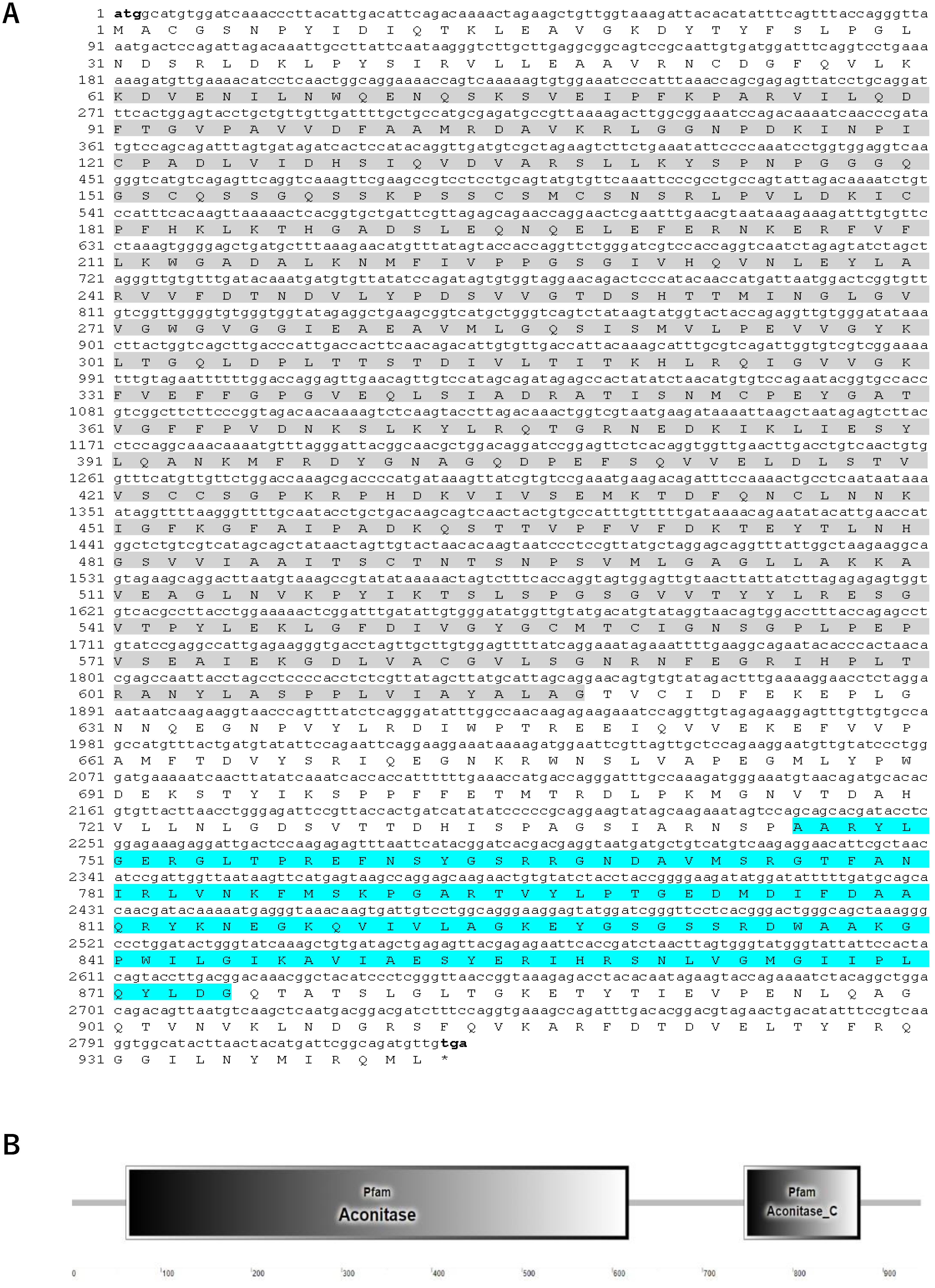
2.3. Clone and Bioinformatic Analyses of SbIRP-1
2.4. RT-qPCR Analysis of SbIRP-1 and SbFn mRNA
2.5. SbIRP-1 Recombinant Protein Expression and Polyclonal Antibody Preparation
2.6. The Knockdown of SbIRP-1
2.7. Electrophoretic Mobility Shift Assay
2.8. RNA Immunoprecipitation
2.9. Western Blot
2.10. Statistical Analysis
3. Results
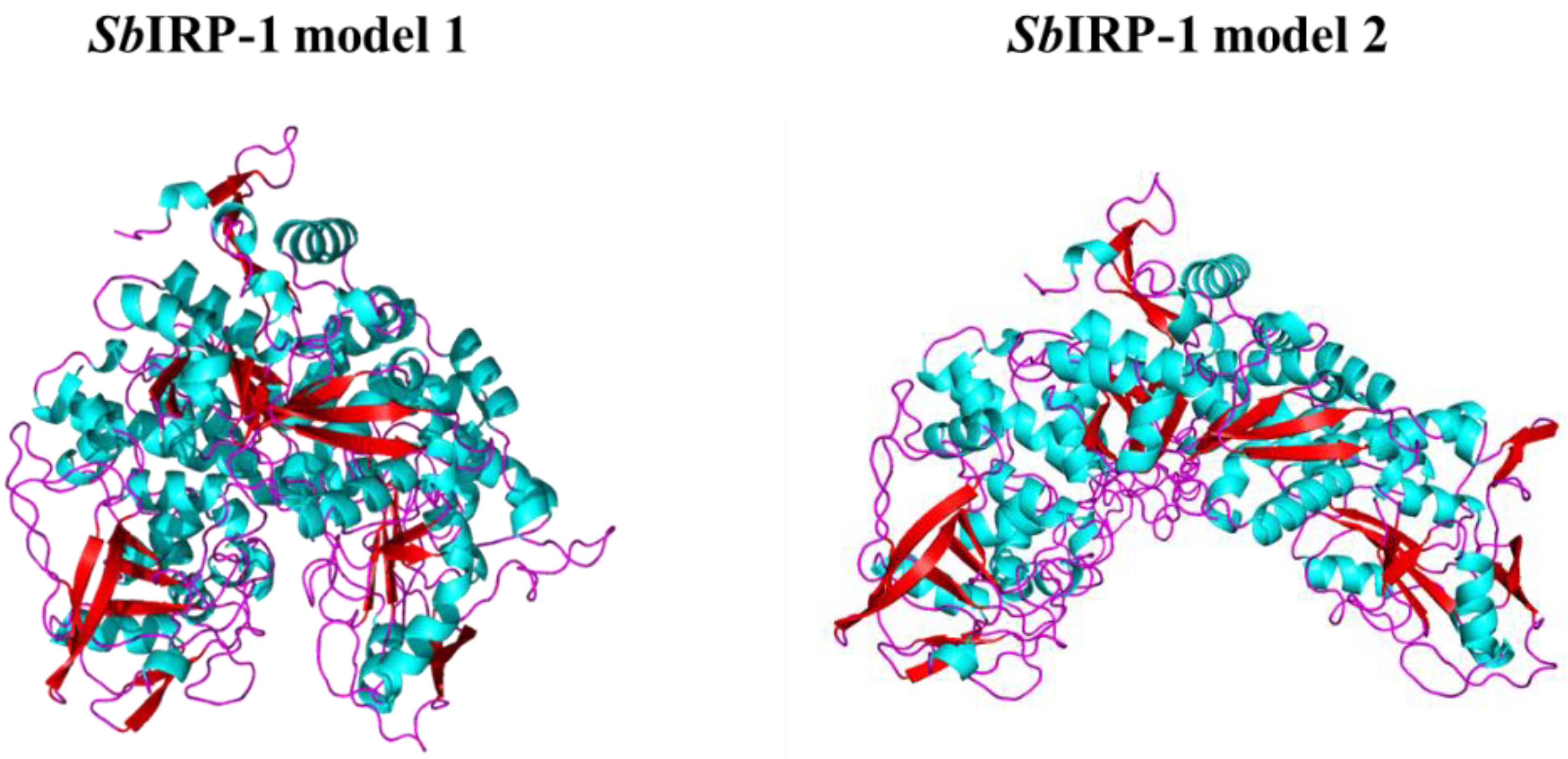
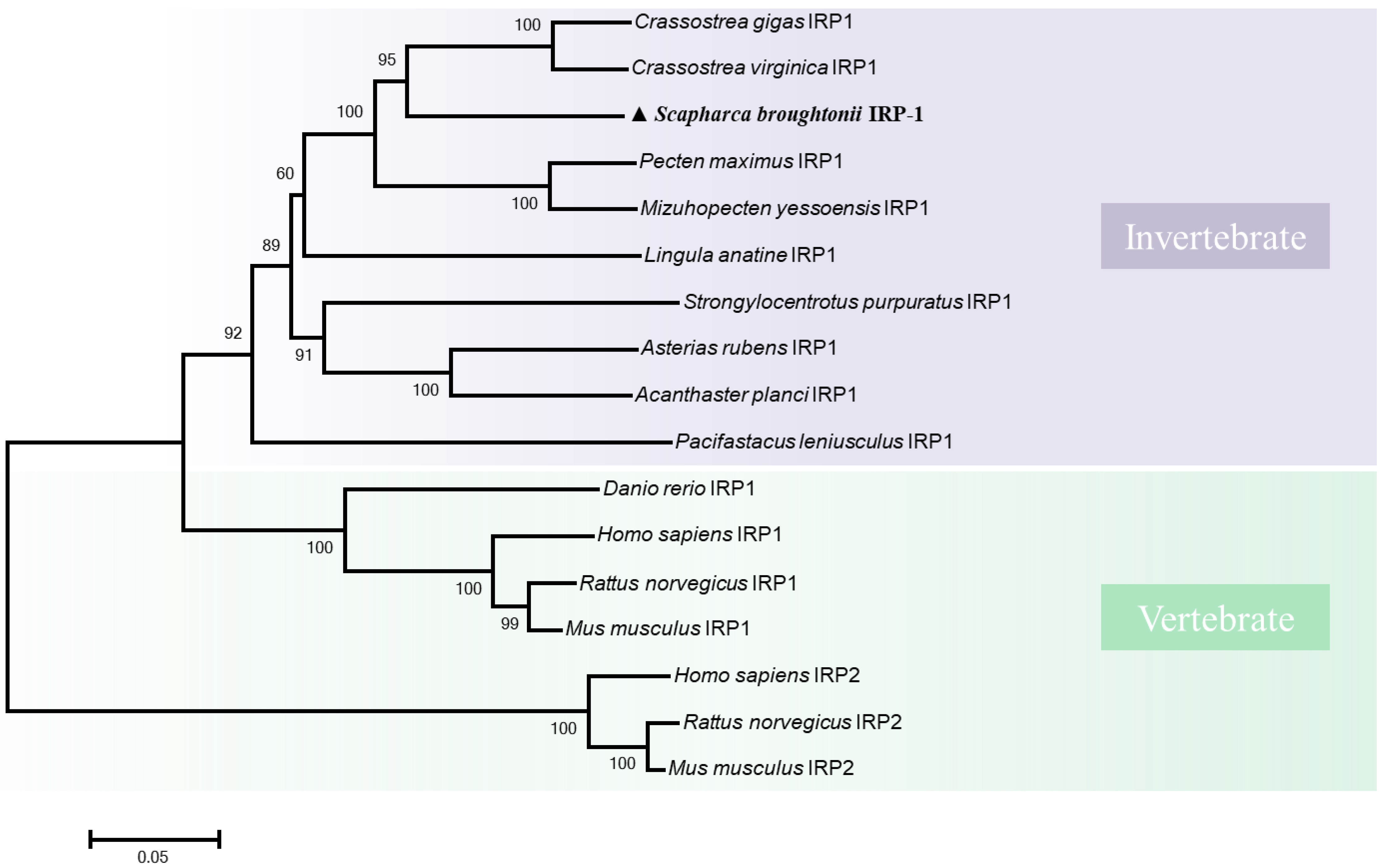
3.1. Molecular Characterization of SbIRP-1 Statistical Analysis
3.2. Tissue Distribution and Expression Profile Post OsHV-1 Infection of SbIRP-1
3.3. Recombinant Expression, Purification and Antiserum Preparation of SbIRP-1
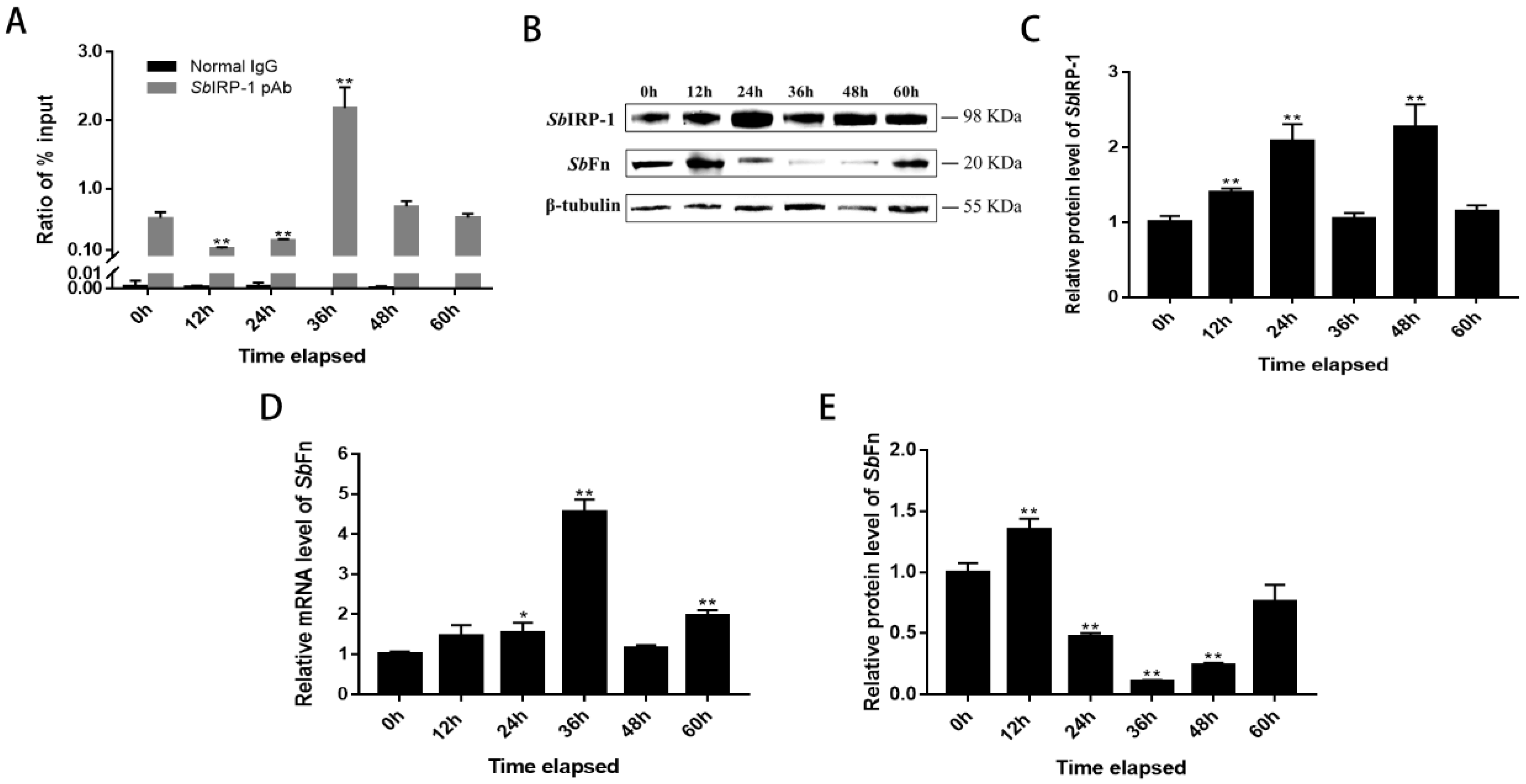
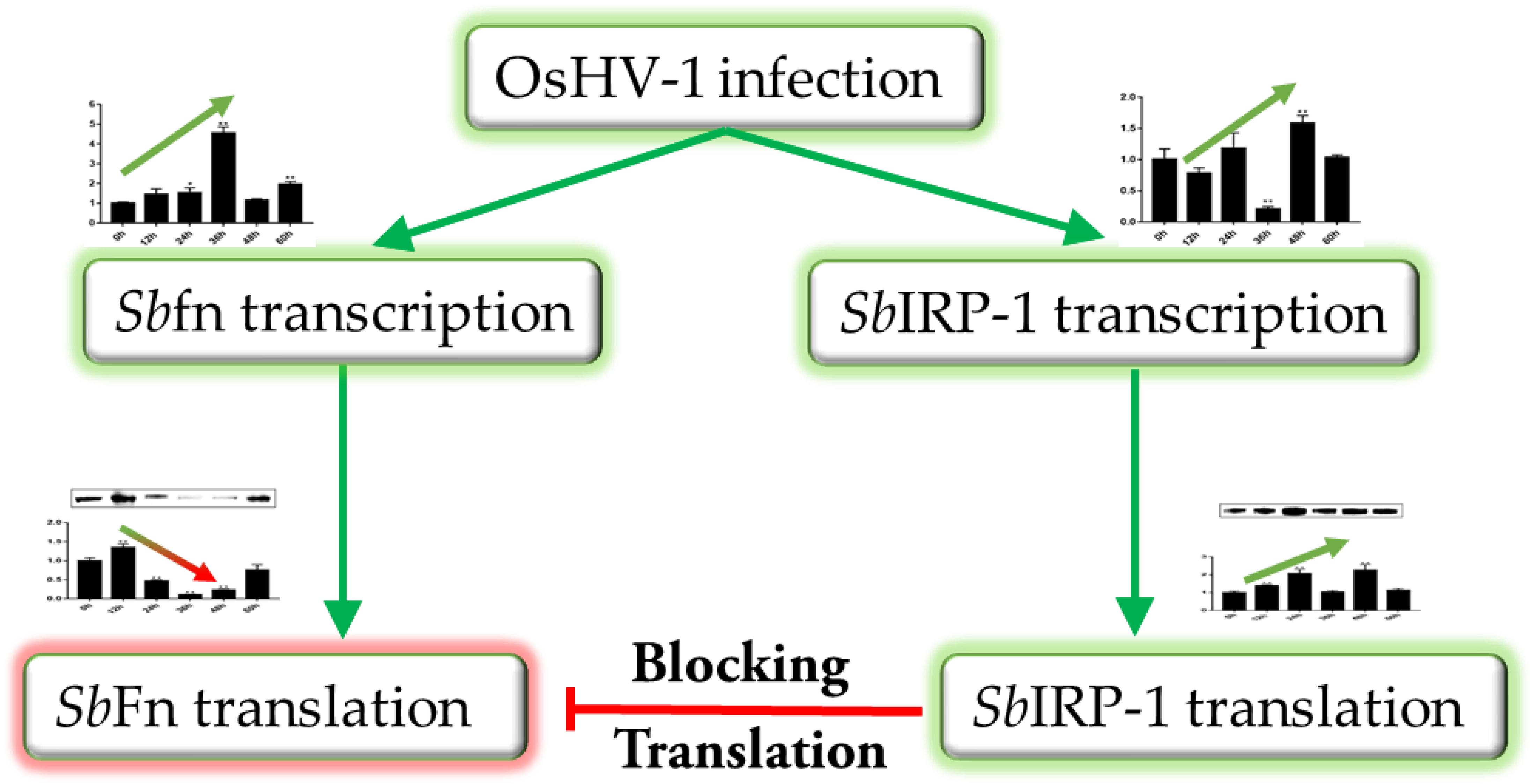
3.4. The Role of SbIRP-1 on the Post-Transcriptional Regulation of SbFn
3.5. The Blocking Effect of SbIRP-1 on the Translation of SbFn Post OsHV-1 Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffettone, C.; De Martino, L.; Irace, C.; Santamaria, R.; Pagnini, U.; Iovane, G.; Colonna, A. Expression of iron-related proteins during infection by bovine herpes virus type-1. J. Cell. Biochem. 2008, 104, 213–223. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef]
- Boodhoo, N.; Kamble, N.; Sharif, S.; Behboudi, S. Glutaminolysis and glycolysis are essential for optimal replication of Marek’s disease virus. Virol. J. 2020, 94, e01680-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbe, J. Ribonucleotide reductases: Amazing and confusing. J. Biol. Chem. 1990, 265, 5329–5332. [Google Scholar] [CrossRef]
