Response of Astrocyte Subpopulations Following Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
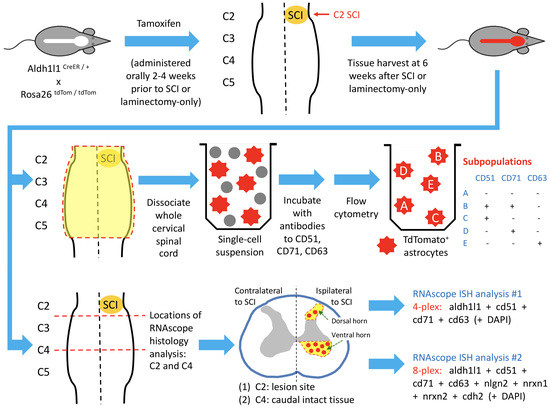
2.1. Animals
2.2. Spinal Cord Hemisection
2.3. Dissociation for Flow Cytometry
2.4. Human Tissue
2.5. HiPlex RNAscope
2.6. Microscopy
2.7. Quantification of RNAscope Puncta
2.8. Statistical Analyses
3. Results
3.1. Astrocyte Subpopulations Can Be Isolated from Adult Spinal Cord Using Flow Cytometry
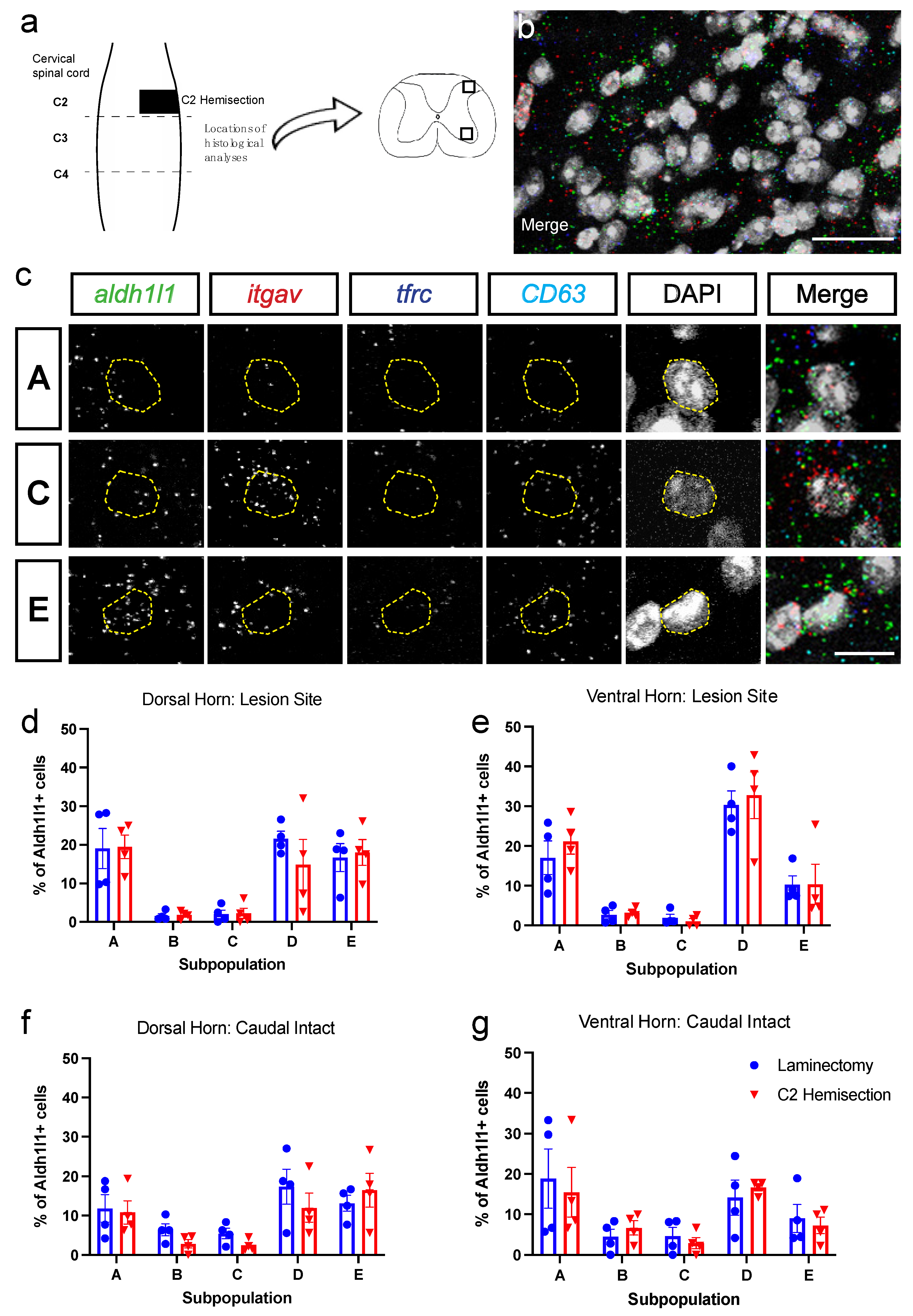
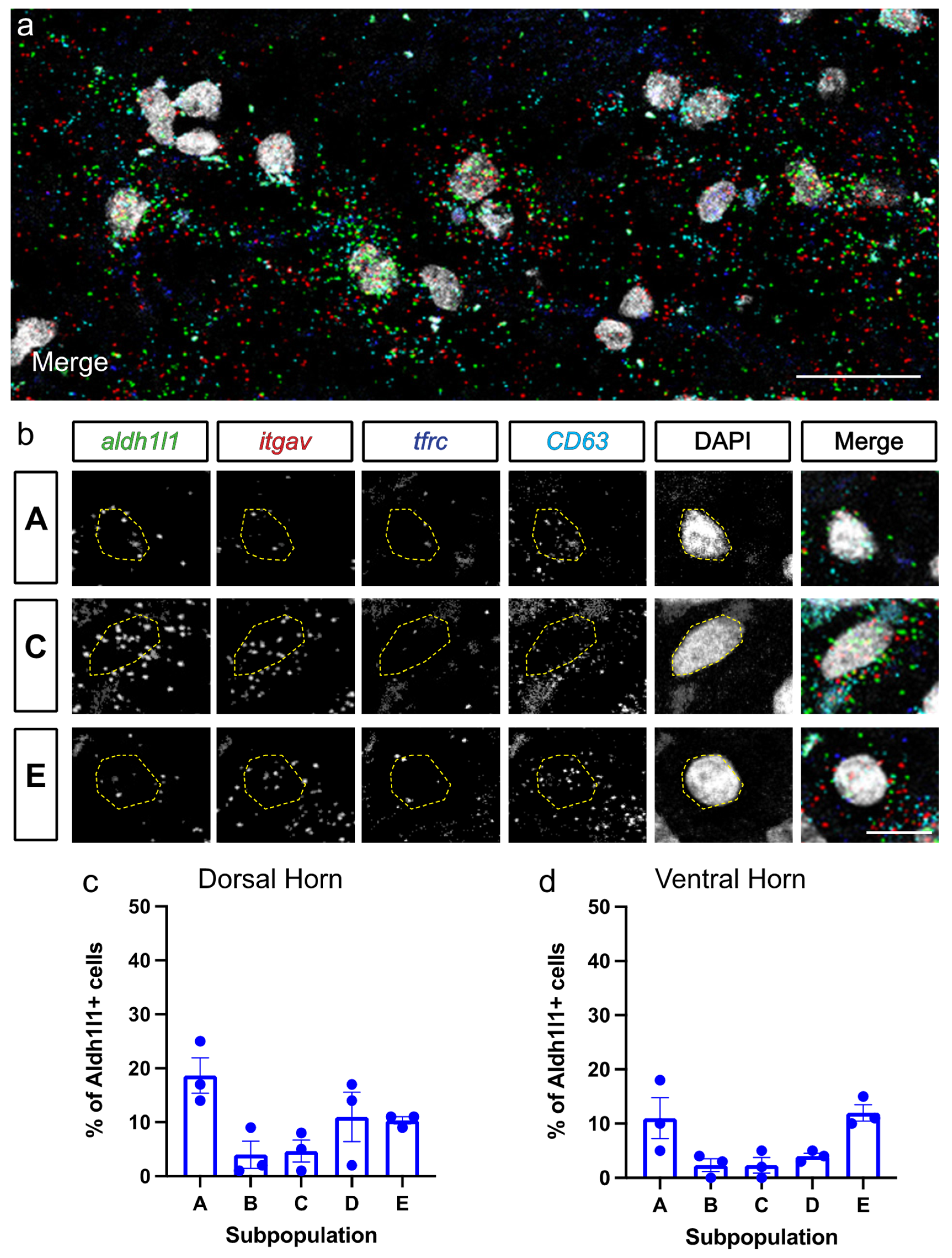
3.2. Astrocyte Subpopulations Can Be Identified in Spinal Cord via RNAscope in Situ Hybridization
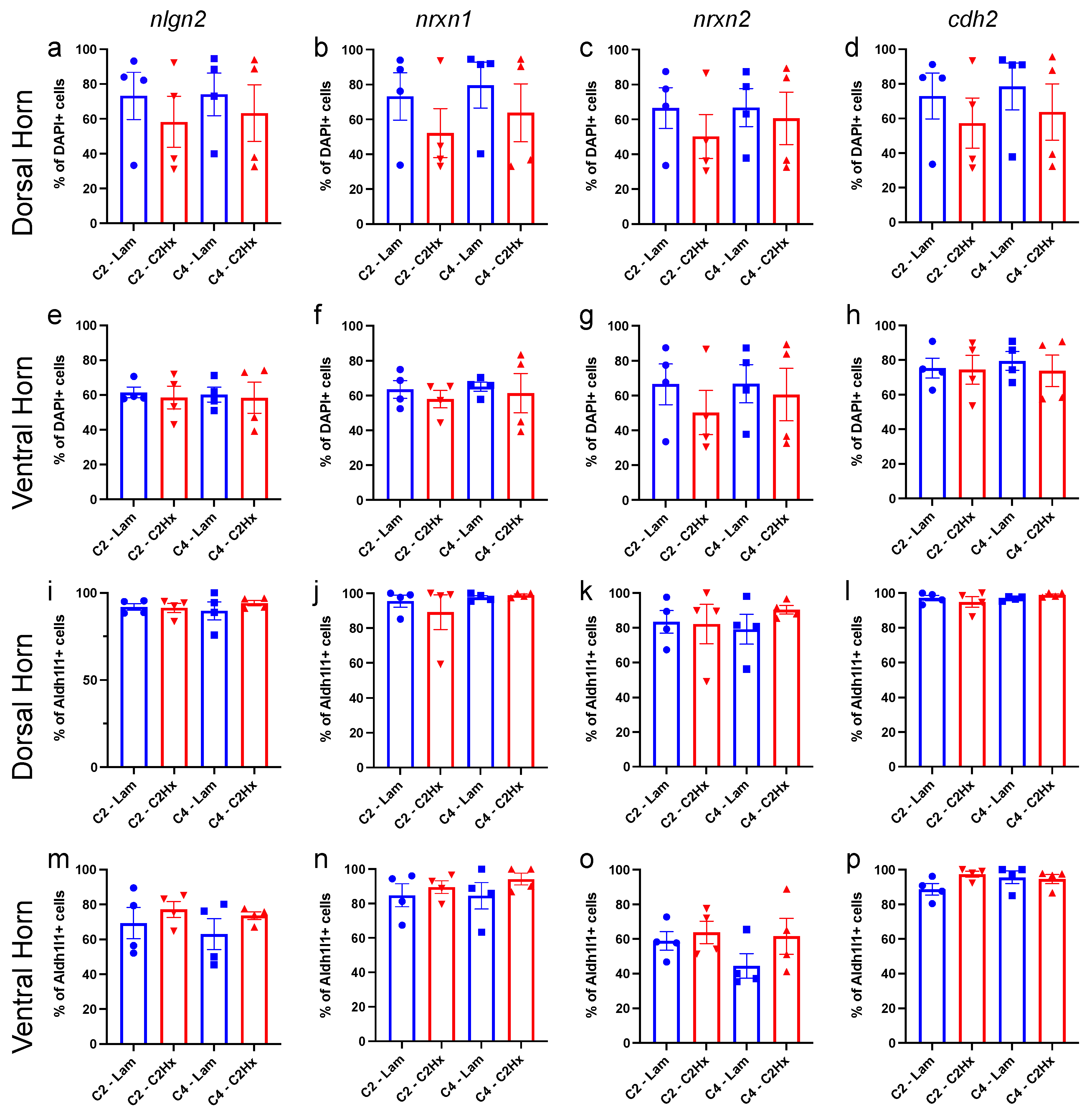
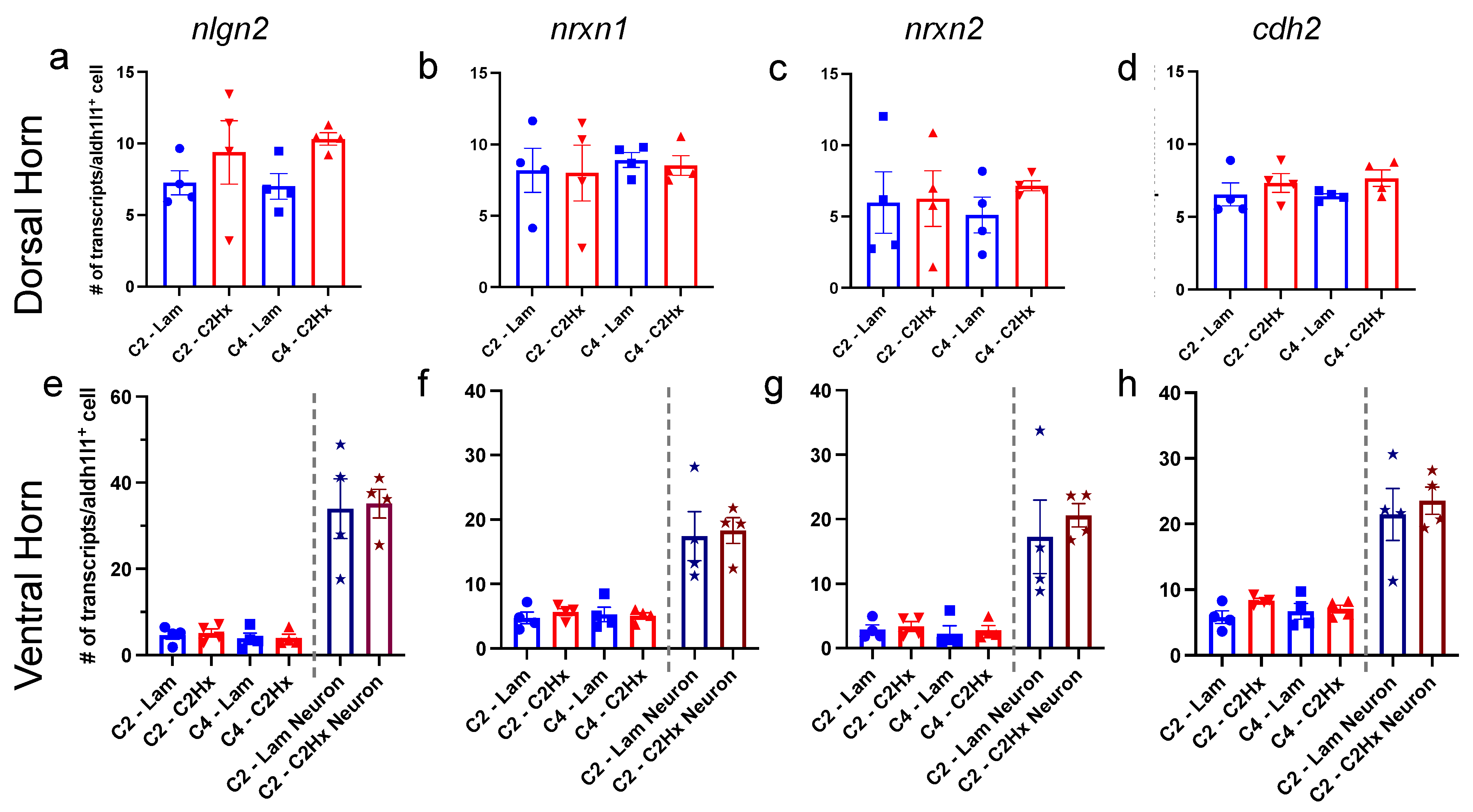
3.3. Expression of Synapse Formation-Associated Genes in Spinal Cord Astrocytes
3.4. Astrocyte Subpopulation Characterization in Human Spinal Cord
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aldh1l1 | aldehyde dehydrogenase 1 family member L1 |
| C2 | cervical spinal cord level 2 |
| C2HX | C2 hemisection |
| cd63 | CD63 antigen (CD63) |
| cdh2 | cadherin 2 |
| CNS | central nervous system |
| FACS | fluorescence activated cell sorting |
| itgav | integrin alpha-V (CD51) |
| ISH | in situ hybridization |
| Lam | laminectomy-only |
| nlgn2 | neuroligin 2 |
| nrxn1 | neurexin 1 |
| nrxn2 | neurexin 2 |
| SCI | spinal cord injury |
| tdTom | tdTomato |
| tfrc | transferring receptor-1 (CD71) |
References
