Partial Disassembly of the Nuclear Pore Complex Proteins during Semi-Closed Mitosis in Dictyostelium discoideum
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning
2.2. Cell Culture
2.3. Immunofluorescence and Microscopic Imaging
2.4. Live Cell Imaging
3. Results
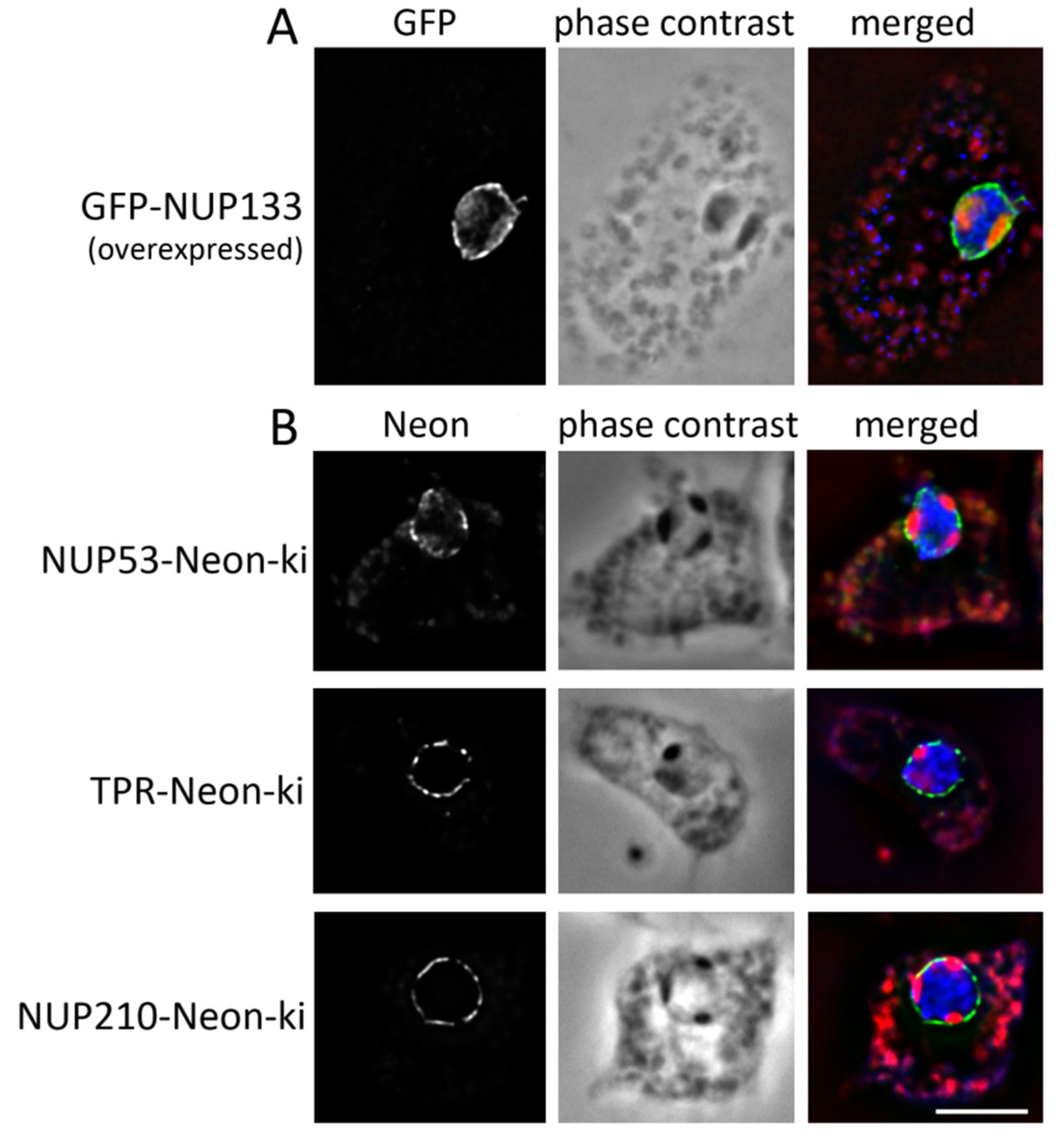
3.1. Nuclear Pore Complexes Are Excluded from Nucleoli
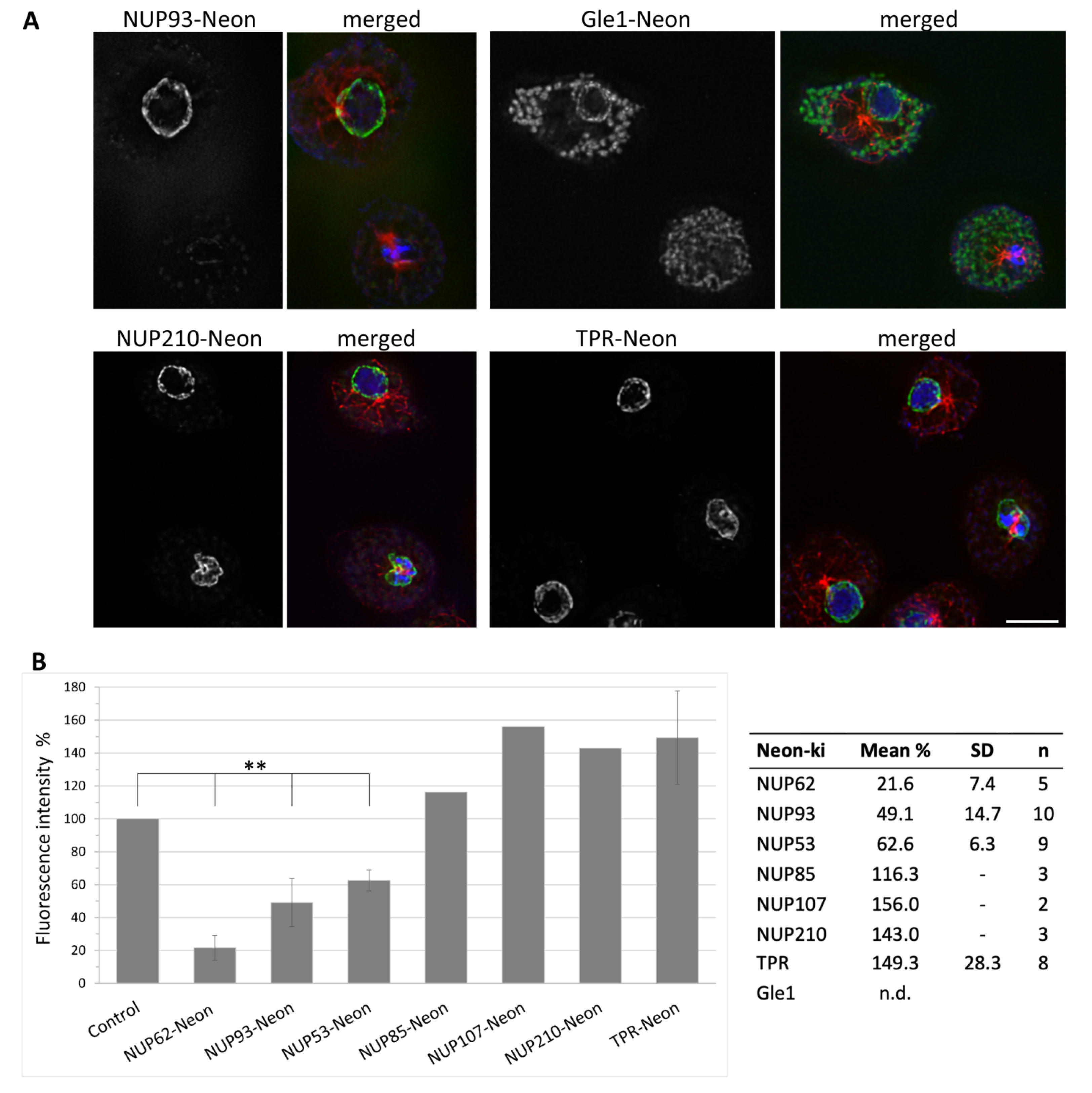
3.2. Some NUPs Localize to the Centrosome
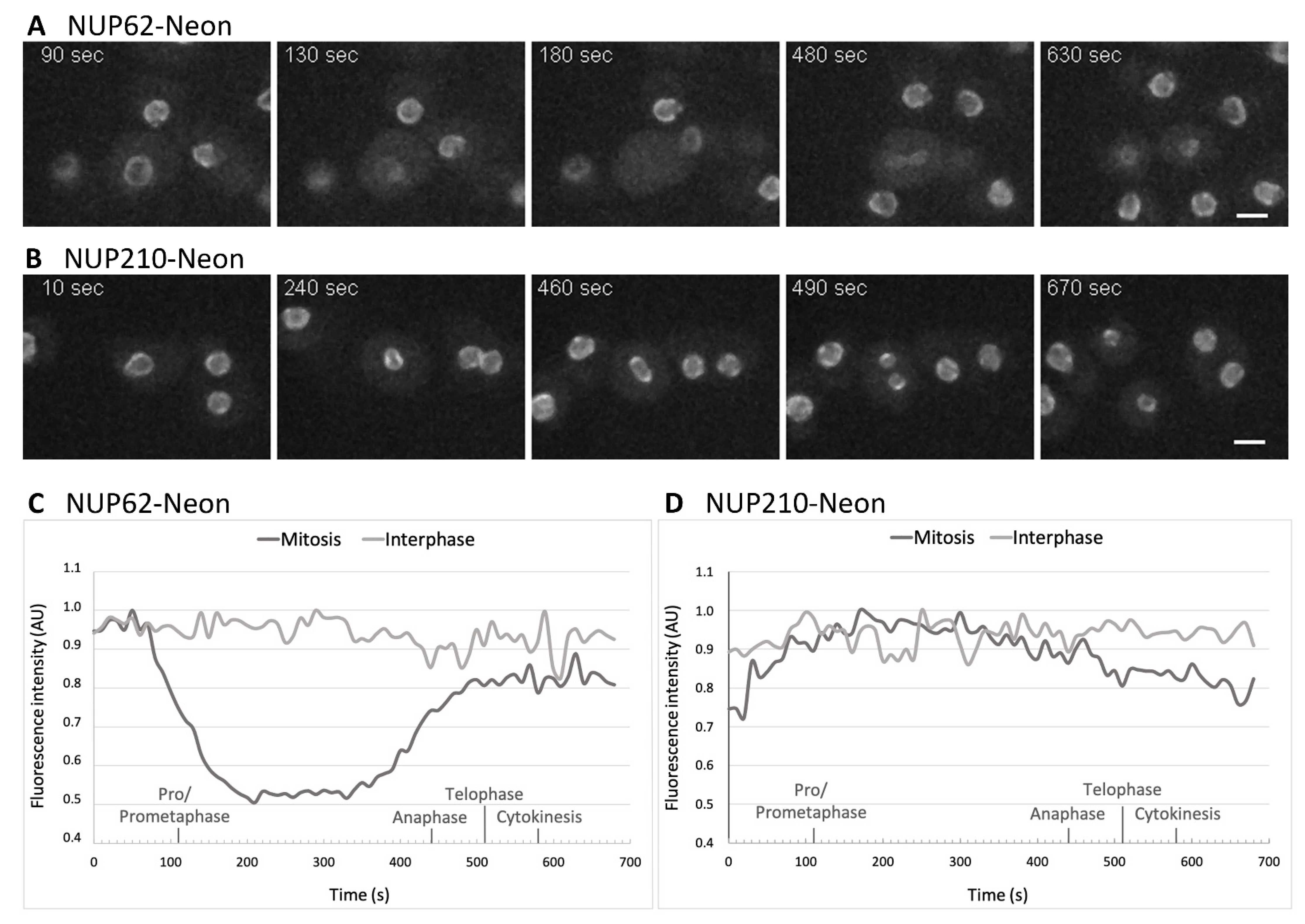
3.3. NUPs Show Divergent Behaviour during Mitosis
3.4. During Mitosis Some NUPs Concentrate at Spindle Poles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Güttinger, S.; Laurell, E.; Kutay, U. Orchestrating Nuclear Envelope Disassembly and Reassembly during Mitosis. Nat. Rev. Mol. Cell Biol. 2009, 10, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Kubai, D.F. The Evolution of the Mitotic Spindle. Int. Rev. Cytol. 1975, 43, 167–227. [Google Scholar] [CrossRef]
- Batsios, P.; Gräf, R.; Koonce, M.P.; Larochelle, D.A.; Meyer, I. Nuclear Envelope Organization in Dictyostelium discoideum. Int. J. Dev. Biol. 2019, 63, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Samereier, M.; Meyer, I.; Koonce, M.P.; Gräf, R. Live Cell-Imaging Techniques for Analyses of Microtubules in Dictyostelium. Methods Cell Biol. 2010, 97, 341–357. [Google Scholar] [CrossRef]
- O’Day, D.H.; Budniak, A. Nucleocytoplasmic Protein Translocation during Mitosis in the Social Amoebozoan Dictyostelium discoideum. Biol. Rev. Camb. Philos. Soc. 2015, 90, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Meyer, I.; Peter, T.; Batsios, P.; Kuhnert, O.; Krüger-Genge, A.; Camurça, C.; Gräf, R. CP39, CP75 and CP91 Are Major Structural Components of the Dictyostelium Centrosome’s Core Structure. Eur. J. Cell Biol. 2017, 96, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Osmani, A.H.; Davies, J.; Liu, H.