Locomotor Exercise Enhances Supraspinal Control of Lower-Urinary-Tract Activity to Improve Micturition Function after Contusive Spinal-Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
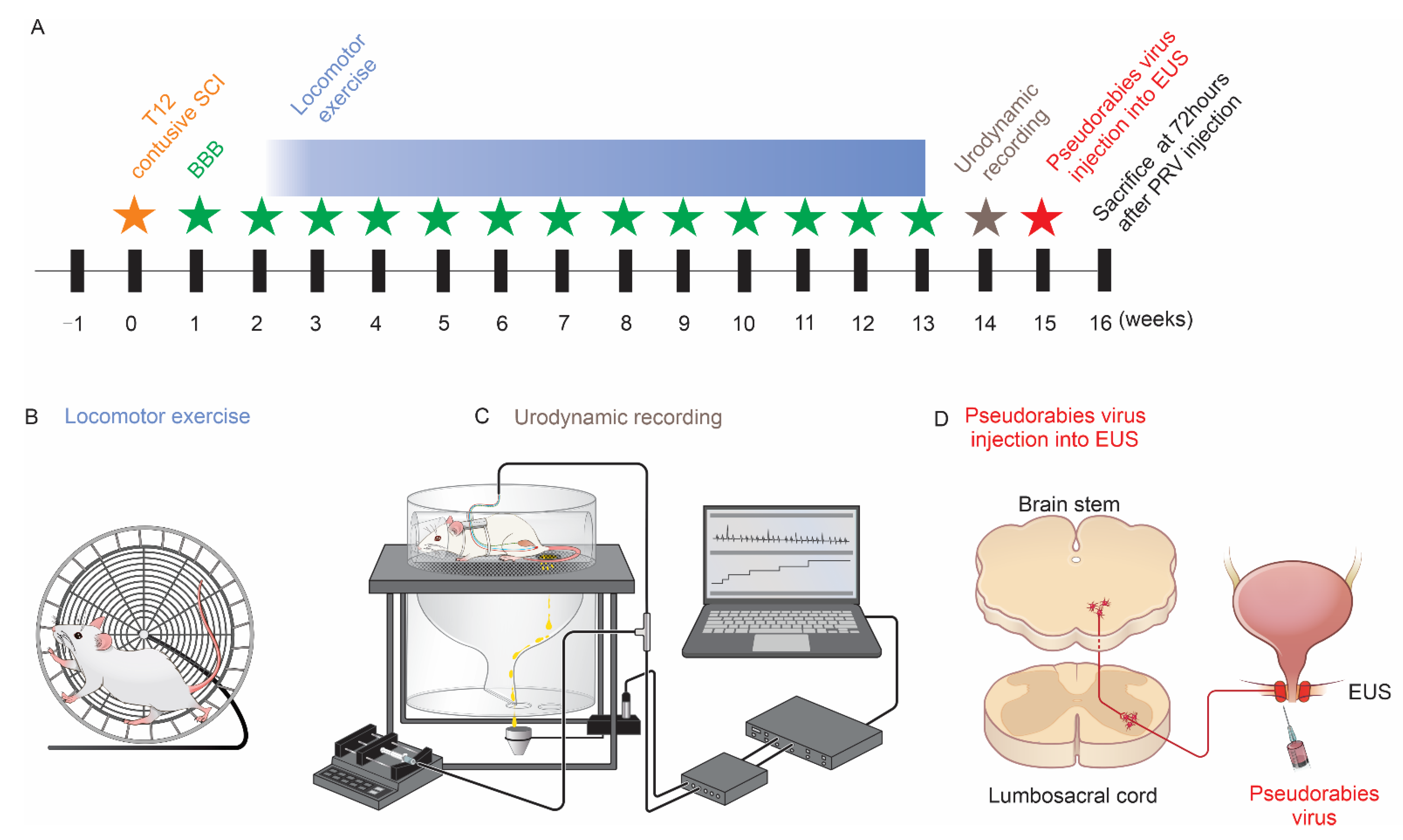
2.2. Contusive SCI
2.3. Basso, Beattie and Bresnahan (BBB) Locomotion Scale
2.4. Forced Running Wheel Locomotor Exercise
2.5. Bladder CMG and EUS EMG in Fully Awake Rats
2.6. PRV-614 Injection
2.7. Tissue Processing and Histology
2.8. Masson Trichrome Staining and Quantification for Bladder Pathology
2.9. Quantitative Analysis
2.10. Statistical Analysis
3. Results
3.1. Locomotor Exercise Promotes the Recovery of Hindlimb Function
3.2. Locomotor Exercise Improves Voiding Efficiency
3.3. Locomotor Exercise Improves Bladder and EUS Reflexes
3.4. Locomotor Exercise Improves the Pathological Changes in Bladder Morphology
3.5. Locomotor Exercise Increases the Extent of Spared Spinal-Cord Tissue
3.6. Locomotor Exercise Promotes Serotonergic Axon Density in EUS Motoneuron Vicinity
3.7. Locomotor Exercise Enhances Supraspinal-EUS Motoneuron Innervation
3.8. Correlation between Supraspinal Control and Voiding Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Dalton, L.; Wolfe and the Spinal Cord Injury Rehabilitation Evidence (SCIRE) Research Team. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma. 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourbeau, D.; Bolon, A.; Creasey, G.; Dai, W.; Fertig, B.; French, J.; Jeji, T.; Kaiser, A.; Kouznetsov, R.; Rabchevsky, A.; et al. Needs, priorities, and attitudes of individuals with spinal cord injury toward nerve stimulation devices for bladder and bowel function: A survey. Spinal. Cord. 2020, 58, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.P.; Wallner, L.P.; Forchheimer, M.B.; Clemens, J.Q.; Dunn, R.L.; Rodriguez, G.; Chen, D.; Horton, J., 3rd; Tate, D.G. Medical and psychosocial complications associated with method of bladder management after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2011, 92, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.J.; Herrity, A.N.; Smith, R.R.; Willhite, A.; Harrison, B.J.; Petruska, J.C.; Harkema, S.J.; Hubscher, C.H. Novel multi-system functional gains via task specific training in spinal cord injured male rats. J. Neurotrauma 2014, 31, 819–833. [Google Scholar] [CrossRef] [Green Version]
- Hubscher, C.H.; Herrity, A.N.; Williams, C.S.; Montgomery, L.R.; Willhite, A.M.; Angeli, C.A.; Harkema, S.J. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS ONE 2018, 13, e0190998. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.; Roppolo, J.R.; de Groat, W.C. Spinal reflex control of micturition after spinal cord injury. Restor. Neurol. Neurosci. 2006, 24, 69–78. [Google Scholar]
- Beaumont, E.; Kaloustian, S.; Rousseau, G.; Cormery, B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 2008, 62, 147–154. [Google Scholar] [CrossRef]
