Generating Membrane Curvature at the Nuclear Pore: A Lipid Point of View
Abstract
1. Introduction
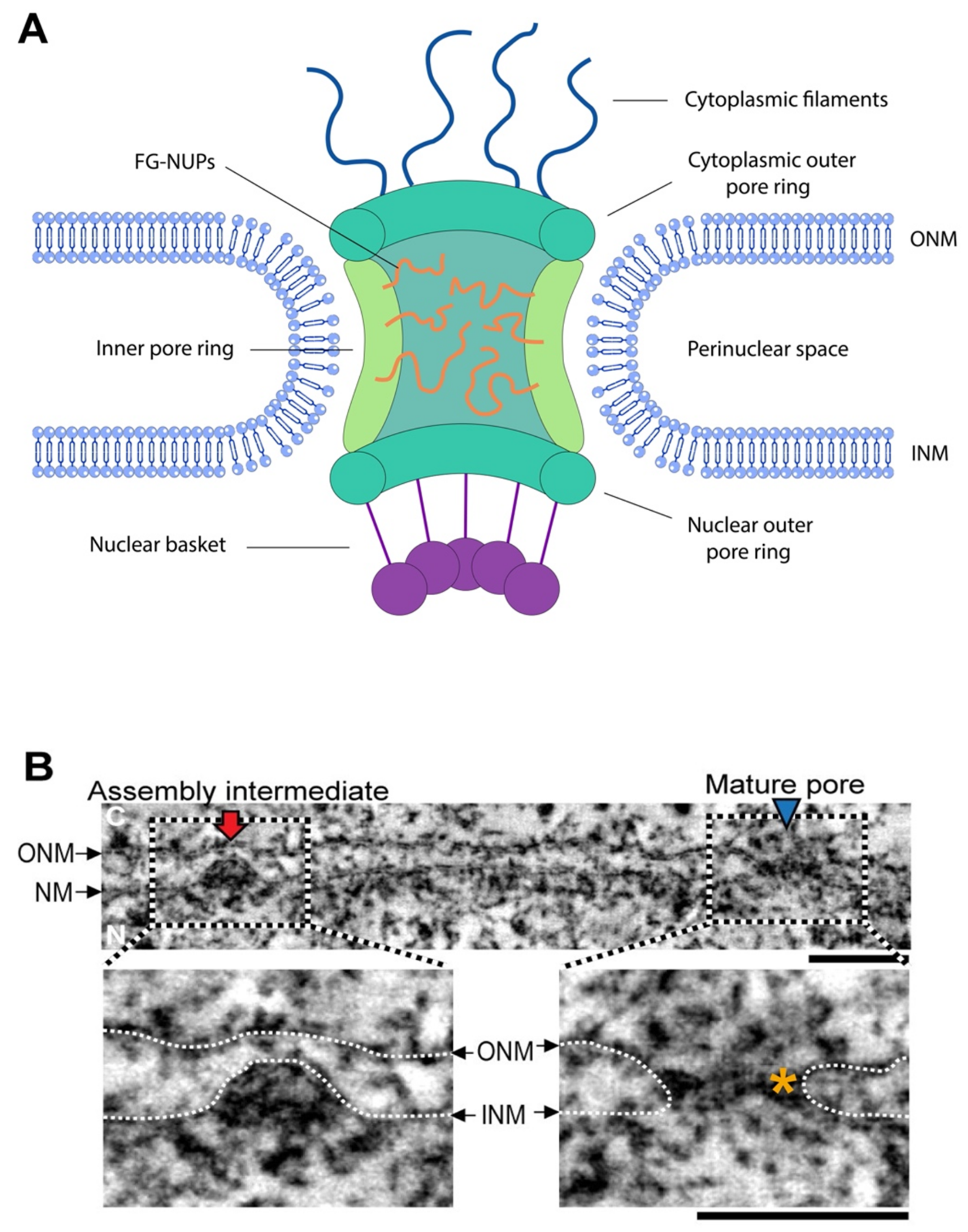
2. NPC Formation
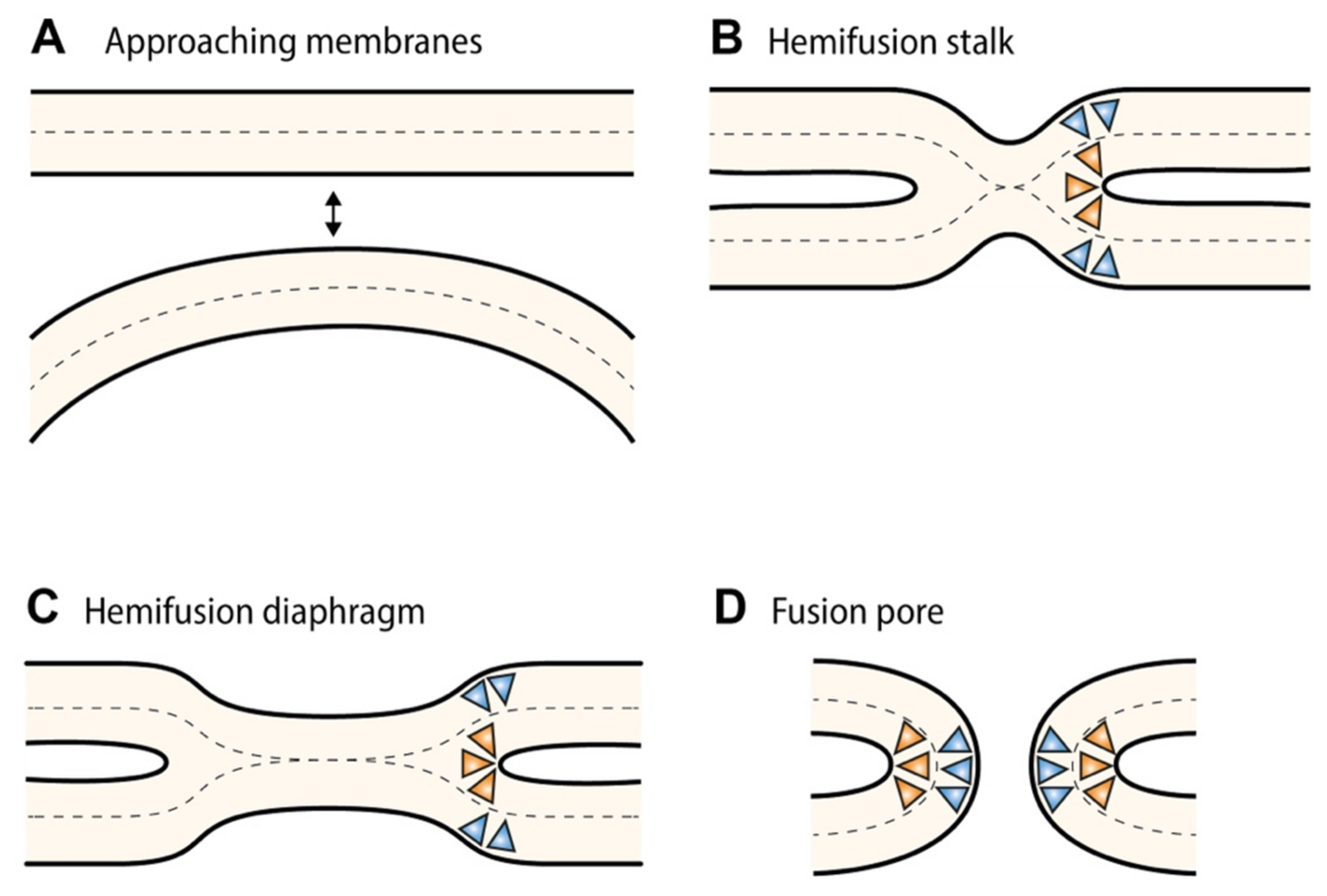
3. Membrane Curvature
4. Proteins and Membrane Curvature
5. Lipids and Membrane Curvature
5.1. Lipid Shape and Membrane Curvature Are Intrinsically Coupled
5.2. Transbilayer Lipid Asymmetry Is a Major Driving Force for Membrane Curvature
5.3. Lipid-Packing Defects Facilitate Insertion of Curvature-Inducing Proteins
6. Lipid Composition of the Nuclear Envelope: Implications for Membrane Curvature
6.1. The Nuclear Envelope Has Low Cholesterol and High Polyunsaturated Fatty Acid Content
6.2. The Inner and Outer Nuclear Membrane Are Compositionally Distinct
7. Lipid Homeostasis at the Nuclear Envelope: Implications for Membrane Curvature
7.1. INM Possesses Intrinsic Metabolic Activity and Is Enriched in Cone-Shaped DAG
7.2. Phospholipase C Activity Can Induce Negative Membrane Curvature and Fusion Events
7.3. Sphingomyelinases Can Promote Negative Curvature by Local Ceramide Generation
7.4. The Sphingomyelinase SMPD4 Localizes to the Nuclear Pore and Interacts with Nucleoporins
7.5. Yeast Acetyl CoA Carboxylase (acc1/mtr7) Links Very-Long-Chain Fatty Acid Synthesis to Membrane Curvature at the Nuclear Pore
8. Towards a Lipid-Inclusive Model of Nuclear Pore Formation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Magistris, P.; Antonin, W. The Dynamic Nature of the Nuclear Envelope. Curr. Biol. 2018, 28, R487–R497. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, R.; Holzenburg, A.; Buhle, E.L.; Jarnik, M.; Engel, A.; Aebi, U. Correlation between Structure and Mass Distribution of the Nuclear Pore Complex and of Distinct Pore Complex Components. J. Cell Biol. 1990, 110, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Rout, M.P.; Blobel, G. Isolation of the Yeast Nuclear Pore Complex. J. Cell Biol. 1993, 123, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Fernandez-Martinez, J.; Nudelman, I.; Shi, Y.; Zhang, W.; Raveh, B.; Herricks, T.; Slaughter, B.D.; Hogan, J.; Upla, P.; et al. Integrative Structure and Functional Anatomy of a Nuclear Pore Complex. Nature 2018, 555, 475. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Stuwe, T.; Schilbach, S.; Rundlet, E.J.; Perriches, T.; Mobbs, G.; Fan, Y.; Thierbach, K.; Huber, F.M.; Collins, L.N.; et al. Architecture of the Nuclear Pore Complex Symmetric Core. Science 2016, 352, aaf1015. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, J.; Mosalaganti, S.; von Appen, A.; Teimer, R.; DiGuilio, A.L.; Wan, W.; Bui, K.H.; Hagen, W.J.; Briggs, J.A.G.; Glavy, J.S.; et al. Molecular Architecture of the Inner Ring Scaffold of the Human Nuclear Pore Complex. Science 2016, 352, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Ellenberg, J. Mechanisms of Nuclear Pore Complex Assembly—Two Different Ways of Building One Molecular Machine. FEBS Lett. 2018, 592, 475–488. [Google Scholar] [CrossRef]
- Weberruss, M.; Antonin, W. Perforating the Nuclear Boundary—How Nuclear Pore Complexes Assemble. J. Cell Sci. 2016, 129, 4439–4447. [Google Scholar] [CrossRef]
- Boettcher, B.; Barral, Y. The Cell Biology of Open and Closed Mitosis. Nucleus 2013, 4, 160–165. [Google Scholar] [CrossRef]
- Daigle, N.; Beaudouin, J.; Hartnell, L.; Imreh, G.; Hallberg, E.; Lippincott-Schwartz, J.; Ellenberg, J. Nuclear Pore Complexes Form Immobile Networks and Have a Very Low Turnover in Live Mammalian Cells. J. Cell Biol. 2001, 154, 71–84. [Google Scholar] [CrossRef]