-L.; Nile, A.; Osmani, S.A. Systematic Deletion and Mitotic Localization of the Nuclear Pore Complex Proteins of Aspergillus Nidulans. Mol. Biol. Cell 2006, 17, 4946–4961. [Google Scholar] [CrossRef]
- Frenkiel-Krispin, D.; Maco, B.; Aebi, U.; Medalia, O. Structural Analysis of a Metazoan Nuclear Pore Complex Reveals a Fused Concentric Ring Architecture. J. Mol. Biol. 2010, 395, 578–586. [Google Scholar] [CrossRef]
- Lange, A.; Mills, R.E.; Lange, C.J.; Stewart, M.; Devine, S.E.; Corbett, A.H. Classical Nuclear Localization Signals: Definition, Function, and Interaction with Importin Alpha. J. Biol. Chem. 2007, 282, 5101–5105. [Google Scholar] [CrossRef]
- Franke, W.W.; Scheer, U.; Krohne, G.; Jarasch, E.D. The Nuclear Envelope and the Architecture of the Nuclear Periphery. J. Cell Biol. 1981, 91, 39s–50s. [Google Scholar] [CrossRef]
- Panté, N.; Aebi, U. Sequential Binding of Import Ligands to Distinct Nucleopore Regions during Their Nuclear Import. Science 1996, 273, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Förster, F.; Ecke, M.; Plitzko, J.M.; Melchior, F.; Gerisch, G.; Baumeister, W.; Medalia, O. Nuclear Pore Complex Structure and Dynamics Revealed by Cryoelectron Tomography. Science 2004, 306, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Lucić, V.; Förster, F.; Baumeister, W.; Medalia, O. Snapshots of Nuclear Pore Complexes in Action Captured by Cryo-Electron Tomography. Nature 2007, 449, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Medalia, O. Structural and Functional Insights into Nucleocytoplasmic Transport. Histol. Histopathol. 2008, 23, 1025–1033. [Google Scholar] [CrossRef]