- Furlan, J.C.; Fehlings, M.G. Hyponatremia in the acute stage after traumatic cervical spinal cord injury: Clinical and neuroanatomic evidence for autonomic dysfunction. Spine 2009, 34, 501–511. [Google Scholar] [CrossRef]
- Wade, C.E. Response, regulation, and actions of vasopressin during exercise: A review. Med. Sci. Sports Exerc. 1984, 16, 506–511. [Google Scholar] [CrossRef]
- Leicht, C.A.; Goosey-Tolfrey, V.L.; Bishop, N.C. Spinal cord injury: Known and possible influences on the immune response to exercise. Exerc. Immunol. Rev. 2013, 19, 144–163. [Google Scholar]
- de Groat, W.C.; Araki, I.; Vizzard, M.A.; Yoshiyama, M.; Yoshimura, N.; Sugaya, K.; Tai, C.; Roppolo, J.R. Developmental and injury induced plasticity in the micturition reflex pathway. Behav. Brain Res. 1998, 92, 127–140. [Google Scholar] [CrossRef]
- Reed, W.R.; Shum-Siu, A.; Whelan, A.; Onifer, S.M.; Magnuson, D.S. Anterograde labeling of ventrolateral funiculus pathways with spinal enlargement connections in the adult rat spinal cord. Brain Res. 2009, 1302, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adkins, D.L.; Boychuk, J.; Remple, M.S.; Kleim, J.A. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. 2006, 101, 1776–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- van den Brand, R.; Heutschi, J.; Barraud, Q.; DiGiovanna, J.; Bartholdi, K.; Huerlimann, M.; Friedli, L.; Vollenweider, I.; Moraud, E.M.; Duis, S.; et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012, 336, 1182–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrity, A.N.; Aslan, S.C.; Ugiliweneza, B.; Mohamed, A.Z.; Hubscher, C.H.; Harkema, S.J. Improvements in bladder function following activity-based recovery training with epidural stimulation after chronic spinal cord injury. Front. Syst. Neurosci. 2020, 14, 614691. [Google Scholar] [CrossRef] [PubMed]
- Engesser-Cesar, C.; Anderson, A.J.; Basso, D.M.; Edgerton, V.R.; Cotman, C.W. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma 2005, 22, 157–171. [Google Scholar] [CrossRef]
- Smith, R.R.; Brown, E.H.; Shum-Siu, A.; Whelan, A.; Burke, D.A.; Benton, R.L.; Magnuson, D.S. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J. Neurotrauma 2009, 26, 1017–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadekawa, K.; Yoshimura, N.; Majima, T.; Wada, N.; Shimizu, T.; Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Sugaya, K.; Yoshiyama, M. Characterization of bladder and external urethral activity in mice with or without spinal cord injury—A comparison study with rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R752–R758. [Google Scholar] [CrossRef]
- Nadelhaft, I.; Vera, P.L. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J. Comp. Neurol. 1996, 375, 502–517. [Google Scholar] [CrossRef]