- Yang, L.; Guan, T.; Gerace, L. Integral Membrane Proteins of the Nuclear Envelope Are Dispersed throughout the Endoplasmic Reticulum during Mitosis. J. Cell Biol. 1997, 137, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Belgareh, N.; Rabut, G.; Baï, S.W.; van Overbeek, M.; Beaudouin, J.; Daigle, N.; Zatsepina, O.V.; Pasteau, F.; Labas, V.; Fromont-Racine, M.; et al. An Evolutionarily Conserved NPC Subcomplex, Which Redistributes in Part to Kinetochores in Mammalian Cells. J. Cell Biol. 2001, 154, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Tan, S.-H.; Karpova, T.S.; McNally, J.G.; Dasso, M. SUMO-1 Targets RanGAP1 to Kinetochores and Mitotic Spindles. J. Cell Biol. 2002, 156, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Steyer, A.M.; Schorb, M.; Hériché, J.-K.; Hossain, M.J.; Sethi, S.; Kueblbeck, M.; Schwab, Y.; Beck, M.; Ellenberg, J. Postmitotic Nuclear Pore Assembly Proceeds by Radial Dilation of Small Membrane Openings. Nat. Struct. Mol. Biol. 2018, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Maul, G.G.; Price, J.W.; Lieberman, M.W. Formation and Distribution of Nuclear Pore Complexes in Interphase. J. Cell Biol. 1971, 51, 405–418. [Google Scholar] [CrossRef]
- Otsuka, S.; Bui, K.H.; Schorb, M.; Hossain, M.J.; Politi, A.Z.; Koch, B.; Eltsov, M.; Beck, M.; Ellenberg, J. Nuclear Pore Assembly Proceeds by an Inside-out Extrusion of the Nuclear Envelope. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.M.; Talamas, J.A.; Hetzer, M.W. Cell Cycle Dependent Differences in Nuclear Pore Complex Assembly in Metazoa. Cell 2010, 141, 1030–1041. [Google Scholar] [CrossRef]
- Bassereau, P.; Jin, R.; Baumgart, T.; Deserno, M.; Dimova, R.; Frolov, V.A.; Bashkirov, P.V.; Grubmüller, H.; Jahn, R.; Risselada, H.J.; et al. The 2018 Biomembrane Curvature and Remodeling Roadmap. J. Phys. D Appl. Phys. 2018, 51, 343001. [Google Scholar] [CrossRef]
- Antonin, W. Nuclear Envelope: Membrane Bending for Pore Formation? Curr. Biol. 2009, 19, R410–R412. [Google Scholar] [CrossRef]
- Fischer, T.M. Bending Stiffness of Lipid Bilayers. I. Bilayer Couple or Single-Layer Bending? Biophys. J. 1992, 63, 1328–1335. [Google Scholar] [CrossRef][Green Version]
- Zimmerberg, J.; Kozlov, M.M. How Proteins Produce Cellular Membrane Curvature. Nat. Rev. Mol. Cell Biol 2006, 7, 9–19. [Google Scholar] [CrossRef]
- Boucrot, E.; Pick, A.; Çamdere, G.; Liska, N.; Evergren, E.; McMahon, H.T.; Kozlov, M.M. Membrane Fission Is Promoted by Insertion of Amphipathic Helices and Is Restricted by Crescent BAR Domains. Cell 2012, 149, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; McMahon, H.T.; Kozlov, M.M. The Hydrophobic Insertion Mechanism of Membrane Curvature Generation by Proteins. Biophys. J. 2008, 95, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.G.; Evans, P.R.; McMahon, H.T. BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.; Schekman, R. COPII-Mediated Vesicle Formation at a Glance. J. Cell Sci. 2011, 124, 1–4. [Google Scholar] [CrossRef]
- Kirchhausen, T. Three Ways to Make a Vesicle. Nat. Rev. Mol. Cell Biol. 2000, 1, 187–198. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular Mechanism and Physiological Functions of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, N.; Cibulka, J.; Mendiburo, M.J.; Romanauska, A.; Schneider, M.; Köhler, A. Nuclear Pore Basket Proteins Are Tethered to the Nuclear Envelope and Can Regulate Membrane Curvature. Dev. Cell 2015, 33, 285–298. [Google Scholar] [CrossRef]
- Vollmer, B.; Schooley, A.; Sachdev, R.; Eisenhardt, N.; Schneider, A.M.; Sieverding, C.; Madlung, J.; Gerken, U.; Macek, B.; Antonin, W. Dimerization and Direct Membrane Interaction of Nup53 Contribute to Nuclear Pore Complex Assembly. EMBO J. 2012, 31, 4072–4084. [Google Scholar] [CrossRef]
- Dawson, T.R.; Lazarus, M.D.; Hetzer, M.W.; Wente, S.R. ER Membrane-Bending Proteins Are Necessary for de Novo Nuclear Pore Formation. J. Cell Biol. 2009, 184, 659–675. [Google Scholar] [CrossRef]