- Xiong, H.; Rivero, F.; Euteneuer, U.; Mondal, S.; Mana-Capelli, S.; Larochelle, D.; Vogel, A.; Gassen, B.; Noegel, A.A. Dictyostelium Sun-1 Connects the Centrosome to Chromatin and Ensures Genome Stability. Traffic Cph. Den. 2008, 9, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Schulz, I.; Erle, A.; Gräf, R.; Krüger, A.; Lohmeier, H.; Putzler, S.; Samereier, M.; Weidenthaler, S. Identification and Cell Cycle-Dependent Localization of Nine Novel, Genuine Centrosomal Components in Dictyostelium discoideum. Cell Motil. Cytoskelet. 2009, 66, 915–928. [Google Scholar] [CrossRef]
- Pitzen, V.; Askarzada, S.; Gräf, R.; Meyer, I. CDK5RAP2 Is an Essential Scaffolding Protein of the Corona of the Dictyostelium Centrosome. Cells 2018, 7, 32. [Google Scholar] [CrossRef]
- Gräf, R.; Euteneuer, U.; Ho, T.-H.; Rehberg, M. Regulated Expression of the Centrosomal Protein DdCP224 Affects Microtubule Dynamics and Reveals Mechanisms for the Control of Supernumerary Centrosome Number. Mol. Biol. Cell 2003, 14, 4067–4074. [Google Scholar] [CrossRef]
- Wehland, J.; Willingham, M.C.; Sandoval, I.V. A Rat Monoclonal Antibody Reacting Specifically with the Tyrosylated Form of Alpha-Tubulin. I. Biochemical Characterization, Effects on Microtubule Polymerization in vitro, and Microtubule Polymerization and Organization in Vivo. J. Cell Biol. 1983, 97, 1467–1475. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Gräf, R.; Grafe, M.; Meyer, I.; Mitic, K.; Pitzen, V. The Dictyostelium Centrosome. Cells 2021, 10, 2657. [Google Scholar] [CrossRef] [PubMed]