- Horst, M.; Heutschi, J.; van den Brand, R.; Andersson, K.E.; Gobet, R.; Sulser, T.; Courtine, G.; Eberli, D. Multisystem neuroprosthetic training improves bladder function after severe spinal cord injury. J. Urol. 2013, 189, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.K.; Titsworth, W.L.; Zhang, Y.P.; Xhafa, A.I.; Shields, C.B.; Xu, X.M. Characterizing phospholipase A2-induced spinal cord injury-a comparison with contusive spinal cord injury in adult rats. Transl. Stroke Res. 2011, 2, 608–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, T.J.; Turner, L.M. Cytoarchitecture of the rat spinal cord. J. Physiol. 1972, 222, 123P–125P. [Google Scholar] [PubMed]
- Heise, C.; Kayalioglu, G. Cytoarchitecture of the Spinal Cord. In Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas; Academic Press: Cambridge, MA, USA, 2009; pp. 64–93. [Google Scholar] [CrossRef]
- Ogier, R.; Tribollet, E.; Suarez, P.; Raggenbass, M. Identified motoneurons involved in sexual and eliminative functions in the rat are powerfully excited by vasopressin and tachykinins. J. Neurosci. 2006, 26, 10717–10726. [Google Scholar] [CrossRef] [Green Version]
- Thor, K.B. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: Implications for treating stress urinary incontinence. Urology 2003, 62, 3–9. [Google Scholar] [CrossRef]
- Maggi, C.A.; Giuliani, S.; Santicioli, P.; Meli, A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am. J. Physiol. 1986, 251, R250–R257. [Google Scholar] [CrossRef]
- Mersdorf, A.; Schmidt, R.A.; Tanagho, E.A. Urodynamic evaluation and electrical and pharmacologic neurostimulation. The rat model. Urol. Res. 1993, 21, 199–209. [Google Scholar] [CrossRef]
- Ahn, J.; Saltos, T.M.; Tom, V.J.; Hou, S. Transsynaptic tracing to dissect supraspinal serotonergic input regulating the bladder reflex in rats. Neurourol. Urodyn. 2018, 37, 2487–2494. [Google Scholar] [CrossRef]
- Pikov, V.; Gillis, R.A.; Jasmin, L.; Wrathall, J.R. Assessment of lower urinary tract functional deficit in rats with contusive spinal cord injury. J. Neurotrauma 1998, 15, 375–386. [Google Scholar] [CrossRef]
- Ni, J.; Cao, N.; Wang, X.; Zhan, C.; Si, J.; Gu, B.; Andersson, K.E. The serotonin (5-hydroxytryptamine) 5-HT7 receptor is up-regulated in Onuf’s nucleus in rats with chronic spinal cord injury. BJU Int. 2019, 123, 718–725. [Google Scholar] [CrossRef]
- Mitsui, T.; Kanno, Y.; Kitta, T.; Moriya, K.; Nonomura, K. Supraspinal projection of serotonergic and noradrenergic pathways modulates nociceptive transmission in the lower urinary tract of rats. Low Urin. Tract. Symptoms 2016, 8, 186–190. [Google Scholar] [CrossRef] [PubMed]