- Kiseleva, E.; Morozova, K.N.; Voeltz, G.K.; Allen, T.D.; Goldberg, M.W. Reticulon 4a/NogoA Locates to Regions of High Membrane Curvature and May Have a Role in Nuclear Envelope Growth. J. Struct. Biol. 2007, 160, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.W.; Wiese, C.; Allen, T.D.; Wilson, K.L. Dimples, Pores, Star-Rings, and Thin Rings on Growing Nuclear Envelopes: Evidence for Structural Intermediates in Nuclear Pore Complex Assembly. J. Cell Sci. 1997, 110, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Eisenhardt, N.; Redolfi, J.; Antonin, W. Interaction of Nup53 with Ndc1 and Nup155 Is Required for Nuclear Pore Complex Assembly. J. Cell Sci. 2014, 127, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Laudermilch, E.; Tsai, P.-L.; Graham, M.; Turner, E.; Zhao, C.; Schlieker, C. Dissecting Torsin/Cofactor Function at the Nuclear Envelope: A Genetic Study. Mol. Biol. Cell 2016, 27, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
- Rampello, A.J.; Laudermilch, E.; Vishnoi, N.; Prophet, S.M.; Shao, L.; Zhao, C.; Lusk, C.P.; Schlieker, C. Torsin ATPase Deficiency Leads to Defects in Nuclear Pore Biogenesis and Sequestration of MLF2. J. Cell Biol. 2020, 219, e201910185. [Google Scholar] [CrossRef]
- Demircioglu, F.E.; Zheng, W.; McQuown, A.J.; Maier, N.K.; Watson, N.; Cheeseman, I.M.; Denic, V.; Egelman, E.H.; Schwartz, T.U. The AAA + ATPase TorsinA Polymerizes into Hollow Helical Tubes with 8.5 Subunits per Turn. Nat. Commun. 2019, 10, 3262. [Google Scholar] [CrossRef]
- Henne, W.M.; Stenmark, H.; Emr, S.D. Molecular Mechanisms of the Membrane Sculpting ESCRT Pathway. Cold Spring Harb. Perspect. Biol. 2013, 5, a016766. [Google Scholar] [CrossRef]
- Webster, B.M.; Colombi, P.; Jäger, J.; Lusk, C.P. Surveillance of Nuclear Pore Complex Assembly by ESCRT-III/Vps4. Cell 2014, 159, 388–401. [Google Scholar] [CrossRef]
- Webster, B.M.; Lusk, C.P. ESCRTs Breach the Nuclear Border. Nucleus 2015, 6, 197–202. [Google Scholar] [CrossRef][Green Version]
- Koch, B.A.; Staley, E.; Jin, H.; Yu, H.-G. The ESCRT-III Complex Is Required for Nuclear Pore Complex Sequestration and Regulates Gamete Replicative Lifespan in Budding Yeast Meiosis. Nucleus 2020, 11, 219–236. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Kozlov, M.M. Mechanics of Membrane Fusion. Nat. Struct. Mol. Biol. 2008, 15, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Watson, H. Biological Membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Frolov, V.A.; Shnyrova, A.V.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef]
- Chen, Y.; Okur, H.I.; Lütgebaucks, C.; Roke, S. Zwitterionic and Charged Lipids Form Remarkably Different Structures on Nanoscale Oil Droplets in Aqueous Solution. Langmuir 2018, 34, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.; Faller, R. Examining the Contributions of Lipid Shape and Headgroup Charge on Bilayer Behavior. Biophys. J. 2008, 95, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.Y.; Kinnunen, P.K. Poly(Ethylene Glycol)-Induced and Temperature-Dependent Phase Separation in Fluid Binary Phospholipid Membranes. Biophys. J. 1995, 68, 525–535. [Google Scholar] [CrossRef]
- Hirama, T.; Lu, S.M.; Kay, J.G.; Maekawa, M.; Kozlov, M.M.; Grinstein, S.; Fairn, G.D. Membrane Curvature Induced by Proximity of Anionic Phospholipids Can Initiate Endocytosis. Nat. Commun 2017, 8, 1393. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.I.; Bitbol, A.-F.; Seigneuret, M.; Staneva, G.; Kodama, A.; Sakuma, Y.; Kawakatsu, T.; Imai, M.; Puff, N. PH Sensing by Lipids in Membranes: The Fundamentals of PH-Driven Migration, Polarization and Deformations of Lipid Bilayer Assemblies. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2042–2063. [Google Scholar] [CrossRef]
- Pedersen, U.R.; Leidy, C.; Westh, P.; Peters, G.H. The Effect of Calcium on the Properties of Charged Phospholipid Bilayers. Biochim. Biophys. Acta 2006, 1758, 573–582. [Google Scholar] [CrossRef]
- Graham, T.R.; Kozlov, M.M. Interplay of Proteins and Lipids in Generating Membrane Curvature. Curr. Opin. Cell Biol. 2010, 22, 430–436. [Google Scholar] [CrossRef]