- Miné-Hattab, J.; Taddei, A. Physical Principles and Functional Consequences of Nuclear Compartmentalization in Budding Yeast. Curr. Opin. Cell Biol. 2019, 58, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Galy, V.; Gadal, O.; Fromont-Racine, M.; Romano, A.; Jacquier, A.; Nehrbass, U. Nuclear Retention of Unspliced MRNAs in Yeast Is Mediated by Perinuclear Mlp1. Cell 2004, 116, 63–73. [Google Scholar] [CrossRef]
- Niepel, M.; Molloy, K.R.; Williams, R.; Farr, J.C.; Meinema, A.C.; Vecchietti, N.; Cristea, I.M.; Chait, B.T.; Rout, M.P.; Strambio-De-Castillia, C. The Nuclear Basket Proteins Mlp1p and Mlp2p Are Part of a Dynamic Interactome Including Esc1p and the Proteasome. Mol. Biol. Cell 2013, 24, 3920–3938. [Google Scholar] [CrossRef]
- Batsios, P.; Ren, X.; Baumann, O.; Larochelle, D.A.; Gräf, R. Src1 Is a Protein of the Inner Nuclear Membrane Interacting with the Dictyostelium Lamin NE81. Cells 2016, 5, 13. [Google Scholar] [CrossRef]
- Linder, M.I.; Köhler, M.; Boersema, P.; Weberruss, M.; Wandke, C.; Marino, J.; Ashiono, C.; Picotti, P.; Antonin, W.; Kutay, U. Mitotic Disassembly of Nuclear Pore Complexes Involves CDK1- and PLK1-Mediated Phosphorylation of Key Interconnecting Nucleoporins. Dev. Cell 2017, 43, 141–156.e7. [Google Scholar] [CrossRef]
- Kutay, U.; Jühlen, R.; Antonin, W. Mitotic Disassembly and Reassembly of Nuclear Pore Complexes. Trends Cell Biol. 2021, 31, 1019–1033. [Google Scholar] [CrossRef]