- DeFinis, J.H.; Weinberger, J.; Hou, S. Delivery of the 5-HT2A receptor agonist, doi, enhances activity of the sphincter muscle during the micturition reflex in rats after spinal cord injury. Biology 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Engesser-Cesar, C.; Ichiyama, R.M.; Nefas, A.L.; Hill, M.A.; Edgerton, V.R.; Cotman, C.W.; Anderson, A.J. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 2007, 25, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Simoes, A. Urodynamic testing and interpretation. In StatPearls; NCBI: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, M.N.; Belton, A.L.; de Groat, W.C. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 1993, 264, R1157–R1163. [Google Scholar] [CrossRef]
- Breyer, B.N.; Fandel, T.M.; Alwaal, A.; Osterberg, E.C.; Shindel, A.W.; Lin, G.; Tanagho, E.A.; Lue, T.F. Comparison of spinal cord contusion and transection: Functional and histological changes in the rat urinary bladder. BJU Int. 2017, 119, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Pinilla, F.; Ying, Z.; Opazo, P.; Roy, R.R.; Edgerton, V.R. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 2001, 13, 1078–1084. [Google Scholar] [CrossRef]
- Perreau, V.M.; Adlard, P.A.; Anderson, A.J.; Cotman, C.W. Exercise-induced gene expression changes in the rat spinal cord. Gene Expr. 2005, 12, 107–121. [Google Scholar] [CrossRef]
- Nadelhaft, I.; Vera, P.L.; Card, J.P.; Miselis, R.R. Central nervous system neurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci. Lett. 1992, 143, 271–274. [Google Scholar] [CrossRef]
- Karnup, S.V.; de Groat, W.C. Propriospinal neurons of L3-L4 segments involved in control of the rat external urethral sphincter. Neuroscience 2020, 425, 12–28. [Google Scholar] [CrossRef]
- de Groat, W.C. Plasticity of bladder reflex pathways during postnatal development. Physiol. Behav. 2002, 77, 689–692. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp. Neurol. 2012, 235, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Sui, T.; Wang, D.V.; Hou, S.; Cao, X.; Peng, K.; Xu, Z.; Xu, X. Locomotor Exercise Enhances Supraspinal Control of Lower-Urinary-Tract Activity to Improve Micturition Function after Contusive Spinal-Cord Injury. Cells 2022, 11, 1398. https://doi.org/10.3390/cells11091398
Deng L, Sui T, Wang DV, Hou S, Cao X, Peng K, Xu Z, Xu X. Locomotor Exercise Enhances Supraspinal Control of Lower-Urinary-Tract Activity to Improve Micturition Function after Contusive Spinal-Cord Injury. Cells. 2022; 11(9):1398. https://doi.org/10.3390/cells11091398
Chicago/Turabian StyleDeng, Lingxiao, Tao Sui, Dong V. Wang, Shaoping Hou, Xiaojian Cao, Kaiwen Peng, Zaocheng Xu, and Xiaoming Xu. 2022. "Locomotor Exercise Enhances Supraspinal Control of Lower-Urinary-Tract Activity to Improve Micturition Function after Contusive Spinal-Cord Injury" Cells 11, no. 9: 1398. https://doi.org/10.3390/cells11091398
APA StyleDeng, L., Sui, T., Wang, D. V., Hou, S., Cao, X., Peng, K., Xu, Z., & Xu, X. (2022). Locomotor Exercise Enhances Supraspinal Control of Lower-Urinary-Tract Activity to Improve Micturition Function after Contusive Spinal-Cord Injury. Cells, 11(9), 1398. https://doi.org/10.3390/cells11091398