- Christie, M.P.; Johnstone, B.A.; Tweten, R.K.; Parker, M.W.; Morton, C.J. Cholesterol-Dependent Cytolysins: From Water-Soluble State to Membrane Pore. Biophys. Rev. 2018, 10, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Kozlov, M.M.; McMahon, H.T. How Synaptotagmin Promotes Membrane Fusion. Science 2007, 316, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Vamparys, L.; Gautier, R.; Vanni, S.; Bennett, W.F.D.; Tieleman, D.P.; Antonny, B.; Etchebest, C.; Fuchs, P.F.J. Conical Lipids in Flat Bilayers Induce Packing Defects Similar to That Induced by Positive Curvature. Biophys. J. 2013, 104, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Drin, G.; Antonny, B. Amphipathic Helices and Membrane Curvature. FEBS Lett. 2010, 584, 1840–1847. [Google Scholar] [CrossRef]
- Drin, G.; Casella, J.-F.; Gautier, R.; Boehmer, T.; Schwartz, T.U.; Antonny, B. A General Amphipathic Alpha-Helical Motif for Sensing Membrane Curvature. Nat. Struct. Mol. Biol. 2007, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Pranke, I.M.; Morello, V.; Bigay, J.; Gibson, K.; Verbavatz, J.-M.; Antonny, B.; Jackson, C.L. α-Synuclein and ALPS Motifs Are Membrane Curvature Sensors Whose Contrasting Chemistry Mediates Selective Vesicle Binding. J. Cell Biol. 2011, 194, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Pinot, M.; Vanni, S.; Ambroggio, E.; Guet, D.; Goud, B.; Manneville, J.-B. Feedback between Membrane Tension, Lipid Shape and Curvature in the Formation of Packing Defects. bioRxiv 2021. [Google Scholar] [CrossRef]
- Vanni, S.; Hirose, H.; Barelli, H.; Antonny, B.; Gautier, R. A Sub-Nanometre View of How Membrane Curvature and Composition Modulate Lipid Packing and Protein Recruitment. Nat. Commun. 2014, 5, 4916. [Google Scholar] [CrossRef]
- Neitcheva, T.; Peeva, D. Phospholipid Composition, Phospholipase A2 and Sphingomyelinase Activities in Rat Liver Nuclear Membrane and Matrix. Int. J. Biochem. Cell Biol. 1995, 27, 995–1001. [Google Scholar] [CrossRef]
- Keenan, T.W.; Berezney, R.; Crane, F.L. Lipid Composition of Further Purified Bovine Liver Nuclear Membranes. Lipids 1972, 7, 212–215. [Google Scholar] [CrossRef]
- Khandwala, A.S.; Kasper, C.B. The Fatty Acid Composition of Individual Phospholipids from Rat Liver Nuclear Membrane and Nuclei. J. Biol. Chem. 1971, 246, 6242–6246. [Google Scholar] [CrossRef]
- James, J.L.; Clawson, G.A.; Chan, C.H.; Smuckler, E.A. Analysis of the Phospholipid of the Nuclear Envelope and Endoplasmic Reticulum of Liver Cells by High Pressure Liquid Chromatography. Lipids 1981, 16, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Walch, L.; Verbavatz, J.-M. Lipids and Their Trafficking: An Integral Part of Cellular Organization. Dev. Cell 2016, 39, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Feller, S.E.; Gawrisch, K.; MacKerell, A.D. Polyunsaturated Fatty Acids in Lipid Bilayers: Intrinsic and Environmental Contributions to Their Unique Physical Properties. J. Am. Chem. Soc. 2002, 124, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Raffy, S.; Teissié, J. Control of Lipid Membrane Stability by Cholesterol Content. Biophys. J. 1999, 76, 2072–2080. [Google Scholar] [CrossRef]
- Bigay, J.; Antonny, B. Curvature, Lipid Packing, and Electrostatics of Membrane Organelles: Defining Cellular Territories in Determining Specificity. Dev. Cell 2012, 23, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Pinot, M.; Vanni, S.; Pagnotta, S.; Lacas-Gervais, S.; Payet, L.-A.; Ferreira, T.; Gautier, R.; Goud, B.; Antonny, B.; Barelli, H. Polyunsaturated Phospholipids Facilitate Membrane Deformation and Fission by Endocytic Proteins. Science 2014, 345, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Praper, T.; Sonnen, A.; Viero, G.; Kladnik, A.; Froelich, C.J.; Anderluh, G.; Dalla Serra, M.; Gilbert, R.J.C. Human Perforin Employs Different Avenues to Damage Membranes. J. Biol. Chem. 2011, 286, 2946–2955. [Google Scholar] [CrossRef]
- Vermeer, J.E.M.; van Wijk, R.; Goedhart, J.; Geldner, N.; Chory, J.; Gadella, T.W.J., Jr.; Munnik, T. In Vivo Imaging of Diacylglycerol at the Cytoplasmic Leaflet of Plant Membranes. Plant Cell Physiol. 2017, 58, 1196–1207. [Google Scholar] [CrossRef]