- Hase, M.E.; Cordes, V.C. Direct Interaction with Nup153 Mediates Binding of Tpr to the Periphery of the Nuclear Pore Complex. Mol. Biol. Cell 2003, 14, 1923–1940. [Google Scholar] [CrossRef]
- Fišerová, J.; Maninová, M.; Sieger, T.; Uhlířová, J.; Šebestová, L.; Efenberková, M.; Čapek, M.; Fišer, K.; Hozák, P. Nuclear Pore Protein TPR Associates with Lamin B1 and Affects Nuclear Lamina Organization and Nuclear Pore Distribution. Cell. Mol. Life Sci. CMLS 2019, 76, 2199–2216. [Google Scholar] [CrossRef]
- Schulz, I.; Baumann, O.; Samereier, M.; Zoglmeier, C.; Gräf, R. Dictyostelium Sun1 Is a Dynamic Membrane Protein of Both Nuclear Membranes and Required for Centrosomal Association with Clustered Centromeres. Eur. J. Cell Biol. 2009, 88, 621–638. [Google Scholar] [CrossRef]
- Lau, C.K.; Giddings, T.H.; Winey, M. A Novel Allele of Saccharomyces Cerevisiae NDC1 Reveals a Potential Role for the Spindle Pole Body Component Ndc1p in Nuclear Pore Assembly. Eukaryot. Cell 2004, 3, 447–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- West, R.R.; Vaisberg, E.V.; Ding, R.; Nurse, P.; McIntosh, J.R. Cut11(+): A Gene Required for Cell Cycle-Dependent Spindle Pole Body Anchoring in the Nuclear Envelope and Bipolar Spindle Formation in Schizosaccharomyces Pombe. Mol. Biol. Cell 1998, 9, 2839–2855. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jin, Z.; Zhang, X.; Shen, N.; Wang, J.; Zhao, Y.; Mei, L. Nup62, Associated with Spindle Microtubule Rather than Spindle Matrix, Is Involved in Chromosome Alignment and Spindle Assembly during Mitosis. Cell Biol. Int. 2016, 40, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-L.; Lai, J.-H.; Lin, T.-F.; Yang, W.-S.; Juang, Y.-L. NUP62 Is Required for the Maintenance of the Spindle Assembly Checkpoint and Chromosomal Stability. Int. J. Biochem. Cell Biol. 2020, 128, 105843. [Google Scholar] [CrossRef]