- y Cajal, S.R. Histologie du Système nerveux de l’homme & des Vertébrés: Cervelet, Cerveau Moyen, rétine, Couche Optique, Corps strié, écorce Cérébrale Générale & régionale, Grand Sympathique; A. Maloine: Paris, French, 1911; Volume 2. (In French) [Google Scholar]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.D.R.; Petrova, R.; Eng, L.; Joyner, A.L. Sonic Hedgehog Regulates Discrete Populations of Astrocytes in the Adult Mouse Forebrain. J. Neurosci. 2010, 30, 13597–13608. [Google Scholar] [CrossRef]
- Blanco-Suarez, E.; Liu, T.-F.; Kopelevich, A.; Allen, N.J. Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 2018, 100, 1116–1132.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John Lin, C.C.; Yu, K.; Hatcher, A.; Huang, T.W.; Lee, H.K.; Carlson, J.; Weston, M.C.; Chen, F.; Zhang, Y.; Zhu, W.; et al. Identifi-cation of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 2017, 20, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayraktar, O.A.; Bartels, T.; Holmqvist, S.; Kleshchevnikov, V.; Martirosyan, A.; Polioudakis, D.; Ben Haim, L.; Young, A.M.H.; Batiuk, M.Y.; Prakash, K.; et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ tran-scriptomic map. Nat. Neurosci. 2020, 23, 500–509. [Google Scholar] [CrossRef]
- A Hill, S.; Blaeser, A.S.; A Coley, A.; Xie, Y.; A Shepard, K.; Harwell, C.C.; Gao, W.-J.; Garcia, A.D.R. Sonic hedgehog signaling in astrocytes mediates cell type-specific synaptic organization. eLife 2019, 8. [Google Scholar] [CrossRef]