- Xie, X.; Wu, G.; Lu, Z.-H.; Ledeen, R.W. Potentiation of a Sodium-Calcium Exchanger in the Nuclear Envelope by Nuclear GM1 Ganglioside. J. Neurochem 2002, 81, 1185–1195. [Google Scholar] [CrossRef]
- Ledeen, R.; Wu, G. GM1 in the Nuclear Envelope Regulates Nuclear Calcium through Association with a Nuclear Sodium-Calcium Exchanger. J. Neurochem. 2007, 103, 126–134. [Google Scholar] [CrossRef]
- Dasgupta, R.; Miettinen, M.S.; Fricke, N.; Lipowsky, R.; Dimova, R. The Glycolipid GM1 Reshapes Asymmetric Biomembranes and Giant Vesicles by Curvature Generation. Proc. Natl. Acad. Sci. USA 2018, 115, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Van Gorkom, L.C.M.; Cheetham, J.J.; Epand, R.M. Ganglioside GD1a Generates Domains of High Curvature in Phosphatidylethanolamine Liposomes as Determined by Solid State 31P-NMR Spectroscopy. Chem. Phys. Lipids 1995, 76, 103–108. [Google Scholar] [CrossRef]
- Alroy, J.; Merk, F.B.; Goyal, V.; Ucci, A. Heterogeneous Distribution of Filipin-Sterol Complexes in Nuclear Membranes. Biochim. Biophys. Acta BBA-Biomembr. 1981, 649, 239–243. [Google Scholar] [CrossRef]
- Kim, J.; Okada, Y. Asymmetric Distribution and Temperature-Dependent Clustering of Filipin-Sterol Complexes in the Nuclear Membrane of Ehrlich Ascites Tumor Cells. Eur. J. Cell Biol. 1983, 29, 244–252. [Google Scholar]
- Albi, E.; Villani, M. Nuclear Lipid Microdomains Regulate Cell Function. Commun. Integr. Biol. 2009, 2, 23–24. [Google Scholar] [CrossRef]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Viola Magni, M.; Albi, E. Lipid Microdomains in Cell Nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef]
- Ivankin, A.; Kuzmenko, I.; Gidalevitz, D. Cholesterol Mediates Membrane Curvature during Fusion Events. Phys. Rev. Lett. 2012, 108, 238103. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Huang, H. Evidence of Cholesterol Accumulated in High Curvature Regions: Implication Ot the Curvature Elastic Energy for Lipid Mixtures. Biophys. J. 2007, 92, 2819–2830. [Google Scholar] [CrossRef]
- Rodal, S.K.; Skretting, G.; Garred, O.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of Cholesterol with Methyl-Beta-Cyclodextrin Perturbs Formation of Clathrin-Coated Endocytic Vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef]
- Subtil, A.; Gaidarov, I.; Kobylarz, K.; Lampson, M.A.; Keen, J.H.; McGraw, T.E. Acute Cholesterol Depletion Inhibits Clathrin-Coated Pit Budding. Proc. Natl. Acad. Sci. USA 1999, 96, 6775–6780. [Google Scholar] [CrossRef]
- Kreutzberger, A.J.B.; Kiessling, V.; Tamm, L.K. High Cholesterol Obviates a Prolonged Hemifusion Intermediate in Fast SNARE-Mediated Membrane Fusion. Biophys. J. 2015, 109, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Kim, S.-A.; Lu, X.; Su, Z.; Kim, S.K.; Shin, Y.-K. Fusion Step-Specific Influence of Cholesterol on SNARE-Mediated Membrane Fusion. Biophys. J. 2009, 96, 1839–1846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chlanda, P.; Mekhedov, E.; Waters, H.; Schwartz, C.L.; Fischer, E.R.; Ryham, R.J.; Cohen, F.S.; Blank, P.S.; Zimmerberg, J. The Hemifusion Structure Induced by Influenza Virus Haemagglutinin Is Determined by Physical Properties of the Target Membranes. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bu, B.; Crowe, M.; Diao, J.; Ji, B.; Li, D. Cholesterol Suppresses Membrane Leakage by Decreasing Water Penetrability. Soft Matter 2018, 14, 5277–5282. [Google Scholar] [CrossRef]
- Risselada, H.J.; Bubnis, G.; Grubmüller, H. Expansion of the Fusion Stalk and Its Implication for Biological Membrane Fusion. Proc. Natl. Acad. Sci. USA 2014, 111, 11043–11048. [Google Scholar] [CrossRef]
- Devaux, P.F.; Herrmann, A.; Ohlwein, N.; Kozlov, M.M. How lipid flippases can modulate membrane structure. Biochim. Biophys. Acta 2008, 1778, 1591–1600. [Google Scholar] [CrossRef]
- Panatala, R.; Hennrich, H.; Holthuis, J.C.M. Inner Workings and Biological Impact of Phospholipid Flippases. J. Cell Sci. 2015, 128, 2021–2032. [Google Scholar] [CrossRef]
