New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation
Abstract
1. Introduction
2. Materials and Methods
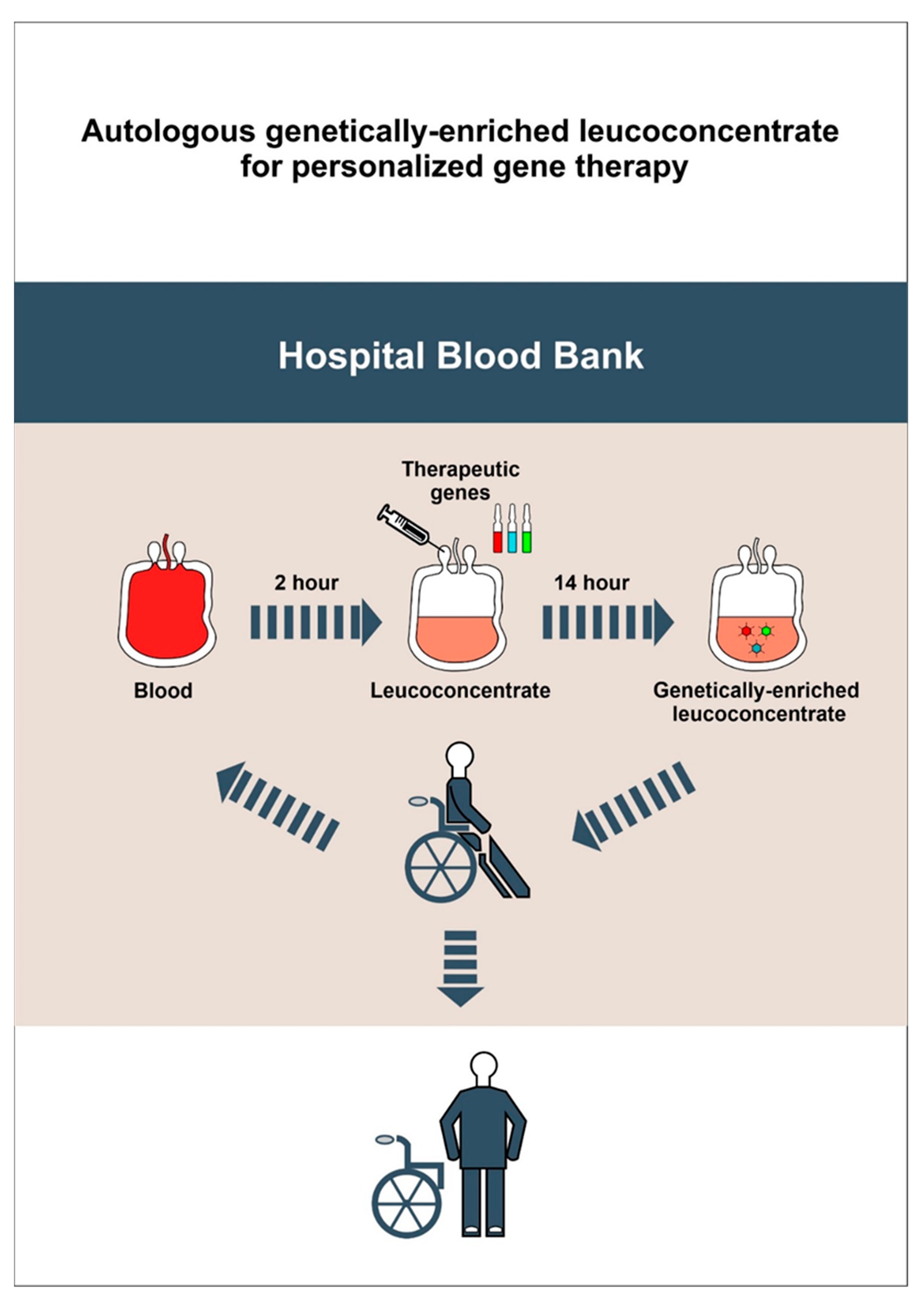
2.1. Preparation of Autologous Genetically-Enriched Leucoconcentrate
2.1.1. Chimeric Ad5/35F Viral Vectors Construction
2.1.2. Blood Collection
2.1.3. Leucoconcentrate Preparation
2.1.4. Transduction of the Leucoconcentrate with Chimeric Ad5/35F Viral Vectors
2.1.5. Complete Blood Count
2.2. Transgene Expression Analysis in Genetically-Enriched Leucoconcentrate In Vitro
2.2.1. Flow Cytometric Analysis
2.2.2. RT-PCR Assay
2.2.3. ELISA
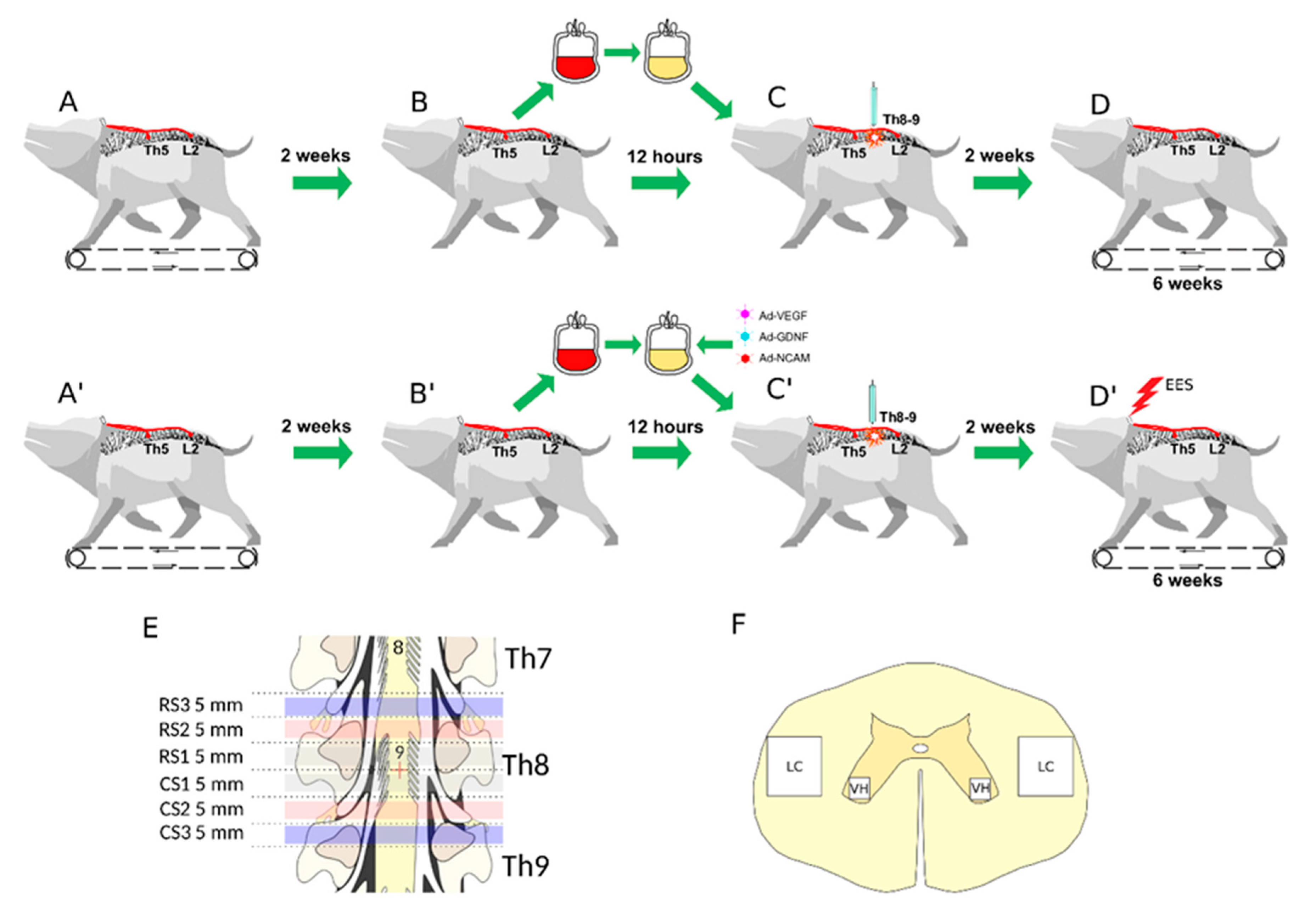
2.3. Animals and Treatment
2.3.1. Anaesthesia and Postoperative Care
2.3.2. Electrode Implantation
2.3.3. Training on the Treadmill
2.3.4. Spinal Cord Injury
2.3.5. Autoinfusion of Genetically-Enriched Leucoconcentrate
2.3.6. Epidural Electrical Stimulation
2.3.7. Experimental Groups
2.4. Post-Traumatic Spinal cord Functional Recovery
2.4.1. Porcine Thoracic Injury Behavioural Scale
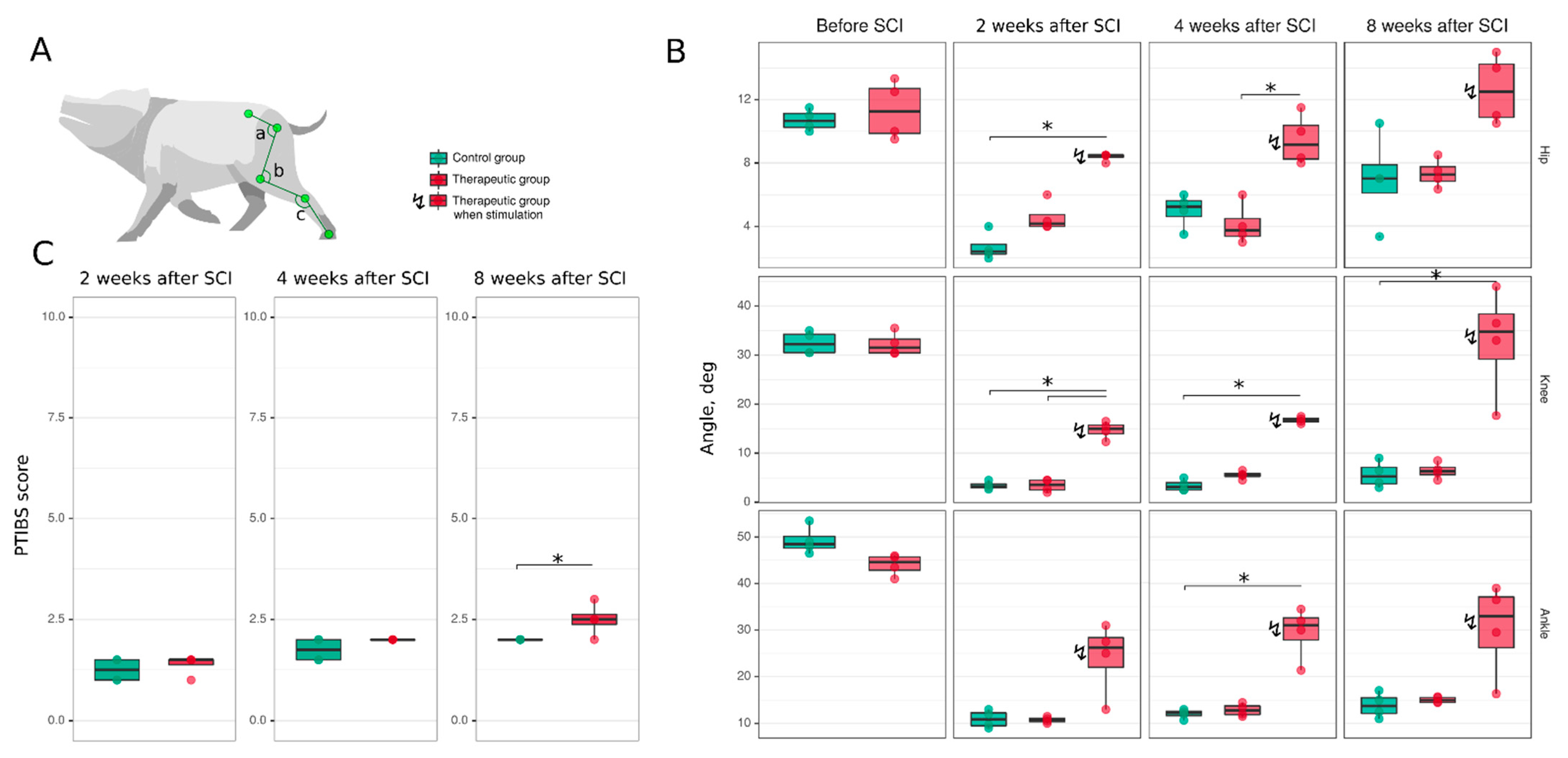
2.4.2. Joint Kinematics
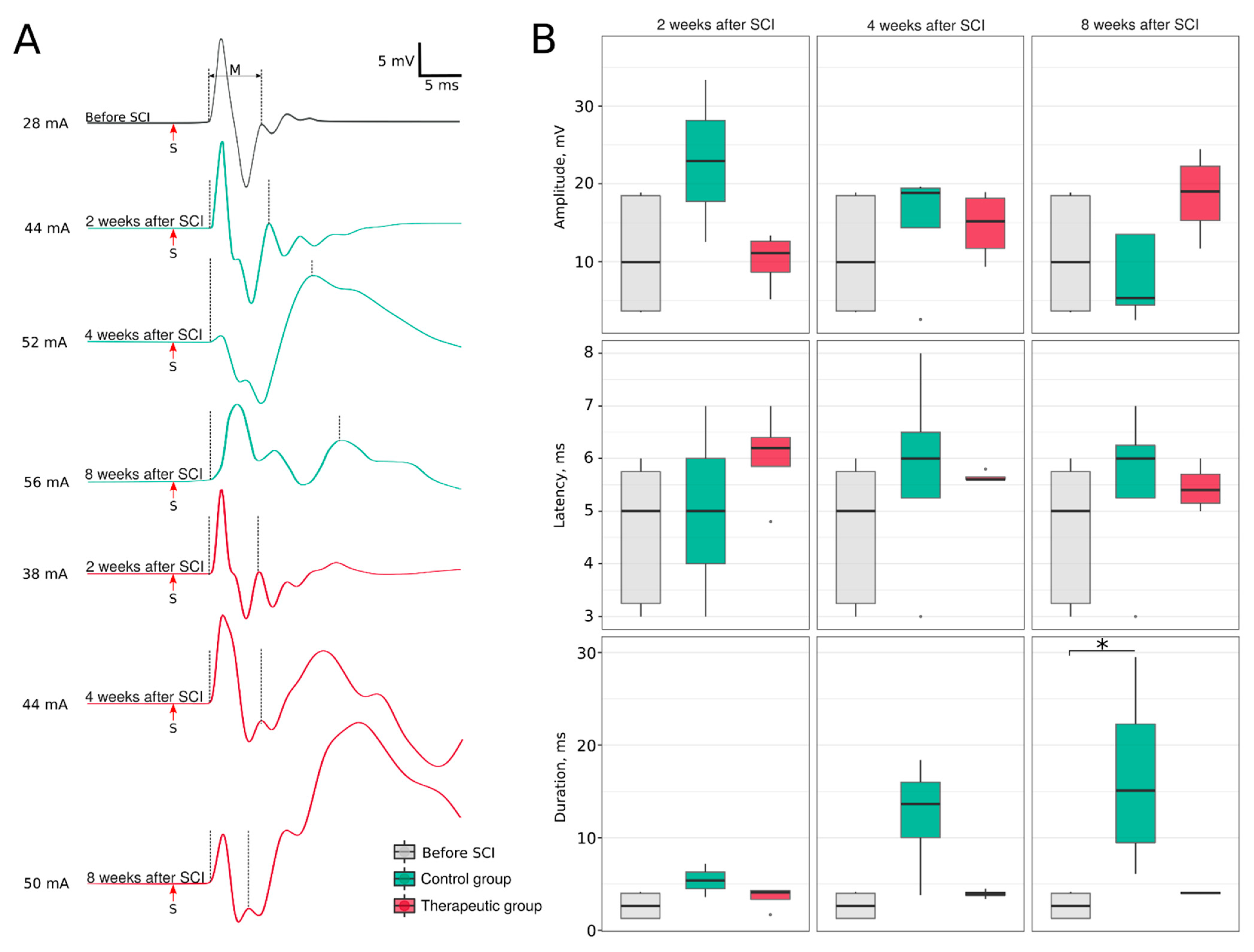
2.4.3. Electrophysiological Study
2.5. Post-Traumatic Spinal Cord Molecular and Cellular Changes
2.5.1. Sample Collection
2.5.2. Immunofluorescence Staining
2.5.3. Synaptic Gene Expression
2.6. Statistical Analysis
3. Results
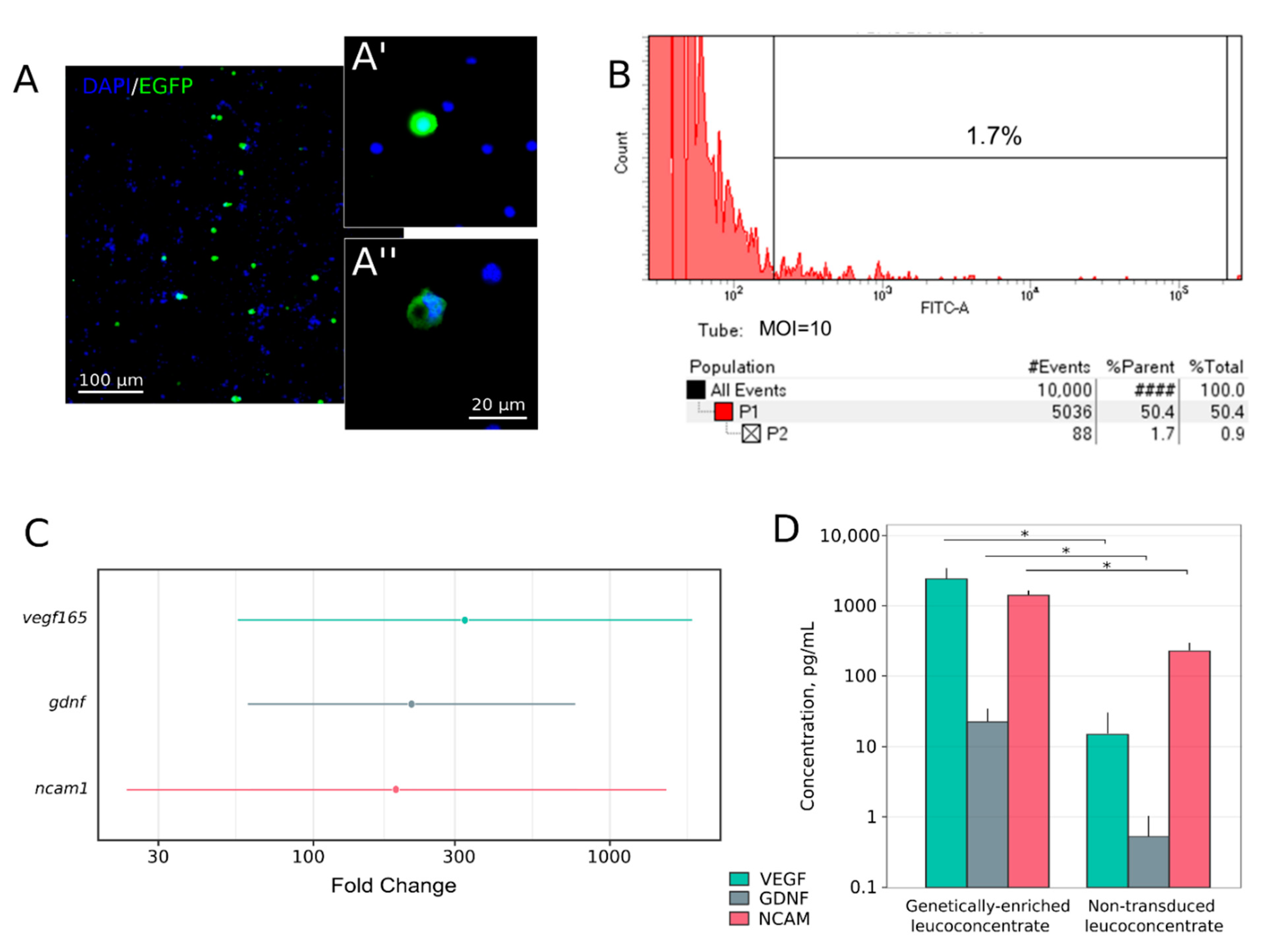
3.1. Complete Blood Count
3.2. Expression of the Recombinant Genes in the Genetically-Enriched Leucoconcentrate
3.3. Hind Limb Locomotor Function Recovery
3.4. Post-Traumatic Spinal Cord Molecular and Cellular Changes
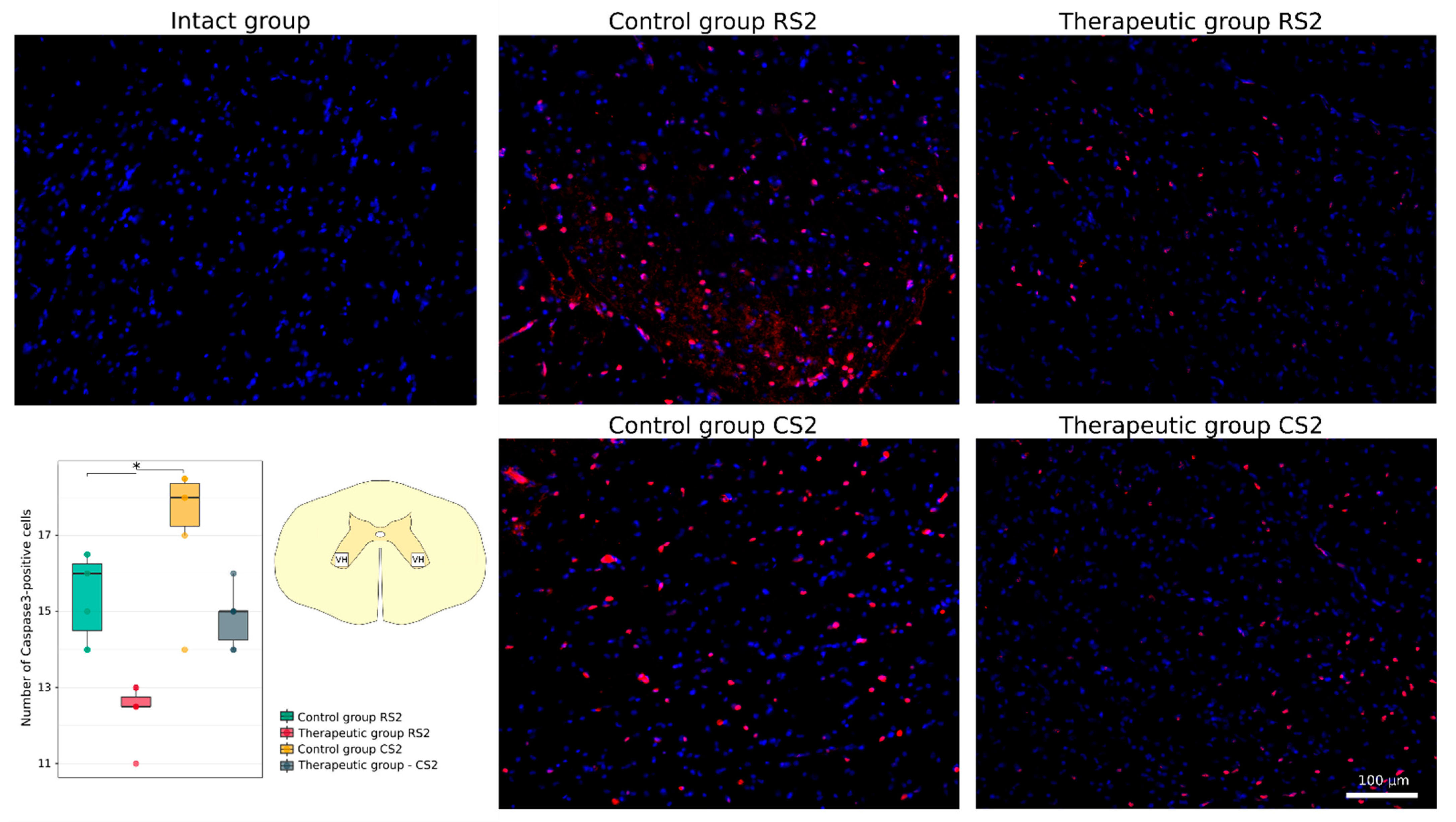
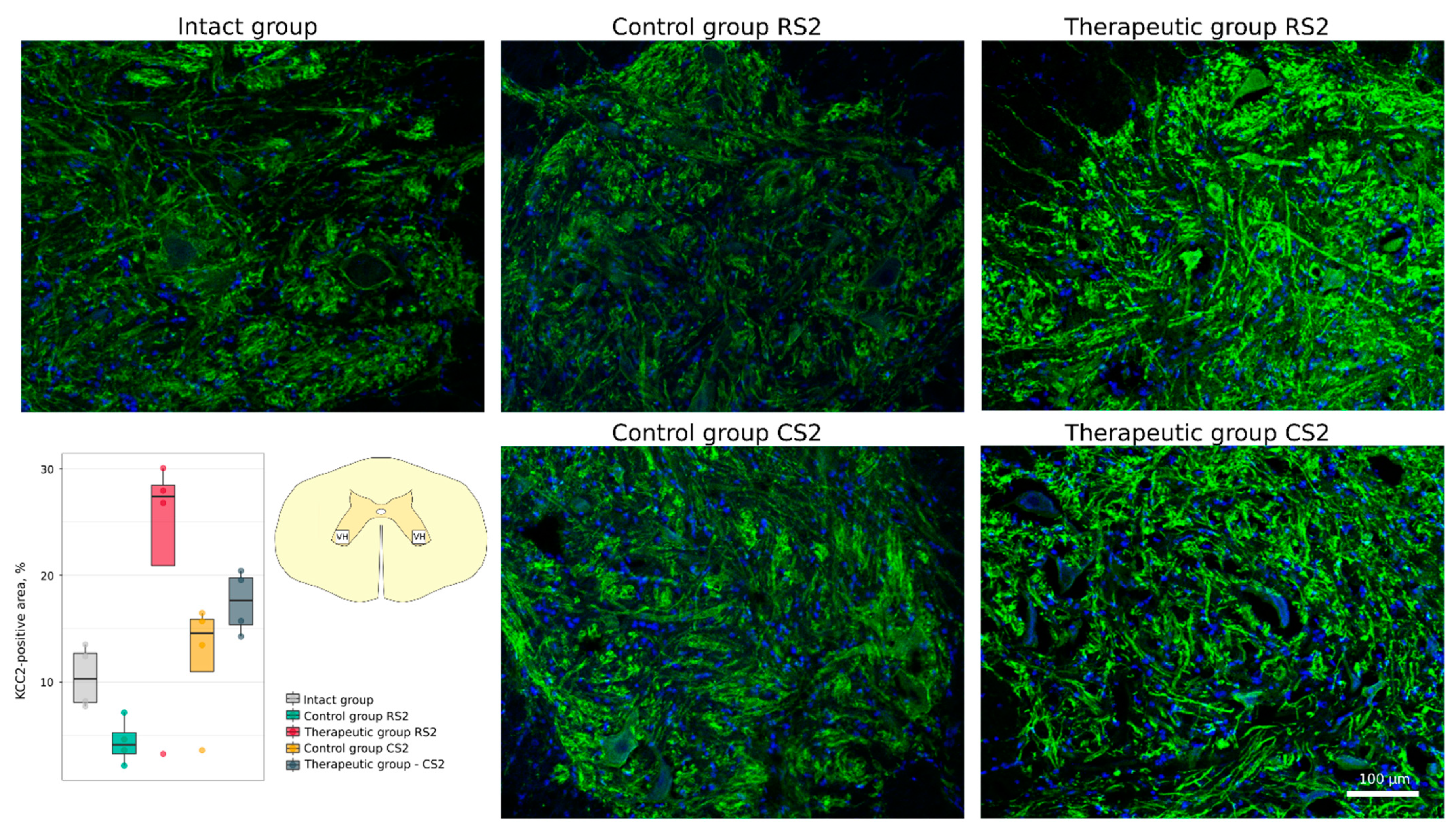
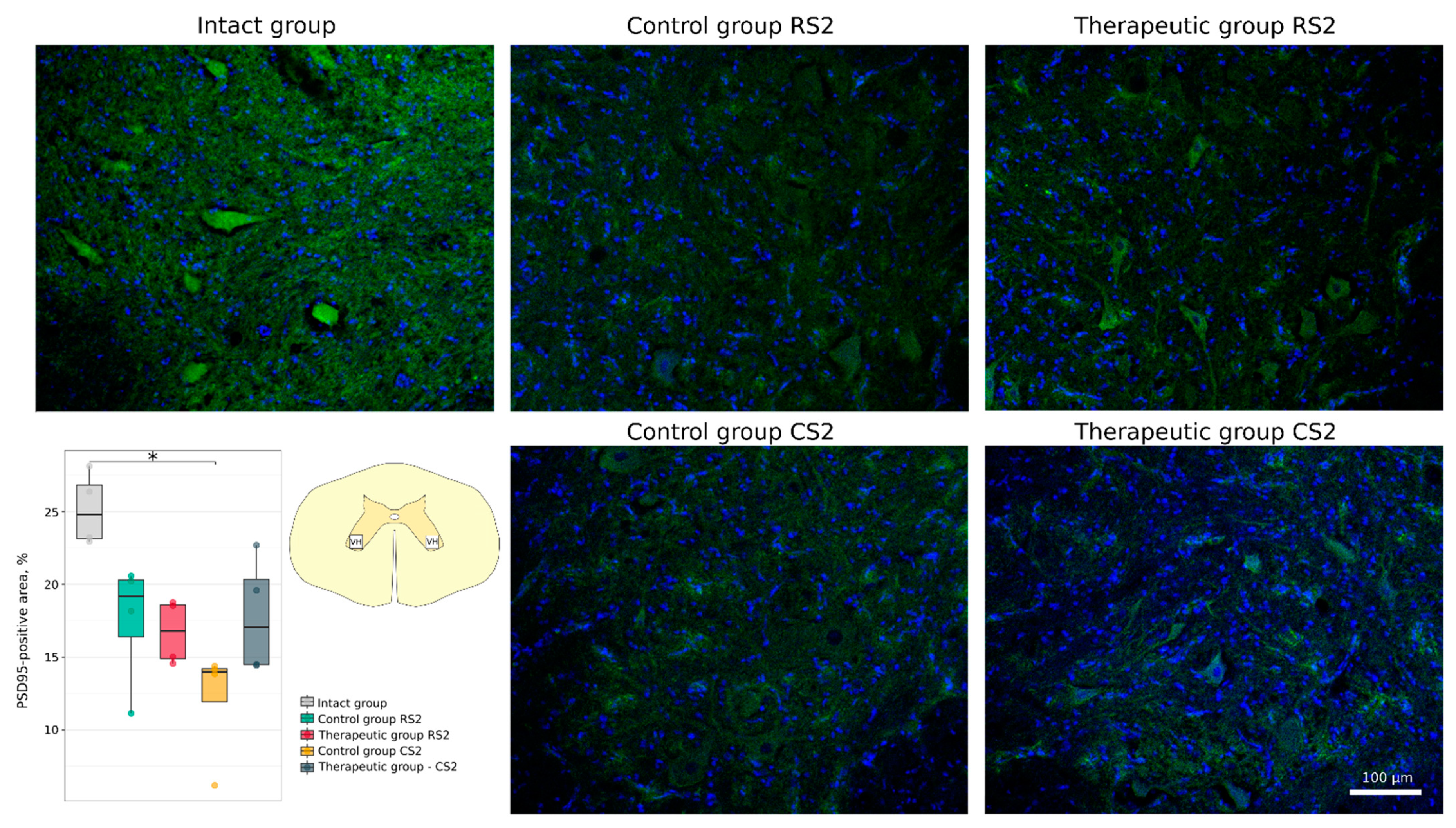
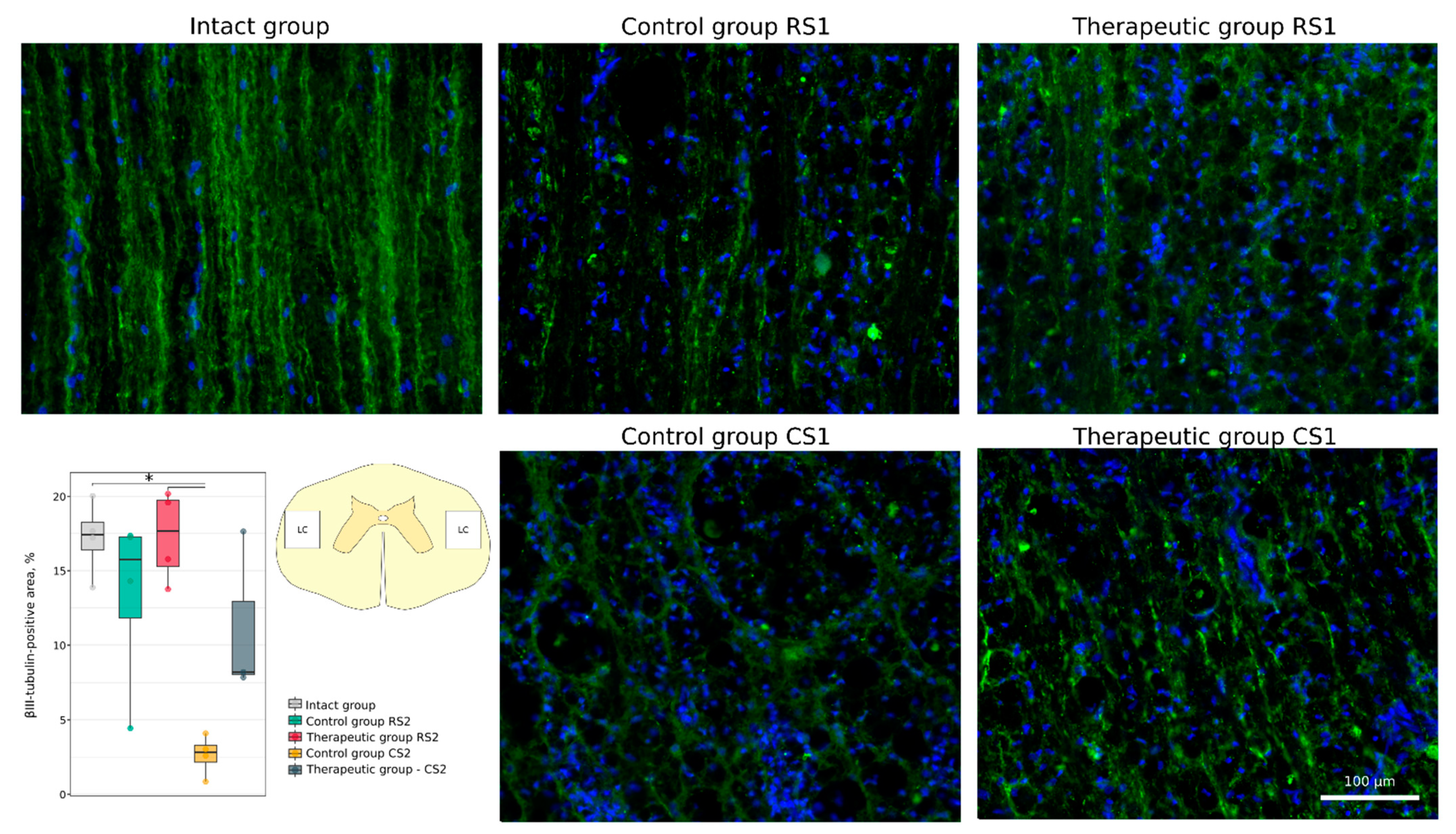
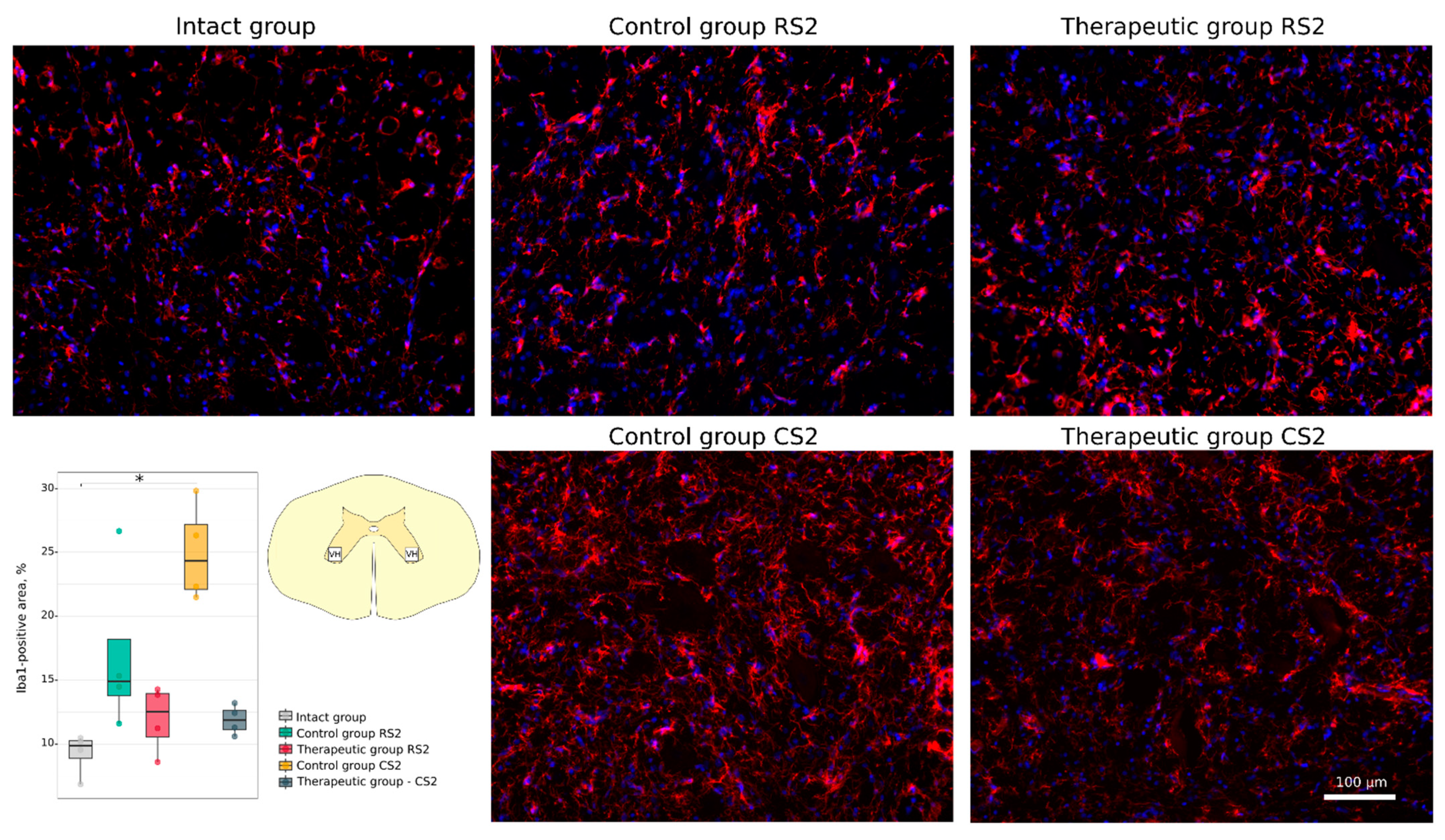
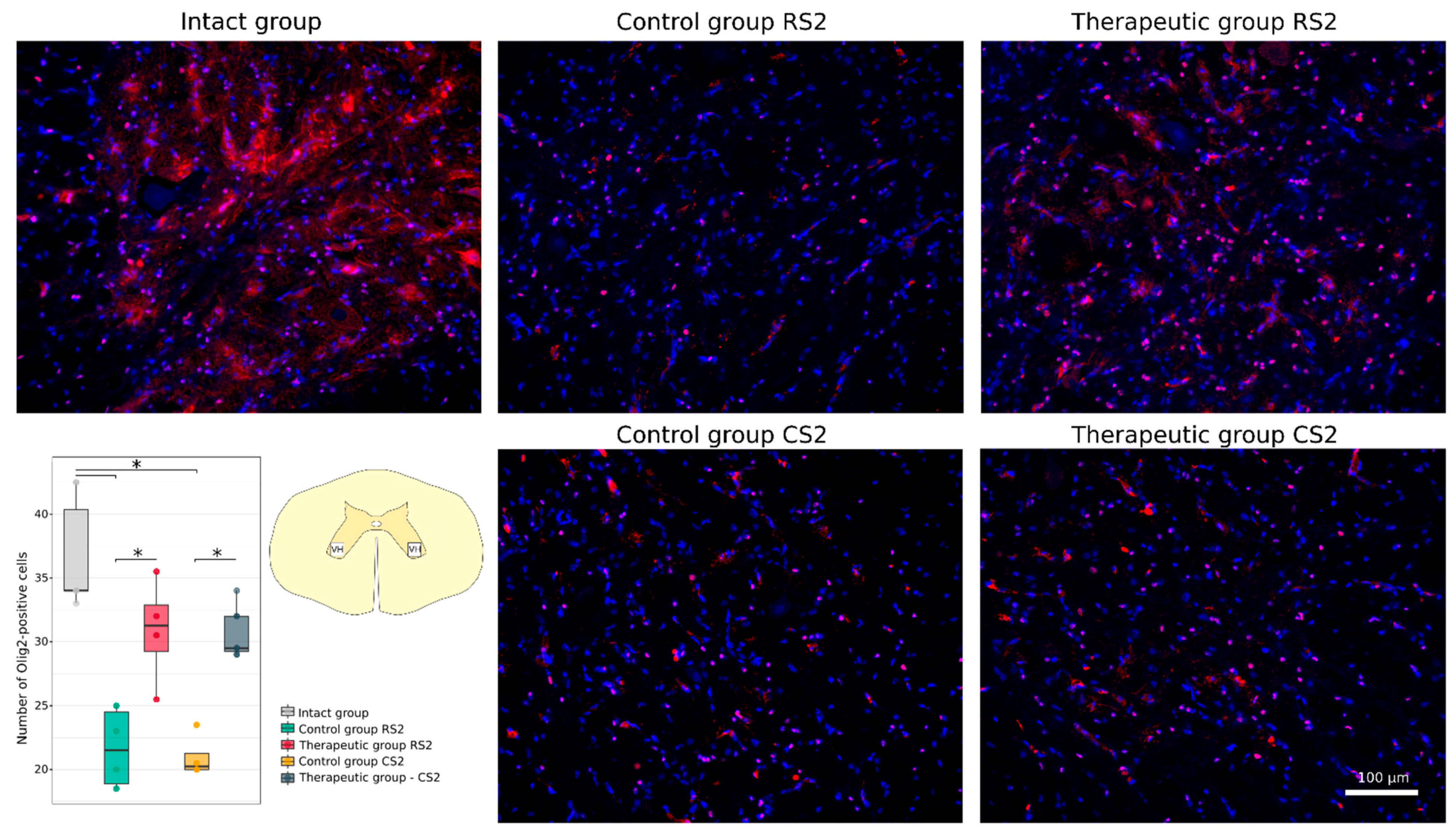
3.4.1. Immunofluorescence Study
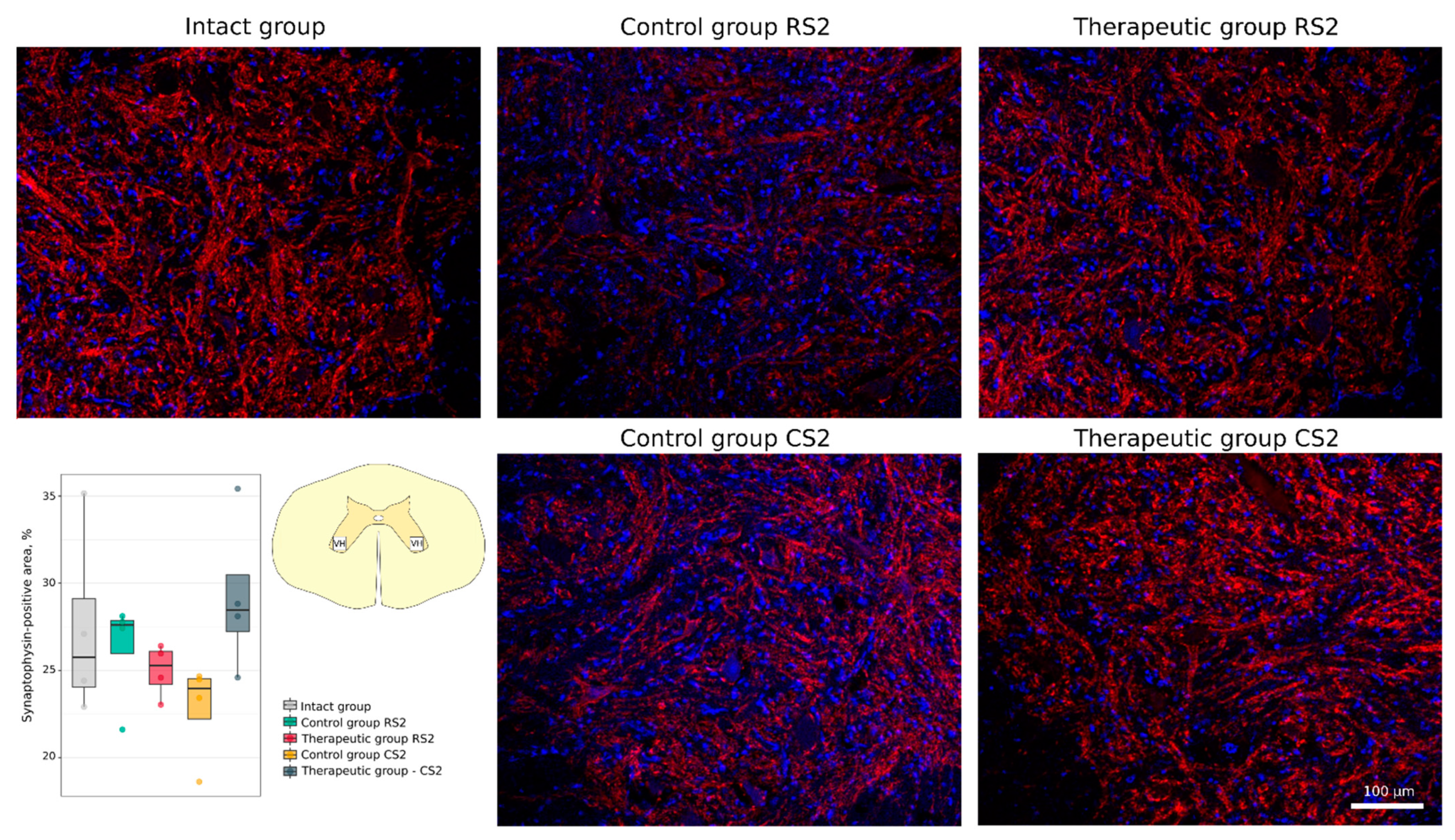
