Calcium Channels in the Heart: Disease States and Drugs
Abstract
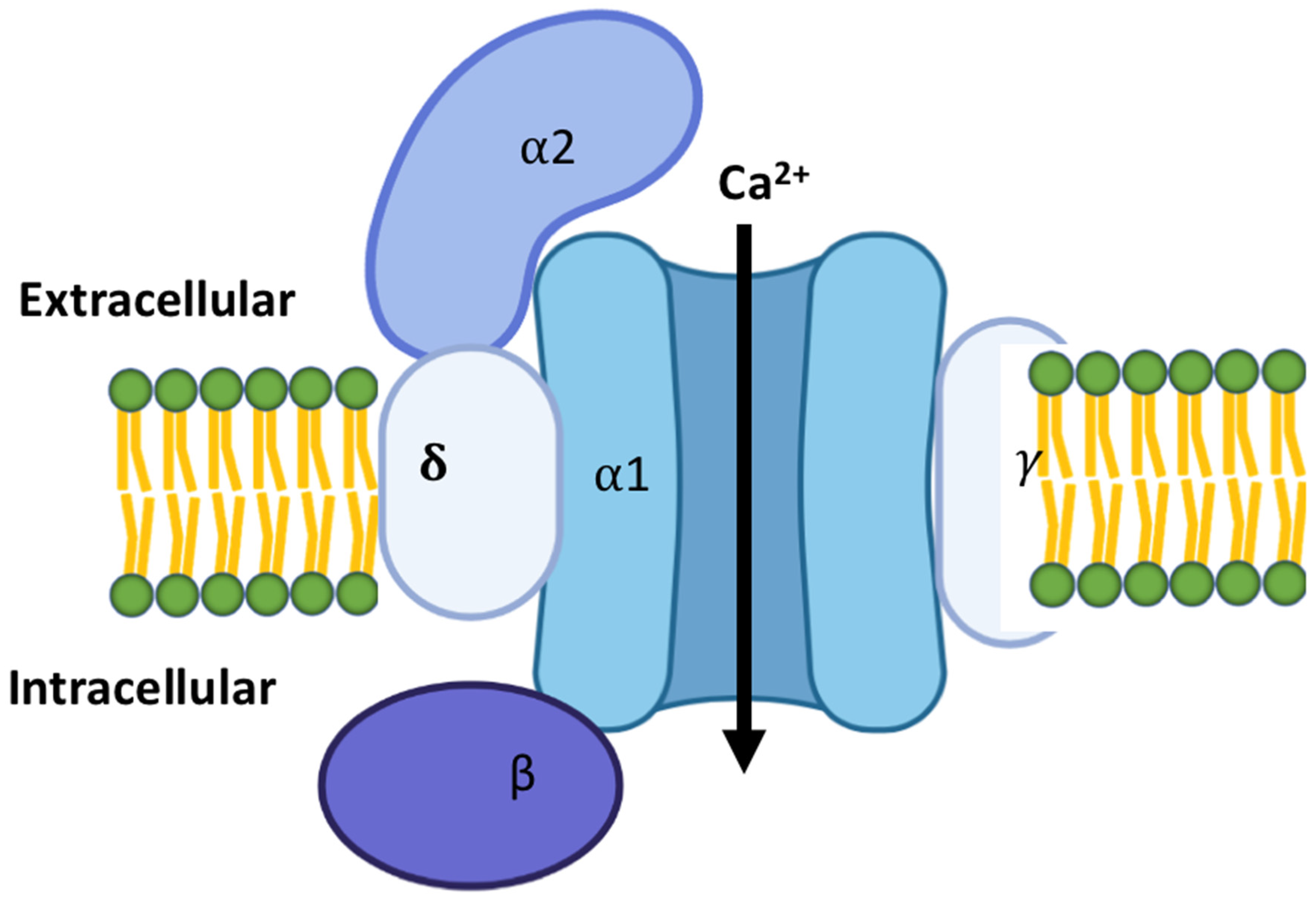
1. Introduction to Cardiac Calcium Channels
2. Types of Calcium Channels in the Cardiac Tissues
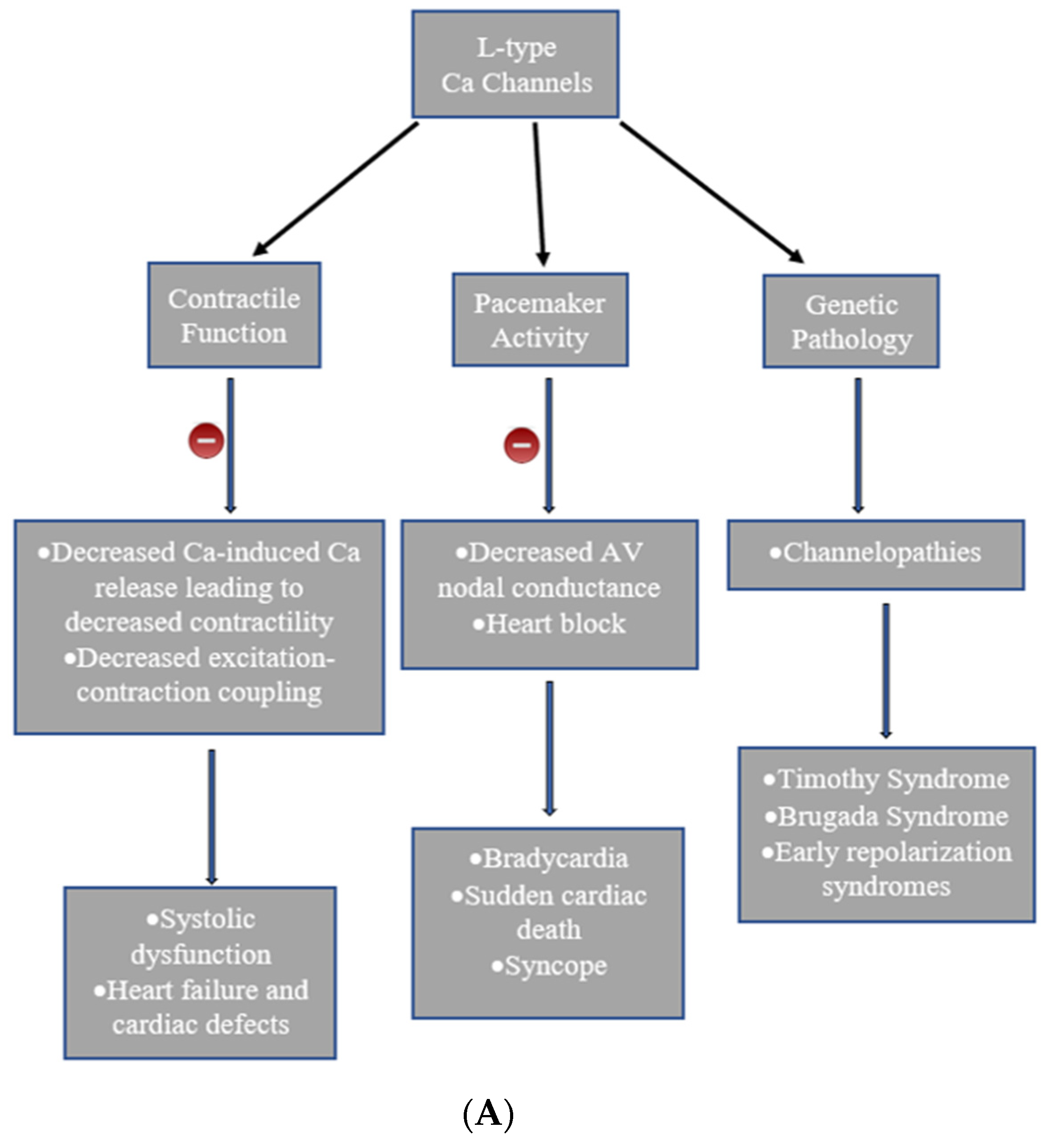
3. Heart Diseases Associated with Calcium Channels
4. Timothy Syndrome
5. Brugada Syndrome
6. Drugs Targeting Calcium Channels
7. Angina
8. Arrhythmia
9. Hypertension
10. Atherosclerosis
11. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukuta, H.; Little, W.C. The Cardiac Cycle and the Physiologic Basis of Left Ventricular Contraction, Ejection, Relaxation, and Filling. Heart Fail. Clin. 2008, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pullan, A.J.; Buist, M.L.; Sands, G.B.; Cheng, L.K.; Smith, N.P. Cardiac electrical activity-from heart to body surface and back again. J. Electrocardiol. 2003, 36, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, J. Compartmentalized Regulations of Ion Channels in the Heart. Biol. Pharm. Bull. 2007, 30, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.O. Cardiac Ion Channels. Circ. Arrhythmia Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.G.; Patel, N.J.; Singh, H. Intracellular Chloride Channels: Novel Biomarkers in Diseases. Front. Physiol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Singh, H. Mitochondrial ion channels in cardiac function. Am. J. Physiol. Physiol. Cell 2021, 321, C812–C825. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, A.; Clementi, S.; Reinbothe, T.; Torrente, A.; Vandael, D.H.; Pirone, A. Structural and functional differences between L-type calcium channels: Crucial issues for future selective targeting. Trends Pharmacol. Sci. 2011, 32, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Karekar, P.; Shah, K.; Hariharan, G.; Fleyshman, M.; Kaur, H.; Singh, H.; Rao, S.G. Drosophila Voltage-Gated Calcium Channel α1-Subunits Regulate Cardiac Function in the Aging Heart. Sci. Rep. 2018, 8, 6910. [Google Scholar] [CrossRef]
- Hansen, P.B.L. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: News from the world of knockout mice. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R227–R237. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A. Calcium channels in the heart. Postgrad. Med. J. 1991, 67 (Suppl. S3), S16–S19. [Google Scholar]