- Gammon, D.B.; Gowrishankar, B.; Duraffour, S.; Andrei, G.; Upton, C.; Evans, D.H. Vaccinia virus–encoded ribonucleotide reductase subunits are differentially required for replication and pathogenesis. PLoS Pathogens 2010, 6, e1000984. [Google Scholar] [CrossRef] [Green Version]
- Romeo, A.M.; Christen, L.; Niles, E.G.; Kosman, D.J. Intracellular chelation of iron by bipyridyl inhibits DNA virus replication: Ribonucleotide reductase maturation as a probe of intracellular iron pools. J. Biol. Chem. 2001, 276, 24301–24308. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.S.; Murphy, W.J.; Wang, D.; O’Brien, S.J.; Parrish, C.R. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. Virol. J. 2001, 75, 3896–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishina, S.; Hino, K.; Korenaga, M.; Vecchi, C.; Pietrangelo, A.; Mizukami, Y.; Furutani, T.; Sakai, A.; Okuda, M.; Hidaka, I. Hepatitis C virus–induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology 2008, 134, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; McCarthy, C.; Drakesmith, H.; Li, D.; Cerundolo, V.; McMichael, A.J.; Screaton, G.R.; Xu, X.N. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 2006, 36, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.W. The MHC class I antigen presentation pathway: Strategies for viral immune evasion. Immunology 2003, 110, 163–169. [Google Scholar] [CrossRef]
- Gu, J.-M.; Lim, S.O.; Oh, S.J.; Yoon, S.-M.; Seong, J.K.; Jung, G. HBx modulates iron regulatory protein 1-mediated iron metabolism via reactive oxygen species. Virus Res. 2008, 133, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.R.; Kühn, L.C.; Scraba, D.G. Induction of Ferritin Synthesis in Cells Infected with Mengo Virus (∗). J. Biol. Chem. 1996, 271, 9851–9857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2013, 1836, 245–254. [Google Scholar] [CrossRef] [PubMed]