3.4.2. Synaptic Gene Expression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, A.; Varma, A.; Banik, N. Recent advances in the pharmacologic treatment of spinal cord injury. Metab. Brain Dis. 2015, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.L.; Fong, A.J.; Otoshi, C.K.; Liang, Y.; Burdick, J.W.; Roy, R.R.; Edgerton, V.R. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J. Neurosci. 2006, 26, 10564–10568. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Bonizzato, M.; Martinez, M. An intracortical neuroprosthesis immediately alleviates walking deficits and improves recovery of leg control after spinal cord injury. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, I.P.; Avelev, V.D.; Nikitin, O.A.; Lavrov, I.A. [Initiation of locomotor activity in spinalized cats by epidural stimulation of the spinal cord]. Ross. Fiziol. zhurnal Im. I.M. Sechenova 2001, 87, 1161–1170. [Google Scholar]
- Lavrov, I.; Dy, C.J.; Fong, A.J.; Gerasimenko, Y.; Courtine, G.G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J. Neurosci. 2008, 28, 6022–6029. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, I.; Musienko, P.E.; Selionov, V.A.; Zdunowski, S.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Activation of spinal locomotor circuits in the decerebrated cat by spinal epidural and/or intraspinal electrical stimulation. Brain Res. 2015, 1600, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Lavrov, I. Spinal Epidural Stimulation Strategies: Clinical Implications of Locomotor Studies in Spinal Rats. Neuroscientist 2017, 23, 664–680. [Google Scholar] [CrossRef]
- Fehlings, M.; Zavvarian, M.M.; Toossi, A.; Khazaei, M.; Hong, J. Novel innovations in cell and gene therapies for spinal cord injury. F1000Research 2020, 9. [Google Scholar]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Pearse, D.D.; Ozawa, H.; Itoi, E.; Bunge, M.B. Schwann cell transplantation for spinal cord injury repair: Its significant therapeutic potential and prospectus. Rev. Neurosci. 2015, 26, 121–128. [Google Scholar] [CrossRef]
- Li, Y.; Field, P.M.; Raisman, G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 1997, 277, 2000–2002. [Google Scholar] [CrossRef]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poon, W.; Liu, Y.; Leung, G.K.K.; Wong, Y.; Feng, Y.; Ng, S.C.P.; Tsang, K.S.; Sun, D.T.F.; Yeung, D.K.; et al. Phase I–II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant. 2016, 25, 1925–1943. [Google Scholar] [CrossRef]