- Sengupta, S.; West, K.O.; Sanghvi, S.; Laliotis, G.; Agosto, L.M.; Lynch, K.W.; Tsichlis, P.N.; Singh, H.; Patrick, K.L.; Guerau-De-Arellano, M. PRMT5 Promotes Symmetric Dimethylation of RNA Processing Proteins and Modulates Activated T Cell Alternative Splicing and Ca2+/NFAT Signaling. ImmunoHorizons 2021, 5, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Triggle, D.J. Calcium channel antagonists: Clinical uses—Past, present and future. Biochem. Pharmacol. 2007, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, D.; Singh, H. Insights into the Role of Mitochondrial Ion Channels in Inflammatory Response. Front. Physiol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Coetzee, W.A. Channel-mediated calcium current in the heart. Cardiovasc. Drugs Ther. 1988, 1, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Betzenhauser, M.J.; Pitt, G.S.; Antzelevitch, C. Calcium Channel Mutations in Cardiac Arrhythmia Syndromes. Curr. Mol. Pharmacol. 2015, 8, 133–142. [Google Scholar] [CrossRef]
- Bers, D.M.; Perez-Reyes, E. Ca channels in cardiac myocytes: Structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 1999, 42, 339–360. [Google Scholar] [CrossRef]
- Nargeot, J.; Lory, P.; Richard, S. Molecular basis of the diversity of calcium channels in cardiovascular tissues. Eur. Heart J. 1997, 18, 15–26. [Google Scholar] [CrossRef]
- Endo, M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977, 57, 71–108. [Google Scholar] [CrossRef]
- Kushner, J.; Ferrer, X.; Marx, O.S. Roles and Regulation of Voltage-gated Calcium Channels in Arrhythmias. J. Innov. Card. Rhythm Manag. 2019, 10, 3874–3880. [Google Scholar] [CrossRef]
- Striessnig, J.; Ortner, N.J.; Pinggera, A. Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets? Curr. Mol. Pharmacol. 2015, 8, 110–122. [Google Scholar] [CrossRef]
- Morrow, J.; Marx, S. Novel approaches to examine the regulation of voltage-gated calcium channels in the heart. Curr. Mol. Pharmacol. 2015, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Yan, C.; Lu, S.; Dong, M.; Yan, N. Structure of the voltage-gated calcium channel Ca v 1.1 complex. Science 2015, 350, aad2395. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.R. Cardiac Intracellular Calcium Release Channels: Role in heart failure. Circ. Res. 2000, 87, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Moosmang, S.; Lenhardt, P.; Haider, N.; Hofmann, F.; Wegener, J.W. Mouse models to study L-type calcium channel function. Pharmacol. Ther. 2005, 106, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Iijima, T. Cardiac T-type Ca2+ channels in the heart. J. Mol. Cell. Cardiol. 