- De Meulenaere, E.; Bailey, J.B.; Tezcan, F.A.; Deheyn, D.D. First biochemical and crystallographic characterization of a fast-performing ferritin from a marine invertebrate. Biochem. J. 2017, 474, 4193–4206. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta (BBA)-Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-Y.; Klausner, R.D.; Rouault, T.A. Translational Repressor Activity Is Equivalent and Is Quantitatively Predicted by in Vitro RNA Binding for Two Iron-responsive Element-binding Proteins, IRP1 and IRP2 (∗). J. Biol. Chem. 1995, 270, 4983–4986. [Google Scholar] [CrossRef] [Green Version]
- Paraskeva, E.; Hentze, M.W. Iron-sulphur clusters as genetic regulatory switches: The bifunctional iron regulatory protein-1. FEBS Lett. 1996, 389, 40–43. [Google Scholar] [CrossRef] [Green Version]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New insights into the role of ferritin in iron homeostasis and neurodegenerative diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar] [CrossRef]
- Hintze, K.; Theil, E. Cellular regulation and molecular interactions of the ferritins. Cell. Mol. Life Sci. 2006, 63, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, P.; Samuelsson, T. Evolution of the iron-responsive element. Rna 2007, 13, 952–966. [Google Scholar] [CrossRef] [Green Version]