- Walthers, C.M.; Seidlits, S.K. Gene delivery strategies to promote spinal cord repair. Biomark. Insights 2015, 2015, 11–29. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Guerrero, A.R.; Johnson, W.E.; Masri, W.E.; Baba, H. Gene therapy strategies for the treatment of spinal cord injury. Ther. Deliv. 2014, 5, 591–607. [Google Scholar] [CrossRef]
- Grill, R.J.; Blesch, A.; Tuszynski, M.H. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp. Neurol. 1997, 148, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Grill, R.; Jones, L.L.; Brant, A.; Blesch, A.; Löw, K.; Lacroix, S.; Lu, P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp. Neurol. 2003, 181, 47–56. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Y.; Zhang, W.; Wang, J.; Wu, J.; Li, J. Co-transplantation of neural stem cells and NT-3-overexpressing schwann cells in transected spinal cord. J. Neurotrauma 2007, 24, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Chen, J.; Lavdas, A.A.; Thomaidou, D.; Schachner, M.; Matsas, R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007, 130, 2159–2174. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; He, Q.; Wang, Y.; Cheng, X.; Howard, R.M.; Zhang, Y.; DeVries, W.H.; Shields, C.B.; Magnuson, D.S.K.; Xu, X.M.; et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J. Neurosci. 2010, 30, 2989–3001. [Google Scholar] [CrossRef]
- Kusano, K.; Enomoto, M.; Hirai, T.; Tsoulfas, P.; Sotome, S.; Shinomiya, K.; Okawa, A. Transplanted neural progenitor cells expressing mutant NT3 promote myelination and partial hindlimb recovery in the chronic phase after spinal cord injury. Biochem. Biophys. Res. Commun. 2010, 393, 812–817. [Google Scholar] [CrossRef]
- Sasaki, M.; Radtke, C.; Tan, A.M.; Zhao, P.; Hamada, H.; Houkin, K.; Honmou, O.; Kocsis, J.D. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 2009, 29, 14932–14941. [Google Scholar] [CrossRef]