- Huang, A.Y.-S.; Woo, J.; Sardar, D.; Lozzi, B.; Huerta, N.A.B.; Lin, C.-C.J.; Felice, D.; Jain, A.; Paulucci-Holthauzen, A.; Deneen, B. Region-Specific Transcriptional Control of Astrocyte Function Oversees Local Circuit Activities. Neuron 2020, 106, 992–1008.e9. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Boisvert, M.M.; Liu, H.; Dowling, C.; A Erikson, G.; Blanco-Suarez, E.; Farhy, C.; Shokhirev, M.N.; Ecker, J.R.; Allen, N.J. Activity-dependent modulation of synapse-regulating genes in astrocytes. eLife 2021, 10. [Google Scholar] [CrossRef]
- Charsar, B.A.; Brinton, M.A.; Locke, K.; Chen, A.Y.; Ghosh, B.; Urban, M.W.; Komaravolu, S.; Krishnamurthy, K.; Smit, R.; Pasinelli, P.; et al. AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury. FASEB J. 2019, 33, 13775–13793. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Sami, A.; Ghosh, B.; Goudsward, H.J.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. Respiratory axon regeneration in the chronically injured spinal cord. Neurobiol. Dis. 2021, 155, 105389. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.W.; Ghosh, B.; Block, C.G.; Charsar, B.A.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. Protein Ty-rosine Phosphatase sigma Inhibitory Peptide Promotes Recovery of Diaphragm Function and Sprouting of Bulbospinal Res-piratory Axons after Cervical Spinal Cord Injury. J. Neurotrauma 2020, 37, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.W.; Ghosh, B.; Block, C.G.; Strojny, L.R.; Charsar, B.A.; Goulão, M.; Komaravolu, S.S.; Smith, G.M.; Wright, M.C.; Li, S.; et al. Long-Distance Axon Regeneration Promotes Recovery of Diaphragmatic Respiratory Function after Spinal Cord Injury. Eneuro 2019, 6. [Google Scholar] [CrossRef]