- Pomorski, T.; Holthuis, J.C.M.; Herrmann, A.; van Meer, G. Tracking down Lipid Flippases and Their Biological Functions. J. Cell Sci. 2004, 117, 805–813. [Google Scholar] [CrossRef]
- Farge, E.; Ojcius, D.M.; Subtil, A.; Dautry-Varsat, A. Enhancement of Endocytosis Due to Aminophospholipid Transport across the Plasma Membrane of Living Cells. Am. J. Physiol. 1999, 276, C725–C733. [Google Scholar] [CrossRef]
- Takada, N.; Naito, T.; Inoue, T.; Nakayama, K.; Takatsu, H.; Shin, H.-W. Phospholipid-Flipping Activity of P4-ATPase Drives Membrane Curvature. EMBO J. 2018, 37. [Google Scholar] [CrossRef]
- Romanauska, A.; Köhler, A. The Inner Nuclear Membrane Is a Metabolically Active Territory That Generates Nuclear Lipid Droplets. Cell 2018, 174, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Carman, G.M.; Han, G.-S. Regulation of phospholipid synthesis in yeast. J. Lipid Res. 2009, 50, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.H.C.; Domart, M.-C.; Peddie, C.; Mantell, J.; Mclaverty, K.; Arabiotorre, A.; Hodgson, L.; Byrne, R.D.; Verkade, P.; Arkill, K.; et al. Acute Depletion of Diacylglycerol from the Cis-Golgi Affects Localized Nuclear Envelope Morphology during Mitosis. J. Lipid Res. 2018, 59, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Siniossoglou, S. Lipins, Lipids and Nuclear Envelope Structure. Traffic Cph. Den. 2009, 10, 1181–1187. [Google Scholar] [CrossRef]
- Siniossoglou, S.; Santos-Rosa, H.; Rappsilber, J.; Mann, M.; Hurt, E. A Novel Complex of Membrane Proteins Required for Formation of a Spherical Nucleus. EMBO J. 1998, 17, 6449–6464. [Google Scholar] [CrossRef]
- Inaba, T.; Kishimoto, T.; Murate, M.; Tajima, T.; Sakai, S.; Abe, M.; Makino, A.; Tomishige, N.; Ishitsuka, R.; Ikeda, Y.; et al. Phospholipase Cβ1 Induces Membrane Tubulation and Is Involved in Caveolae Formation. Proc. Natl. Acad. Sci. USA 2016, 113, 7834–7839. [Google Scholar] [CrossRef]
- Riske, K.A.; Döbereiner, H.-G. Diacylglycerol-Rich Domain Formation in Giant Stearoyl-Oleoyl Phosphatidylcholine Vesicles Driven by Phospholipase C Activity. Biophys. J. 2003, 85, 2351–2362. [Google Scholar] [CrossRef]
- Martelli, A.M.; Gilmour, R.S.; Bertagnolo, V.; Neri, L.M.; Manzoli, L.; Cocco, L. Nuclear Localization and Signalling Activity of Phosphoinositidase C Beta in Swiss 3T3 Cells. Nature 1992, 358, 242–245. [Google Scholar] [CrossRef]
- D’Santos, C.S.; Clarke, J.H.; Irvine, R.F.; Divecha, N. Nuclei Contain Two Differentially Regulated Pools of Diacylglycerol. Curr. Biol. 1999, 9, 437–440. [Google Scholar] [CrossRef]
- Albi, E.; Rossi, G.; Maraldi, N.M.; Magni, M.V.; Cataldi, S.; Solimando, L.; Zini, N. Involvement of Nuclear Phosphatidylinositol-Dependent Phospholipases C in Cell Cycle Progression during Rat Liver Regeneration. J. Cell Physiol 2003, 197, 181–188. [Google Scholar] [CrossRef]
- Barona, T.; Byrne, R.D.; Pettitt, T.R.; Wakelam, M.J.O.; Larijani, B.; Poccia, D.L. Diacylglycerol Induces Fusion of Nuclear Envelope Membrane Precursor Vesicles. J. Biol. Chem. 2005, 280, 41171–41177. [Google Scholar] [CrossRef] [PubMed]
- Schooley, A.; Vollmer, B.; Antonin, W. Building a Nuclear Envelope at the End of Mitosis: Coordinating Membrane Reorganization, Nuclear Pore Complex Assembly, and Chromatin de-Condensation. Chromosoma 2012, 121, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.; Thomas, P.; Michell, R.H.; Allan, P.T.D. Rapid Transbilayer Diffusion of 1,2-Diacylglycerol and Its Relevance to Control of Membrane Curvature. Nature 1978, 276, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Ira; Johnston, L.J. Sphingomyelinase Generation of Ceramide Promotes Clustering of Nanoscale Domains in Supported Bilayer Membranes. Biochim. Biophys. Acta 2008, 1778, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Ohba, T.; Miyata, H.; Ohki, K. Rapid Phase Change of Lipid Microdomains in Giant Vesicles Induced by Conversion of Sphingomyelin to Ceramide. Biochim. Biophys. Acta 2006, 1758, 145–153. [Google Scholar] [CrossRef]