2010, 48, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Zamponi, G.W. T-type calcium channels: From molecule to therapeutic opportunities. Int. J. Biochem. Cell Biol. 2019, 108, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Nilius, B. Biophysics and structure–function relationship of T-type Ca2+ channels. Cell Calcium 2006, 40, 97–114. [Google Scholar] [CrossRef]
- Cribbs, L. T-type calcium channel expression and function in the diseased heart. Channels 2010, 4, 447–452. [Google Scholar] [CrossRef][Green Version]
- Van Eif, V.W.; Stefanovic, S.; Mohan, R.A.; Christoffels, V.M. Gradual differentiation and confinement of the cardiac conduction system as indicated by marker gene expression. Biochim. Biophys. Acta 2019, 1867, 118509. [Google Scholar] [CrossRef]
- Marionneau, C.; Couette, B.; Liu, J.; Li, H.; Mangoni, M.E.; Nargeot, J.; Lei, M.; Escande, D.; Demolombe, S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J. Physiol. 2004, 562, 223–234. [Google Scholar] [CrossRef]
- January, C.T.; Riddle, J.M. Early afterdepolarizations: Mechanism of induction and block. A role for L-type Ca2+ current. Circ. Res. 1989, 64, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Seisenberger, C.; Specht, V.; Welling, A.; Platzer, J.; Pfeifer, A.; Kühbandner, S.; Striessnig, J.; Klugbauer, N.; Feil, R.; Hofmann, F. Functional Embryonic Cardiomyocytes after Disruption of the L-type α1C (Ca 1.2) Calcium Channel Gene in the Mouse. J. Biol. Chem. 2000, 275, 39193–39199. [Google Scholar] [CrossRef]
- Bidaud, I.; Lory, P. Hallmarks of the channelopathies associated with L-type calcium channels: A focus on the Timothy mutations in Cav1.2 channels. Biochimie 2011, 93, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, C.; Antzelevitch, C. Phenotypical Manifestations of Mutations in the Genes Encoding Subunits of the Cardiac Voltage–Dependent L-Type Calcium Channel. Circ. Res. 2011, 108, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, H.; Meister, E.M.; Theile, H. The heart-hand syndrome. A new variant of disorders of heart conduction and syndactylia including osseous changes in hands and feet. Kinderarztl. Prax. 1992, 60, 54–56. [Google Scholar] [PubMed]
- Splawski, I.; Timothy, K.W.; Decher, N.; Kumar, P.; Sachse, F.; Beggs, A.; Sanguinetti, M.C.; Keating, M.T. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 8089–8096. [Google Scholar] [CrossRef]