- Brown, E.V.; Falnikar, A.; Heinsinger, N.; Cheng, L.; Andrews, C.E.; DeMarco, M.; Lepore, A.C. Cervical spinal cord injury-induced neuropathic pain in male mice is associated with a persistent pro-inflammatory macrophage/microglial response in the superficial dorsal horn. Exp. Neurol. 2021, 343, 113757. [Google Scholar] [CrossRef] [PubMed]
- Goulao, M.; Ghosh, B.; Urban, M.W.; Sahu, M.; Mercogliano, C.; Charsar, B.A.; Komaravolu, S.; Block, C.G.; Smith, G.M.; Wright, M.C.; et al. Astrocyte progenitor transplantation promotes regeneration of bulbospinal res-piratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia 2019, 67, 452–466. [Google Scholar] [PubMed]
- Li, K.; Javed, E.; Scura, D.; Hala, T.J.; Seetharam, S.; Falnikar, A.; Richard, J.-P.; Chorath, A.; Maragakis, N.J.; Wright, M.C.; et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 2015, 271, 479–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive as-trogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Lu, T.-Y.; Chai, H.; Xu, J.; Huang, B.; Golshani, P.; Coppola, G.; Khakh, B.S. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron 2016, 92, 1181–1195. [Google Scholar] [CrossRef] [Green Version]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2009, 13, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Vidensky, S.; Jin, L.; Jie, C.; Lorenzini, I.; Frankl, M.; Rothstein, J.D. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 2010, 59, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. J. Spinal. Cord. Med. 2013, 36, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef] [Green Version]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef]