- Cao, L.; Liu, L.; Chen, Z.Y.; Wang, L.M.; Ye, J.L.; Qiu, H.Y.; Lu, C.L.; He, C. Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain 2004, 127, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Islam, R.; Cuellar, C.A.; Silvernail, J.L.; Knudsen, B.; Curley, D.E.; Strickland, T.; Manske, E.; Suwan, P.T.; Latypov, T.; et al. Newly regenerated axons through a cell-containing biomaterial scaffold promote reorganization of spinal circuitry and restoration of motor functions with epidural electrical stimulation. npj Regen. Med. 2021, 6, 66. [Google Scholar] [CrossRef]
- Fadeev, F.O.; Bashirov, F.V.; Markosyan, V.A.; Izmailov, A.A.; Povysheva, T.V.; Sokolov, M.E.; Kuznetsov, M.S.; Eremeev, A.A.; Salafutdinov, I.I.; Rizvanov, A.A.; et al. Combination of epidural electrical stimulation with ex vivo triple gene therapy for spinal cord injury: A proof of principle study in a rat model. Neural Regen. Res. 2021, 16, 550–560. [Google Scholar] [CrossRef]
- Fadeev, F.; Eremeev, A.; Bashirov, F.; Shevchenko, R.; Izmailov, A.; Markosyan, V.; Sokolov, M.; Kalistratova, J.; Khalitova, A.; Garifulin, R.; et al. Combined supra-and sub-lesional epidural electrical stimulation for restoration of the motor functions after spinal cord injury in mini pigs. Brain Sci. 2020, 10, 744. [Google Scholar] [CrossRef]
- Islamov, R.; Bashirov, F.; Fadeev, F.; Shevchenko, R.; Izmailov, A.; Markosyan, V.; Sokolov, M.; Kuznetsov, M.; Davleeva, M.; Garifulin, R.; et al. Epidural Stimulation Combined with Triple Gene Therapy for Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2020, 21, 8896. [Google Scholar] [CrossRef]
- Izmailov, A.A.; Povysheva, T.V.; Bashirov, F.V.; Sokolov, M.E.; Fadeev, F.O.; Garifulin, R.R.; Naroditsky, B.S.; Logunov, D.Y.; Salafutdinov, I.I.; Chelyshev, Y.A.; et al. Spinal Cord Molecular and Cellular Changes Induced by Adenoviral Vector- and Cell-Mediated Triple Gene Therapy after Severe Contusion. Front. Pharmacol. 2017, 8, 813. [Google Scholar] [CrossRef]