- Lind, M.I.; Missirlis, F.; Melefors, O.; Uhrigshardt, H.; Kirby, K.; Phillips, J.P.; Söderhäll, K.; Rouault, T.A. Of two cytosolic aconitases expressed in Drosophila, only one functions as an iron-regulatory protein. J. Biol. Chem. 2006, 281, 18707–18714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Dimopoulos, G.; Wolf, A.; Minana, B.; Kafatos, F.; Winzerling, J.J. Cloning and molecular characterization of two mosquito iron regulatory proteins. Insect Biochem. Mol. Biol. 2002, 32, 579–589. [Google Scholar] [CrossRef]
- Huang, T.S.; Melefors, O.; Lind, M.I.; Söderhäll, K. An atypical iron-responsive element (IRE) within crayfish ferritin mRNA and an iron regulatory protein 1 (IRP1)-like protein from crayfish hepatopancreas. Insect Biochem. Mol. Biol. 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Prochazkova, P.; Škanta, F.; Roubalová, R.; Šilerová, M.; Dvořák, J.; Bilej, M. Involvement of the iron regulatory protein from Eisenia andrei earthworms in the regulation of cellular iron homeostasis. Plos ONE 2014, 9, e109900. [Google Scholar] [CrossRef]
- Zhang, D.; Albert, D.; Kohlhepp, P.; Pham, D.Q.D.; Winzerling, J.J. Repression of Manduca sexta ferritin synthesis by IRP1/IRE interaction. Insect Mol. Biol. 2001, 10, 531–539. [Google Scholar] [CrossRef]
- Zhang, D.; Ferris, C.; Gailer, J.; Kohlhepp, P.; Winzerling, J.J. Manduca sexta IRP1: Molecular characterization and in vivo response to iron. Insect Biochem. Mol. Biol. 2001, 32, 85–96. [Google Scholar] [CrossRef]
- Davison, A.J. Evolution of the herpesviruses. Vet. Microbiol. 2002, 86, 69–88. [Google Scholar] [CrossRef]
- Martenot, C.; Oden, E.; Travaillé, E.; Malas, J.-P.; Houssin, M. Detection of different variants of Ostreid Herpesvirus 1 in the Pacific oyster, Crassostrea gigas between 2008 and 2010. Virus Res. 2011, 160, 25–31. [Google Scholar] [CrossRef]