- Albi, E.; Lazzarini, R.; Viola Magni, M. Phosphatidylcholine/Sphingomyelin Metabolism Crosstalk inside the Nucleus. Biochem. J. 2008, 410, 381–389. [Google Scholar] [CrossRef]
- Dreger, M.; Bengtsson, L.; Schöneberg, T.; Otto, H.; Hucho, F. Nuclear Envelope Proteomics: Novel Integral Membrane Proteins of the Inner Nuclear Membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 11943–11948. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Baboo, S.; Lindsay, C.; Brusman, L.; Martinez-Bartolomé, S.; Tapia, O.; Zhang, X.; Yates, J.R.; Gerace, L. Identification of New Transmembrane Proteins Concentrated at the Nuclear Envelope Using Organellar Proteomics of Mesenchymal Cells. Nucleus 2019, 10, 126–143. [Google Scholar] [CrossRef]
- Magini, P.; Smits, D.J.; Vandervore, L.; Schot, R.; Columbaro, M.; Kasteleijn, E.; van der Ent, M.; Palombo, F.; Lequin, M.H.; Dremmen, M.; et al. Loss of SMPD4 Causes a Developmental Disorder Characterized by Microcephaly and Congenital Arthrogryposis. Am. J. Hum. Genet. 2019, 105, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.A.; He, Q.; Ponnusamy, S.; Ogretmen, B.; Huang, Y.; Sheikh, M.S. Neutral Sphingomyelinase-3 Is a DNA Damage and Nongenotoxic Stress-Regulated Gene That Is Deregulated in Human Malignancies. Mol. Cancer Res. 2008, 6, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Krut, O.; Wiegmann, K.; Kashkar, H.; Yazdanpanah, B.; Krönke, M. Novel Tumor Necrosis Factor-Responsive Mammalian Neutral Sphingomyelinase-3 Is a C-Tail-Anchored Protein. J. Biol. Chem. 2006, 281, 13784–13793. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Smith, J.; Horrell, E.M.W.; McLean, J.B.; Deevska, G.M.; Bonnell, M.R.; Nikolova-Karakashian, M.N.; Reid, M.B. Neutral sphingomyelinase-3 mediates TNF-stimulated oxidant activity in skeletal muscle. Redox Biol. 2014, 2, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Tsukaguchi, H.; Koshimizu, E.; Nakazato, H.; Itoh, K.; Kuraoka, S.; Komohara, Y.; Shiina, M.; Nakamura, S.; Kitajima, M.; et al. Homozygous Splicing Mutation in NUP133 Causes Galloway-Mowat Syndrome. Ann. Neurol. 2018, 84, 814–828. [Google Scholar] [CrossRef]
- Hein, M.Y.; Hubner, N.C.; Poser, I.; Cox, J.; Nagaraj, N.; Toyoda, Y.; Gak, I.A.; Weisswange, I.; Mansfeld, J.; Buchholz, F.; et al. A Human Interactome in Three Quantitative Dimensions Organized by Stoichiometries and Abundances. Cell 2015, 163, 712–723. [Google Scholar] [CrossRef]
- Schneiter, R.; Hitomi, M.; Ivessa, A.S.; Fasch, E.V.; Kohlwein, S.D.; Tartakoff, A.M. A Yeast Acetyl Coenzyme A Carboxylase Mutant Links Very-Long-Chain Fatty Acid Synthesis to the Structure and Function of the Nuclear Membrane-Pore Complex. Mol. Cell Biol. 1996, 16, 7161–7172. [Google Scholar] [CrossRef]
- Thaller, D.J.; Patrick Lusk, C. Fantastic Nuclear Envelope Herniations and Where to Find Them. Biochem. Soc. Trans. 2018, 46, 877–889. [Google Scholar] [CrossRef]
- Kohlwein, S.D.; Eder, S.; Oh, C.S.; Martin, C.E.; Gable, K.; Bacikova, D.; Dunn, T. Tsc13p Is Required for Fatty Acid Elongation and Localizes to a Novel Structure at the Nuclear-Vacuolar Interface in Saccharomyces Cerevisiae. Mol. Cell Biol. 2001, 21, 109–125. [Google Scholar] [CrossRef]
- Sipos, G.; Reggiori, F.; Vionnet, C.; Conzelmann, A. Alternative Lipid Remodelling Pathways for Glycosylphosphatidylinositol Membrane Anchors in Saccharomyces Cerevisiae. EMBO J. 1997, 16, 3494–3505. [Google Scholar] [CrossRef]
- Schneiter, R.; Brügger, B.; Amann, C.M.; Prestwich, G.D.; Epand, R.F.; Zellnig, G.; Wieland, F.T.; Epand, R.M. Identification and Biophysical Characterization of a Very-Long-Chain-Fatty-Acid-Substituted Phosphatidylinositol in Yeast Subcellular Membranes. Biochem. J. 2004, 381, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.; Atkinson, A.E.; Hodge, C.A.; Cottier, S.; Martínez-Montañés, F.; Maithel, S.; Mène-Saffrané, L.; Cole, C.N.; Schneiter, R. Yeast Integral Membrane Proteins Apq12, Brl1, and Brr6 Form a Complex Important for Regulation of Membrane Homeostasis and Nuclear Pore Complex Biogenesis. Eukaryot. Cell 2015, 14, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, J.J.; Hodge, C.A.; Cole, C.N. The yeast integral membrane protein Apq12 potentially links membrane dynamics to assembly of nuclear pore complexes. J. Cell Biol. 2007, 178, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Kops, A.D.B.; Guthrie, C. An essential nuclear envelope integral membrane protein, Brr6p, required for nuclear transport. EMBO J. 2001, 20, 4183–4193. [Google Scholar] [CrossRef]