- Adams, W.C.; Gujer, C.; McInerney, G.; Gall, J.G.D.; Petrovas, C.; Karlsson Hedestam, G.B.; Koup, R.A.; Loré, K. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc. Natl. Acad. Sci. USA 2011, 108, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.R.; Bashirov, F.V.F.V.; Sokolov, M.E.M.E.; Izmailov, A.A.A.A.; Fadeev, F.O.F.O.; Markosyan, V.A.V.A.; Davleeva, M.A.M.A.; Zubkova, O.V.O.V.; Smarov, M.M.M.M.; Logunov, D.Y.; et al. Gene-modified leucoconcentrate for personalized ex vivo gene therapy in a mini pig model of moderate spinal cord injury. Neural Regen. Res. 2021, 16, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.; Cummings, D.T.; Evelegh, C.M.; Graham, F.L. Yeast recombinase FLP functions effectively in human cells for constraction of adenovirus vectors. Biotechniques 2000, 29, 524–528. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Krupa, P.; Siddiqui, A.M.; Grahn, P.J.; Islam, R.; Chen, B.K.; Madigan, N.N.; Windebank, A.J.; Lavrov, I.A. The Translesional Spinal Network and Its Reorganization after Spinal Cord Injury. Neuroscientist 2020. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.; Sokolov, M.E.; Bashirov, F.V.; Fadeev, F.O.; Shmarov, M.M.; Naroditskiy, B.S.; Povysheva, T.V.; Shaymardanova, G.F.; Yakupov, R.A.; Chelyshev, Y.A.; et al. A pilot study of cell-mediated gene therapy for spinal cord injury in mini pigs. Neurosci. Lett. 2017, 644, 67–75. [Google Scholar] [CrossRef]
- Lee, J.H.T.; Jones, C.F.; Okon, E.B.; Anderson, L.; Tigchelaar, S.; Kooner, P.; Godbey, T.; Chua, B.; Gray, G.; Hildebrandt, R.; et al. A Novel Porcine Model of Traumatic Thoracic Spinal Cord Injury. J. Neurotrauma 2013, 30, 142–159. [Google Scholar] [CrossRef]
- Harvie, D.S.; Fadiga, L.; Smith, R.T.; Hunter, E.V.; Davis, M.G.; Sterling, M.; Lorimer Moseley, G. Using visuo-kinetic virtual reality to induce illusory spinal movement: The MoOVi Illusion. PeerJ 2017, 1–16. [Google Scholar] [CrossRef][Green Version]
- Friedli, L.; Rosenzweig, E.S.; Barraud, Q.; Schubert, M.; Dominici, N.; Awai, L.; Nielson, J.L.; Musienko, P.; Nout-Lomas, Y.; Zhong, H.; et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci. Transl. Med. 2015, 7, 302ra134. [Google Scholar] [CrossRef]