- Rosani, U.; Varotto, L.; Domeneghetti, S.; Arcangeli, G.; Pallavicini, A.; Venier, P. Dual analysis of host and pathogen transcriptomes in ostreid herpesvirus 1-positive C rassostrea gigas. Environ. Microbiol. 2015, 17, 4200–4212. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Huang, B.; Zhang, H.; Li, C.; Bai, C.; Wang, C. OsHV-1 infection leads to mollusc tissue lesion and iron redistribution, revealing a strategy of iron limitation against pathogen. Metallomics 2019, 11, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Schikorski, D.; Renault, T.; Saulnier, D.; Faury, N.; Moreau, P.; Pépin, J.-F. Experimental infection of Pacific oyster Crassostrea gigas spat by ostreid herpesvirus 1: Demonstration of oyster spat susceptibility. Vet. Res. 2011, 42, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.-M.; Xin, L.-S.; Rosani, U.; Wu, B.; Wang, Q.-C.; Duan, X.-K.; Liu, Z.-H.; Wang, C.-M. Chromosomal-level assembly of the blood clam, Scapharca (Anadara) broughtonii, using long sequence reads and Hi-C. GigaScience 2019, 8, giz067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Loyevsky, M.; Mompoint, F.; Yikilmaz, E.; Altschul, S.F.; Madden, T.; Wootton, J.C.; Kurantsin-Mills, J.; Kassim, O.O.; Gordeuk, V.R.; Rouault, T.A. Expression of a recombinant IRP-like Plasmodium falciparum protein that specifically binds putative plasmodial IREs. Mol. Biochem. Parasitol. 2003, 126, 231–238. [Google Scholar] [CrossRef]