- Mishra, R.K.; Chakraborty, P.; Arnaoutov, A.; Fontoura, B.M.A.; Dasso, M. The Nup107-160 Complex and Gamma-TuRC Regulate Microtubule Polymerization at Kinetochores. Nat. Cell Biol. 2010, 12, 164–169. [Google Scholar] [CrossRef]
- Del Viso, F.; Huang, F.; Myers, J.; Chalfant, M.; Zhang, Y.; Reza, N.; Bewersdorf, J.; Lusk, C.P.; Khokha, M.K. Congenital Heart Disease Genetics Uncovers Context-Dependent Organization and Function of Nucleoporins at Cilia. Dev. Cell 2016, 38, 478–492. [Google Scholar] [CrossRef]
- Jao, L.-E.; Akef, A.; Wente, S.R. A Role for Gle1, a Regulator of DEAD-Box RNA Helicases, at Centrosomes and Basal Bodies. Mol. Biol. Cell 2017, 28, 120–127. [Google Scholar] [CrossRef]
- Busayavalasa, K.; Chen, X.; Farrants, A.-K.Ö.; Wagner, N.; Sabri, N. The Nup155-Mediated Organisation of Inner Nuclear Membrane Proteins Is Independent of Nup155 Anchoring to the Metazoan Nuclear Pore Complex. J. Cell Sci. 2012, 125, 4214–4218. [Google Scholar] [CrossRef]
- Hawryluk-Gara, L.A.; Shibuya, E.K.; Wozniak, R.W. Vertebrate Nup53 Interacts with the Nuclear Lamina and Is Required for the Assembly of a Nup93-Containing Complex. Mol. Biol. Cell 2005, 16, 2382–2394. [Google Scholar] [CrossRef]
- Pitzen, V.; Sander, S.; Baumann, O.; Gräf, R.; Meyer, I. Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae. Cells 2021, 10, 2384. [Google Scholar] [CrossRef]