- Hodge, C.A.; Choudhary, V.; Wolyniak, M.J.; Scarcelli, J.J.; Schneiter, R.; Cole, C.N. Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum. J. Cell Sci. 2010, 123, 141–151. [Google Scholar] [CrossRef]
- Zhang, W.; Neuner, A.; Rüthnick, D.; Sachsenheimer, T.; Lüchtenborg, C.; Brügger, B.; Schiebel, E. Brr6 and Brl1 locate to nuclear pore complex assembly sites to promote their biogenesis. J. Cell Biol. 2018, 217, 877–894. [Google Scholar] [CrossRef]
- Callan, H.G.; Tomlin, S.G. Experimental Studies on Amphibian Oocyte Nuclei. I. Investigation of the Structure of the Nuclear Membrane by Means of the Electron Microscope. Proc. R Soc. Lond. B Ser. Biol. Sci. 1950, 137, 367–378. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Domart, M.-C.; Hobday, T.M.C.; Peddie, C.J.; Chung, G.H.C.; Wang, A.; Yeh, K.; Jethwa, N.; Zhang, Q.; Wakelam, M.J.O.; Woscholski, R.; et al. Acute Manipulation of Diacylglycerol Reveals Roles in Nuclear Envelope Assembly & Endoplasmic Reticulum Morphology. PLoS ONE 2012, 7, e51150. [Google Scholar] [CrossRef]
- Fuertes, G.; Giménez, D.; Esteban-Martín, S.; Sánchez-Muñoz, O.L.; Salgado, J. A Lipocentric View of Peptide-Induced Pores. Eur. Biophys. J. 2011, 40, 399–415. [Google Scholar] [CrossRef]
- Fuertes, G.; Giménez, D.; Esteban-Martin, S.; Garcia-Sáez, A.; Sánchez, O.; Salgado, J. Role of Membrane Lipids for the Activity of Pore Forming Peptides and Proteins. Adv. Exp. Med. Biol 2010, 677, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.W.; Leikin, S.L.; Chernomordik, L.V.; Pastushenko, V.F.; Sokirko, A.I. Reversible Electrical Breakdown of Lipid Bilayers: Formation and Evolution of Pores. Biochim. Biophys. Acta (BBA) - Biomembr. 1988, 940, 275–287. [Google Scholar] [CrossRef]
- Agrawal, A.; Lele, T.P. Mechanics of Nuclear Membranes. J. Cell Sci. 2019, 132, jcs229245. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, C.E.; Allegretti, M.; Rantos, V.; Goetz, S.K.; Obarska-Kosinska, A.; Zagoriy, I.; Halavatyi, A.; Hummer, G.; Mahamid, J.; Kosinski, J.; et al. Nuclear pores dilate and constrict in cellulo. Science 2021, 374, eabd9776. [Google Scholar] [CrossRef]
- Xie, W.; Chojnowski, A.; Boudier, T.; Lim, J.S.; Ahmed, S.; Ser, Z.; Stewart, C.; Burke, B. A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr. Biol. 2016, 26, 2651–2658. [Google Scholar] [CrossRef]
- Li, P.; Meng, Y.; Wang, L.; Di, L.-J. BioID: A Proximity-Dependent Labeling Approach in Proteomics Study. Methods Mol. Biol. Clifton NJ 2018, 1871, 143–151. [Google Scholar] [CrossRef]
- Lauwers, E.; Goodchild, R.; Verstreken, P. Membrane Lipids in Presynaptic Function and Disease. Neuron 2016, 90, 11–25. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, B.W.A.; Piët, A.C.A.; Fornerod, M. Generating Membrane Curvature at the Nuclear Pore: A Lipid Point of View. Cells 2022, 11, 469. https://doi.org/10.3390/cells11030469
Peeters BWA, Piët ACA, Fornerod M. Generating Membrane Curvature at the Nuclear Pore: A Lipid Point of View. Cells. 2022; 11(3):469. https://doi.org/10.3390/cells11030469
Chicago/Turabian StylePeeters, Bas W. A., Alexandra C. A. Piët, and Maarten Fornerod. 2022. "Generating Membrane Curvature at the Nuclear Pore: A Lipid Point of View" Cells 11, no. 3: 469. https://doi.org/10.3390/cells11030469
APA StylePeeters, B. W. A., Piët, A. C. A., & Fornerod, M. (2022). Generating Membrane Curvature at the Nuclear Pore: A Lipid Point of View. Cells, 11(3), 469. https://doi.org/10.3390/cells11030469