- Basilion, J.P.; Rouault, T.A.; Massinople, C.M.; Klausner, R.D.; Burgess, W.H. The iron-responsive element-binding protein: Localization of the RNA-binding site to the aconitase active-site cleft. Proc. Natl. Acad. Sci. USA 1994, 91, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Radisky, E.; Leibold, E. The iron-responsive element binding protein. Purification, cloning, and regulation in rat liver. J. Biol. Chem. 1992, 267, 19005–19010. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [Green Version]
- Hentze, M.W.; Kühn, L.C. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc. Natl. Acad. Sci. USA 1996, 93, 8175–8182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Li, Y.; Li, S.; Tang, T.; Liu, F. Two ferritin genes (MdFerH and MdFerL) are involved in iron homeostasis, antioxidation and immune defense in housefly Musca domestica. J. Insect Physiol. 2020, 124, 104073. [Google Scholar] [CrossRef]
- Tang, T.; Yang, Z.; Li, J.; Yuan, F.; Xie, S.; Liu, F. Identification of multiple ferritin genes in Macrobrachium nipponense and their involvement in redox homeostasis and innate immunity. Fish Shellfish Immunol. 2019, 89, 701–709. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, D.; Tan, K.; Liu, H.; Ye, T.; Li, S.; Ma, H.; Zheng, H. Identification of two ferritin genes and their expression profiles in response to bacterial challenge in noble scallop Chlamys nobilis with different carotenoids content. Fish Shellfish Immunol. 2019, 88, 9–16. [Google Scholar] [CrossRef]
- Simão, M.; Leite, R.B.; Cancela, M.L. Expression of four new ferritins from grooved carpet shell clam Ruditapes decussatus challenged with Perkinsus olseni and metals (Cd, Cu and Zn). Aquat. Toxicol. 2020, 229, 105675. [Google Scholar] [CrossRef]