- Vollmer, B.; Schooley, A.; Sachdev, R.; Eisenhardt, N.; Schneider, A.M.; Sieverding, C.; Madlung, J.; Gerken, U.; Macek, B.; Antonin, W. Dimerization and Direct Membrane Interaction of Nup53 Contribute to Nuclear Pore Complex Assembly. EMBO J. 2012, 31, 4072–4084. [Google Scholar] [CrossRef] [PubMed]
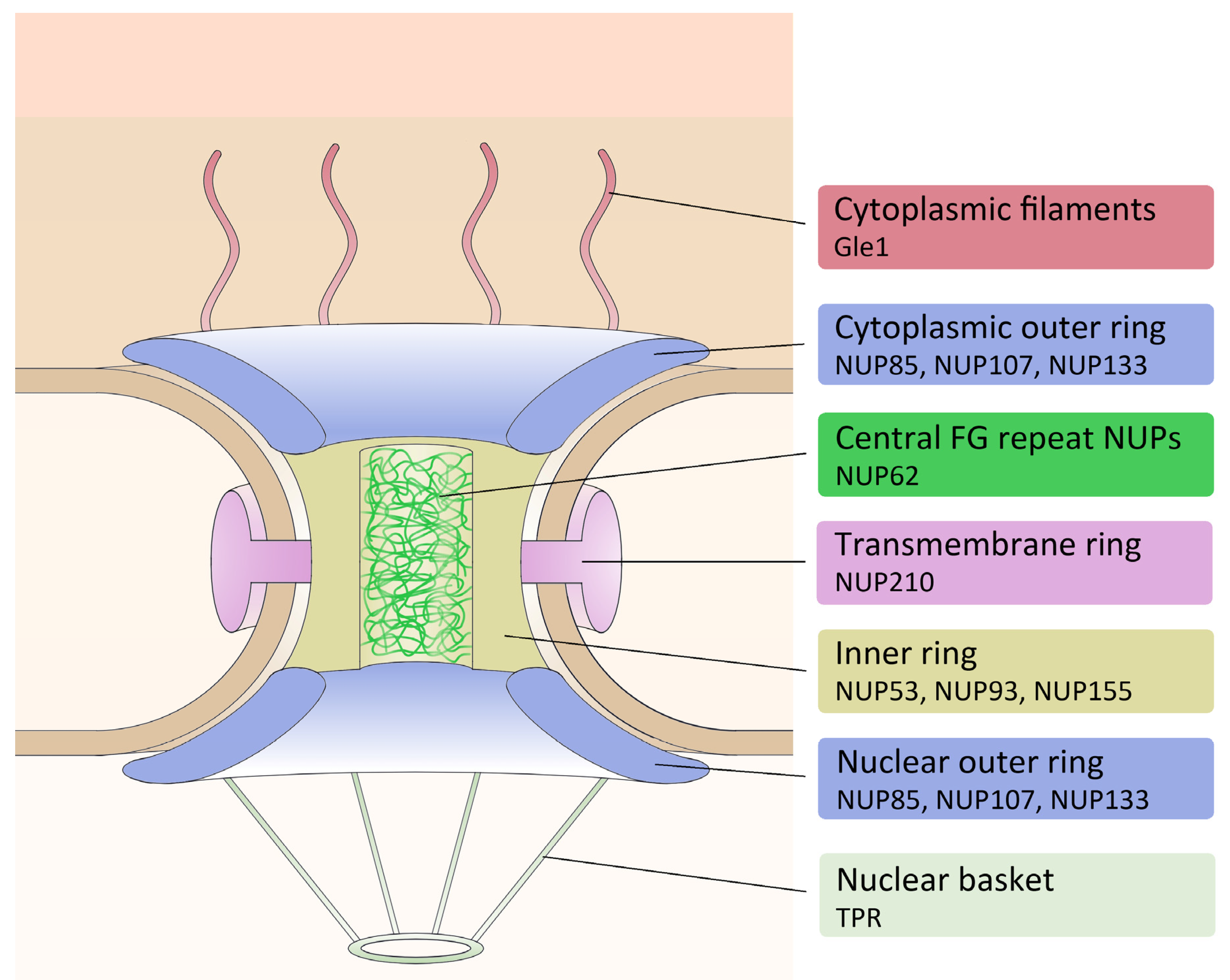


| NPC Complex | Protein | Dictybase ID |
|---|---|---|
| Cytoplasmic filaments | Gle1 | DDB_G0267918 |
| NUP53 | DDB_G0276109 | |
| Inner ring | NUP93 | DDB_G0267480 |
| NUP155 | DDB_G0291163 | |
| NUP85 | DDB_G0285415 | |
| Outer ring | NUP107 | DDB_G0285579 |
| NUP133 | DDB_G0287497 | |
| Central FG | NUP62 | DDB_G0274587 |
| Nuclear basket | TPR | DDB_G0288073 |
| Transmembrane | NUP210 | DDB_G0288545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitic, K.; Grafe, M.; Batsios, P.; Meyer, I. Partial Disassembly of the Nuclear Pore Complex Proteins during Semi-Closed Mitosis in Dictyostelium discoideum. Cells 2022, 11, 407. https://doi.org/10.3390/cells11030407
Mitic K, Grafe M, Batsios P, Meyer I. Partial Disassembly of the Nuclear Pore Complex Proteins during Semi-Closed Mitosis in Dictyostelium discoideum. Cells. 2022; 11(3):407. https://doi.org/10.3390/cells11030407
Chicago/Turabian StyleMitic, Kristina, Marianne Grafe, Petros Batsios, and Irene Meyer. 2022. "Partial Disassembly of the Nuclear Pore Complex Proteins during Semi-Closed Mitosis in Dictyostelium discoideum" Cells 11, no. 3: 407. https://doi.org/10.3390/cells11030407
APA StyleMitic, K., Grafe, M., Batsios, P., & Meyer, I. (2022). Partial Disassembly of the Nuclear Pore Complex Proteins during Semi-Closed Mitosis in Dictyostelium discoideum. Cells, 11(3), 407. https://doi.org/10.3390/cells11030407