- Brugada, R.; Campuzano, O.; Sarquella-Brugada, G.; Brugada, J.; Brugada, P. Brugada Syndrome. Methodist DeBakey Cardiovasc. J. 2014, 10, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Brugada, P.; Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome: A multicenter report. J. Am. Coll. Cardiol. 1992, 20, 1391–1396. [Google Scholar] [CrossRef]
- Brugada, R.; Campuzano, O.; Sarquella-Brugada, G.; Brugada, P.; Brugada, J.; Hong, K. Brugada Syndrome. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Antzelevitch, C. Brugada Syndrome. Pacing Clin. Electrophysiol. 2006, 29, 1130–1159. [Google Scholar] [CrossRef]
- Di Diego, J.M.; Cordeiro, J.; Goodrow, R.J.; Fish, J.M.; Zygmunt, A.C.; Perez, G.; Scornik, F.S.; Antzelevitch, C. Ionic and Cellular Basis for the Predominance of the Brugada Syndrome Phenotype in Males. Circulation 2002, 106, 2004–2011. [Google Scholar] [CrossRef]
- Nielsen, M.W.; Holst, A.G.; Olesen, S.-P.; Olesen, M.S. The genetic component of Brugada syndrome. Front. Physiol. 2013, 4, 179. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Barajas-Martínez, H.; Xia, H.; Zhang, Z.; Chen, G.; Yang, B.; Jiang, H.; Antzelevitch, C.; Hu, D. Clinical and Functional Genetic Characterization of the Role of Cardiac Calcium Channel Variants in the Early Repolarization Syndrome. Front. Cardiovasc. Med. 2021, 8, 680819. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T.; Polster, P. Comparative study of drugs inhibiting the contractile response of isolated vessels of human and animal origin. Therapies 1968, 23, 1209–1220. [Google Scholar]
- Godfriand, T.; Lesne, M. Relative potency of digitoxin and digoxin in relation with their binding to isolated cardiac and smooth muscles. Arch. Int. Pharmacodyn. Ther. 1968, 171, 228–230. [Google Scholar]
- Frishman, W.H. Calcium channel blockers: Differences between subclasses. Am. J. Cardiovasc. Drugs 2007, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- McKeever, R.G.; Hamilton, R.J. Calcium Channel Blockers; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Higginbotham, M.B.; Morris, K.G.; Coleman, R.E.; Cobb, F.R. Chronic stable angina monotherapy: Nifedipine versus propranolol. Am. J. Med. 1989, 86, 1–5. [Google Scholar] [CrossRef]
- Talreja, O.; Cassagnol, M. StatPearls [Internet]. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539883/ (accessed on 29 January 2022).