- Schomberg, D.T.; Miranpuri, G.S.; Chopra, A.; Patel, K.; Meudt, J.J.; Tellez, A.; Resnick, D.K.; Shanmuganayagam, D. Translational relevance of swine models of spinal cord injury. J. Neurotrauma 2017, 34, 541–551. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Schomberg, D.T.; Stan, P.; Chopra, A.; Buttar, S.; Wood, A.; Radzin, A.; Meudt, J.J.; Resnick, D.K.; Shanmuganayagam, D. Comparative Morphometry of the Wisconsin Miniature Swine(TM) Thoracic Spine for Modeling Human Spine in Translational Spinal Cord Injury Research. Ann. Neurosci. 2018, 25, 210–218. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Bogacheva, I.N.; Shcherbakova, N.A.; Makarovskii, A.N. Bioelectric activity of spinal cord in patients with vertebrospinal pathologies. Bull. Exp. Biol. Med. 2001, 132, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, I.; Gerasimenko, Y.P.; Ichiyama, R.M.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J. Neurophysiol. 2006, 96, 1699–1710. [Google Scholar] [CrossRef]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Gerasimenko, Y.; Van Den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Grahn, P.J.; Lavrov, I.A.; Sayenko, D.G.; Van Straaten, M.G.; Gill, M.L.; Strommen, J.A.; Calvert, J.S.; Drubach, D.I.; Beck, L.A.; Linde, M.B.; et al. Enabling Task-Specific Volitional Motor Functions via Spinal Cord Neuromodulation in a Human With Paraplegia. Mayo Clin. Proc. 2017, 92, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and clinical implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, E.D.; Markov, G.J.; Eckalbar, W.L.; George, R.M.; King, J.M.; Tokuyama, M.A.; Geiger, L.A.; Emmert, N.; Ammar, M.J.; Allen, A.N.; et al. Transcriptomic analysis of tail regeneration in the lizard Anolis carolinensis reveals activation of conserved vertebrate developmental and repair mechanisms. PLoS ONE 2014, 9, e105004. [Google Scholar] [CrossRef]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.-H.; Davis, F.G.; et al. A Tissue-Mapped Axolotl De Novo Transcriptome Enables Identification of Limb Regeneration Factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Tillo, M.; Ruhrberg, C.; Mackenzie, F. Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell Adhes. Migr. 2012, 6, 541–546. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Andressoo, J.O. Biology of GDNF and its receptors—Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.; Ferrua, F.; Castagnaro, L.; Pajno, R.; Barzaghi, F.; Giannelli, S.; Dionisio, F.; Brigida, I.; Bonopane, M.; Casiraghi, M.; et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 2016, 128, 45–54. [Google Scholar] [CrossRef] [PubMed]



| Genes and Nucleotide Sequences | Product Length, bp | GC% | Tm, °C |
|---|---|---|---|
| vegf165 F: CTTGCTCTATCTTTCTTTGG R: GCTGCTCTACCTCCACCATG | 400 | 40.00 | 45 |
| 60.00 | 45 | ||
| gdnf F: GGATGTCGTGGCTGTCTGCCTGG R: TGCCCCTCTGGCCTCTCCGACC | 300 | 65.20 | 55 |
| 72.70 | 55 | ||
| ncam1 F: GCCGTGATTGTGTGTGATGT R: GCCTCGTCGTTCTTATCCAC | 450 | 50.00 | 45 |
| 55.00 | 45 | ||
| chat (NM_001001541) F: CCATGCCGGATTCGGAGAA R: CCCATTGGGTACCACAGGAC | 263 | 57.89 | 59.85 |
| 60.00 | 60.03 | ||
| chrm1 (NM_214034) F: GAAAAGCTTGGCTCAGAGGGA R: ATGACATAGTGGGACCGTCG | 260 | 52.38 | 60.27 |
| 55.00 | 59.26 | ||
| gad67 (D31849) F: GCCGACCTCATTGTCCGTAT R: GTGTTCTCCACCCCACACAA | 184 | 55.00 | 59.90 |
| 55.00 | 60.11 | ||
| gabra2 (XM_013978645) F: CACGCCAGAACCCAACAAGA R: GTACATGGCAAAACAAACCAGG | 244 | 55.00 | 60.82 |
| 45.45 | 58.61 | ||
| gapdh (NM_001206359) F: CCGTGTGTTCCGTGCATTG R: TGCCGTGGGTGGAATCATAC | 198 | 57.89 | 60.08 |
| 55.00 | 60.11 |
| Antibody Against: | Host | Dilution | Source |
|---|---|---|---|
| Beta III Tubulin | Rabbit | 1:200 | Abcam (Cat # ab18207) |
| Caspase 3 | Rabbit | 1:200 | Cell Signaling Technology (Cat # 9661) |
| Glial cell-line derived neurotrophic factor (GDNF) | Rabbit | 1:100 | Santa Cruz (Cat # sc-9010) |
| Glial fibrillary acidic protein (GFAP) | Mouse | 1:200 | Santa Cruz (Cat # sc-33673) |
| Ionized calcium binding adaptor molecule 1 (Iba1) | Rabbit | 1:150 | Abcam (Cat # ab178847) |
| The K+–Cl− cotransporter isoform 2 (KCC2) | Rabbit | 1:200 | Abcam (Cat # ab49917) |
| Heat shock protein 27 kDa (Hsp27) | Rabbit | 1:200 | Abcam (Cat # ab12351) |
| Oligodendrocyte transcription factor 2 (Olig2) | Rabbit | 1:100 | Abcam (Cat # ab18258) |