- Coba de la Pena, T.; Carcamo, C.B.; Diaz, M.I.; Winkler, F.M.; Morales-Lange, B.; Mercado, L.; Brokordt, K.B. Cloning and molecular characterization of two ferritins from red abalone Haliotis rufescens and their expressions in response to bacterial challenge at juvenile and adult life stages. Fish Shellfish Immunol. 2018, 82, 279–285. [Google Scholar] [CrossRef]
- Sumithra, T.G.; Neethu, B.R.; Reshma, K.J.; Anusree, V.N.; Reynold, P.; Sanil, N.K. A novel ferritin subunit gene from Asian green mussel, Perna viridis (Linnaeus, 1758). Fish Shellfish Immunol. 2021, 115, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.K.; Pantopoulos, K.; Dandekar, T.; Ackrell, B.A.; Hentze, M.W. Translational regulation of mammalian and Drosophila citric acid cycle enzymes via iron-responsive elements. Proc. Natl. Acad. Sci. USA 1996, 93, 4925–4930. [Google Scholar] [CrossRef] [Green Version]
- Goossen, B.; Hentze, M.W. Position is the critical determinant for function of iron-responsive elements as translational regulators. Mol. Cell. Biol. 1992, 12, 1959–1966. [Google Scholar] [PubMed] [Green Version]
- Laham, N.; Ehrlich, R. Manipulation of iron to determine survival. Immunol. Res. 2004, 30, 15–28. [Google Scholar] [CrossRef]



| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| Gene clone primer | |
| SbIRP-F | ATGGCATGTGGATCAAACCCTTACA |
| SbIRP-R | TCACAACATCTGCCGAATCATGTAG |
| Real-time quantitative PCR primers | |
| qSbIRP-F | GGACTCGGTGTTGTCGGTTGG |
| qSbIRP-R | GACGCAAATGCTTTGTAATGGTC |
| qSbFn-F | ACTCTGCCACCTCTCTTGTTCTG |
| qSbFn-R | TGCCAGTTATGTCTATCAGTCCA |
| qSbRL15-F | AGACCAGACAAAGCCAGAAGAC |
| qSbRL15-R | GCTGAAGTAAGTCCACGCATT |
| Vector construction primers | |