- Elliott, W.J.; Ram, C.V.S. Calcium Channel Blockers. J. Clin. Hypertens. 2011, 13, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Hof, R.P.; Scholtysik, G.; Loutzenhiser, R.; Vuorela, H.J.; Neumann, P. PN 200-110, a new calcium antagonist: Electrophysiological, inotropic, and chronotropic effects on guinea pig myocardial tissue and effects on contraction and calcium uptake of rabbit aorta. J. Cardiovasc. Pharmacol. 1984, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- De Simone, A.; De Pasquale, M.; De Matteis, C.; Canciello, M.; Manzo, M.; Sabino, L.; Alfano, F.; Di Mauro, M.; Campana, A.; De Fabrizio, G.; et al. VErapamil plus antiarrhythmic drugs reduce atrial fibrillation recurrences after an electrical cardioversion (VEPARAF Study). Eur. Heart J. 2003, 24, 1425–1429. [Google Scholar] [CrossRef]
- Mason, R.P.; Marche, P.; Hintze, T.H. Novel Vascular Biology of Third-Generation L-Type Calcium Channel Antagonists: Ancillary actions of amlodipine. Arter. Thromb. Vasc. Biol. 2003, 23, 2155–2163. [Google Scholar] [CrossRef]
- Hernández, R.H.; Armas-Hernández, M.J.; Velasco, M.; Israili, Z.H.; Armas-Padilla, M.C. Calcium Antagonists and Atherosclerosis Protection in Hypertension. Am. J. Ther. 2003, 10, 409–414. [Google Scholar] [CrossRef] [PubMed]

| Drug | Brand Names | Indications |
|---|---|---|
| Non-Dihydropyridine calcium channel blockers | ||
| Diltiazem | Cardizem LA, Dilacor, Tiazac | Hypertension, Angina, Arrythmia |
| Verapamil | Covera-HS, Verelan PM, Calan | Hypertension, Angina, Arrythmia |
| Dihydropyridine calcium channel blockers | ||
| Amlodipine | Norvasc | Hypertension, Angina |
| Felodipine | Plendil | Hypertension |
| Nifedipine | Adalat, Procardia | Hypertension, Angina |
| Nicardipine | Cardene | Hypertension, Angina |
| Nisoldipine | Sular | Hypertension |
| Isradipine | Dynacirc | Hypertension |
| Nimdipine | Nimotop | Hypertension |
| Type of Calcium Channel | Location in Myocardial Cell Types | Targeted Drugs |
|---|---|---|
| Cav1.2 (L-type) | Ventricular Conduction | DHP |
| Cav3.1 (T-type) | Pacemaker Conduction | DHP |
| Cav3.2 (T-type) | Pacemaker Conduction | DHP |
| Cav2.3 (L-type) | Smooth muscle | DHP and non-DHP |
| Cav1.3 (L-type) | Smooth muscle | DHP and non-DHP |
| Calcium store-operated calcium channels | Endothelial cells | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.; Seeley, S.; Schulz, C.; Fisher, J.; Gururaja Rao, S. Calcium Channels in the Heart: Disease States and Drugs. Cells 2022, 11, 943. https://doi.org/10.3390/cells11060943
Shah K, Seeley S, Schulz C, Fisher J, Gururaja Rao S. Calcium Channels in the Heart: Disease States and Drugs. Cells. 2022; 11(6):943. https://doi.org/10.3390/cells11060943
Chicago/Turabian StyleShah, Kajol, Sarah Seeley, Castin Schulz, Jacqueline Fisher, and Shubha Gururaja Rao. 2022. "Calcium Channels in the Heart: Disease States and Drugs" Cells 11, no. 6: 943. https://doi.org/10.3390/cells11060943
APA StyleShah, K., Seeley, S., Schulz, C., Fisher, J., & Gururaja Rao, S. (2022). Calcium Channels in the Heart: Disease States and Drugs. Cells, 11(6), 943. https://doi.org/10.3390/cells11060943