| Neural cell adhesion molecule (NCAM) | Rabbit | 1:100 | Abcam (Cat # ab9272) |
| Postsynaptic density protein 95 kDa (PSD95) | Rabbit | 1:200 | Abcam (Cat # ab18258) |
| Synaptophysin | Rabbit | 1:100 | Abcam (Cat # ab32127) |
| Vascular endothelial growth factor (VEGF) | Rabbit | 1:300 | Santa Cruz (Cat # sc-152) |
| Mouse IgG conjugated with Alexa 488 | Donkey | 1:200 | Invitrogen (Cat # A-21202) |
| Rabbit IgG conjugated with Alexa 488 | Donkey | 1:200 | Invitrogen (Cat # A-21206) |
| Rabbit IgG conjugated with Alexa 647 | Donkey | 1:200 | Invitrogen (Cat # A-31573) |
| Sample | WBC (10⁹/L) | RBC (1012/L) | PLT (10⁹/L) | LYM (10⁹/L) | MON (10⁹/L) | GRAN (10⁹/L) | Volume (mL) |
|---|---|---|---|---|---|---|---|
| VB | 13.2 ± 3.3 | 6.5 ± 0.9 | 710.7 ± 88.8 | 7.0 ± 1.5 | 0.6 ± 0.3 | 5.6 ± 1.6 | 50.0 ± 0.0 |
| GEL | 8.0 ± 3.7 | 0.2 ± 0.2 | 196.0 ± 155.2 | 2.9 ± 2.5 | 0.6 ± 0.4 | 4.5 ± 3.9 | 49.3 ± 14.4 |
| P | 0.0469 | 0.0156 | 0.0156 | 0.0156 | 1 | 0.3750 | 0.9325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamov, R.; Bashirov, F.; Izmailov, A.; Fadeev, F.; Markosyan, V.; Sokolov, M.; Shmarov, M.; Logunov, D.; Naroditsky, B.; Lavrov, I. New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation. Cells 2022, 11, 144. https://doi.org/10.3390/cells11010144
Islamov R, Bashirov F, Izmailov A, Fadeev F, Markosyan V, Sokolov M, Shmarov M, Logunov D, Naroditsky B, Lavrov I. New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation. Cells. 2022; 11(1):144. https://doi.org/10.3390/cells11010144
Chicago/Turabian StyleIslamov, Rustem, Farid Bashirov, Andrei Izmailov, Filip Fadeev, Vage Markosyan, Mikhail Sokolov, Maksim Shmarov, Denis Logunov, Boris Naroditsky, and Igor Lavrov. 2022. "New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation" Cells 11, no. 1: 144. https://doi.org/10.3390/cells11010144
APA StyleIslamov, R., Bashirov, F., Izmailov, A., Fadeev, F., Markosyan, V., Sokolov, M., Shmarov, M., Logunov, D., Naroditsky, B., & Lavrov, I. (2022). New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation. Cells, 11(1), 144. https://doi.org/10.3390/cells11010144