| SbIRP-Nco I | CATGCCATGGGCGCATGTGGATCAAACCCTTACA |
| SbIRP-Xho I | CCGCTCGAGTCAACATCTGCCGAATCATGTAG |
| SbIRP interference | |
| Sense | CCAGGUCAAUCUAGAGUAUTT |
| Anti-sense | AUACUCUAGAUUGACCUGGTT |
| Negative control interference | |
| Sense | UUCUCCGAACGUGUCACGUTT |
| Anti-sense | ACGUGACACGUUCGGAGAATT |
| EMSA probe | |
| SbFn IRE | AUUUGUUUUGCUGCGUCAGUGAACGUACGGACAGCU |
| Mutant SbFn IRE | AUUUGUUUUGUUGCGUuuuUuuACGUACGGACAGCU |
| RNA-ChIP primers | |
| ChIP-F | AACGTACGGACAGCTTGTGA |
| ChIP-R | GTCTTGGTTGTGTTTGAGCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Zhang, X.; Liu, Q.; Bai, C.; Li, C.; Wang, C.; Xin, L. Iron Regulatory Protein 1 Inhibits Ferritin Translation Responding to OsHV-1 Infection in Ark Clams, Scapharca Broughtonii. Cells 2022, 11, 982. https://doi.org/10.3390/cells11060982
Huang B, Zhang X, Liu Q, Bai C, Li C, Wang C, Xin L. Iron Regulatory Protein 1 Inhibits Ferritin Translation Responding to OsHV-1 Infection in Ark Clams, Scapharca Broughtonii. Cells. 2022; 11(6):982. https://doi.org/10.3390/cells11060982
Chicago/Turabian StyleHuang, Bowen, Xiang Zhang, Qin Liu, Changming Bai, Chen Li, Chongming Wang, and Lusheng Xin. 2022. "Iron Regulatory Protein 1 Inhibits Ferritin Translation Responding to OsHV-1 Infection in Ark Clams, Scapharca Broughtonii" Cells 11, no. 6: 982. https://doi.org/10.3390/cells11060982
APA StyleHuang, B., Zhang, X., Liu, Q., Bai, C., Li, C., Wang, C., & Xin, L. (2022). Iron Regulatory Protein 1 Inhibits Ferritin Translation Responding to OsHV-1 Infection in Ark Clams, Scapharca Broughtonii. Cells, 11(6), 982. https://doi.org/10.3390/cells11060982