- Roy, R.R.; Edgerton, V.R. Neurobiological perspective of spasticity as occurs after a spinal cord injury. Exp. Neurol. 2012, 235, 116–122. [Google Scholar] [CrossRef]
- Tan, A.M.; Waxman, S.G. Spinal cord injury, dendritic spine remodeling, and spinal memory mechanisms. Exp. Neurol. 2012, 235, 142–151. [Google Scholar] [CrossRef]
- Kramer, J.L.; Minhas, N.K.; Jutzeler, C.R.; Erskine, E.L.; Liu, L.J.; Ramer, M.S. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J. Neurosci. Res. 2016, 95, 1295–1306. [Google Scholar] [CrossRef]
- Risher, W.C.; Eroglu, C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012, 31, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J. Cell Commun. Signal. 2009, 3, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.J.; Bennett, M.; Foo, L.C.; Wang, G.; Chakraborty, C.; Smith, S.J.; Barres, B.A. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012, 486, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Hormigo, K.M.; Zholudeva, L.; Spruance, V.M.; Marchenko, V.; Cote, M.-P.; Vinit, S.; Giszter, S.; Bezdudnaya, T.; Lane, M.A. Enhancing neural activity to drive respiratory plasticity following cervical spinal cord injury. Exp. Neurol. 2016, 287, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Weidner, N.; Ner, A.; Salimi, N.; Tuszynski, M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA 2001, 98, 3513–3518. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Duale, H.; Rabchevsky, A. Intraspinal sprouting of unmyelinated pelvic afferents after complete spinal cord injury is correlated with autonomic dysreflexia induced by visceral pain. Neuroscience 2009, 159, 369–379. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allahyari, R.V.; Heinsinger, N.M.; Hwang, D.; Jaffe, D.A.; Rasouli, J.; Shiers, S.; Thomas, S.J.; Price, T.J.; Rostami, A.; Lepore, A.C. Response of Astrocyte Subpopulations Following Spinal Cord Injury. Cells 2022, 11, 721. https://doi.org/10.3390/cells11040721
Allahyari RV, Heinsinger NM, Hwang D, Jaffe DA, Rasouli J, Shiers S, Thomas SJ, Price TJ, Rostami A, Lepore AC. Response of Astrocyte Subpopulations Following Spinal Cord Injury. Cells. 2022; 11(4):721. https://doi.org/10.3390/cells11040721
Chicago/Turabian StyleAllahyari, R. Vivian, Nicolette M. Heinsinger, Daniel Hwang, David A. Jaffe, Javad Rasouli, Stephanie Shiers, Samantha J. Thomas, Theodore J. Price, Abdolmohamad Rostami, and Angelo C. Lepore. 2022. "Response of Astrocyte Subpopulations Following Spinal Cord Injury" Cells 11, no. 4: 721. https://doi.org/10.3390/cells11040721
APA StyleAllahyari, R. V., Heinsinger, N. M., Hwang, D., Jaffe, D. A., Rasouli, J., Shiers, S., Thomas, S. J., Price, T. J., Rostami, A., & Lepore, A. C. (2022). Response of Astrocyte Subpopulations Following Spinal Cord Injury. Cells, 11(4), 721. https://doi.org/10.3390/cells11040721