Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Procedures
2.3. Pulmonary Function Tests (PFTs)
2.4. 6-Minute-Walk Test (6MWT)
2.5. Pulmonary Rehabilitation
2.6. EBC Collection
2.7. NMR Sample Preparation and Spectra Acquisition
2.8. Power Analysis
2.9. Multivariate Data Analysis
3. Results
3.1. Patients
3.2. NMR Profiling of EBC
3.3. Correlation of Metabolomics with Clinical Data
3.4. Metabolomics and Walk-Distance Paths
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: Systematic analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m234, Erratum in BMJ 2020, 370, m3150. [Google Scholar] [CrossRef]
- Franssen, F.M.; Alter, P.; Bar, N.; Benedikter, B.J.; Iurato, S.; Maier, D.; Maxheim, M.; Roessler, F.K.; Spruit, M.A.; Vogelmeier, C.F.; et al. Personalized medicine for patients with COPD: Where are we? Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1465–1484. [Google Scholar] [CrossRef]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; ZuWallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64, Erratum in Am. J. Respir. Crit. Care Med. 2014, 189, 1570. [Google Scholar] [CrossRef]
- Agusti, A.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Edwards, L.D.; Lomas, D.A.; MacNee, W.; Miller, B.E.; Rennard, S.; Silverman, E.K.; et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 2010, 11, 122. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Maniscalco, M.; Motta, A. Metabolomics of exhaled breath condensate: A means for phenotyping respiratory diseases? Biomark. Med. 2017, 11, 405–407. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Paris, D.; Maniscalco, M.; Motta, A. Nuclear magnetic resonance-based metabolomics in respiratory medicine. Eur. Respir. J. 2018, 52, 1801107. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Cutignano, A.; Paris, D.; Melck, D.J.; Molino, A.; Fuschillo, S.; Motta, A. Metabolomics of Exhaled Breath Condensate by Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry: A Methodological Approach. Curr. Med. Chem. 2020, 27, 2381–2399. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Motta, A. Clinical and Inflammatory Phenotyping: Can Electronic Nose and NMR-based Metabolomics Work at the Bedside? Arch. Med. Res. 2018, 49, 74–76. [Google Scholar] [CrossRef]
- Maniscalco, M.; Fuschillo, S.; Paris, D.; Cutignano, A.; Sanduzzi, A.; Motta, A. Clinical metabolomics of exhaled breath condensate in chronic respiratory diseases. Adv. Clin. Chem. 2019, 88, 121–149. [Google Scholar]
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [CrossRef] [PubMed]
- Franssen, F.M.; Han, M.K. The ABC of GOLD A-B-C-D. Eur. Respir. J. 2013, 42, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Horowitz, M.B. Perception of breathlessness during exercise in patients with respiratory disease. Med. Sci. Sports Exerc. 1994, 26, 1078–1081. [Google Scholar] [CrossRef]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. ATS/ERS Task Force on Policy in Pulmonary Rehabilitation, An official American Thoracic Society/European Respiratory Society policy statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Zainuldin, R.; Mackey, M.G.; Alison, J.A. Prescribing cycle exercise intensity using moderate symptom levels in chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. Prev. 2016, 36, 195–202. [Google Scholar] [CrossRef]
- de Laurentiis, G.; Paris, D.; Melck, D.; Maniscalco, M.; Marsico, S.; Corso, G.; Motta, A.; Sofia, M. Metabonomic analysis of exhaled breath condensate in adults by nuclear magnetic resonance spectroscopy. Eur. Respir. J. 2008, 32, 1175–1183. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Meng, X.; Donovan, K.J.; Shaka, A.J. SOGGY: Solvent-optimized double gradient spectroscopy for water suppression. A comparison with some existing techniques. J. Magn. Reson. 2007, 184, 263–274. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; D’Amato, M.; Zedda, A.; Sofia, M.; Stellato, C.; Motta, A. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J. Allergy Clin. Immunol. 2017, 139, 1536–1547. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis: Basic Principles and Applications, 3rd ed.; MKS Umetrics AB: Malmö, Sweden, 2013. [Google Scholar]
- Di Costanzo, A.; Paris, D.; Melck, D.; Angiolillo, A.; Corso, G.; Maniscalco, M.; Motta, A. Blood biomarkers indicate that the preclinical stages of Alzheimer’s disease present overlapping molecular features. Sci. Rep. 2020, 10, 15612. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Shindyapina, A.V.; Sheshukova, E.V.; Komarova, T.V. Metabolic methanol: Molecular pathways and physiological roles. Physiol. Rev. 2015, 95, 603–644. [Google Scholar] [CrossRef] [PubMed]
- Persoz, C.; Achard, S.; Momas, I.; Seta, N. Inflammatory response modulation of airway epithelial cells exposed to formaldehyde. Toxicol. Lett. 2012, 211, 159–163. [Google Scholar] [CrossRef]
- Lino-dos-Santos-Franco, A.; Correa-Costa, M.; Durão, A.C.; de Oliveira, A.P.; Breithaupt-Faloppa, A.C.; Bertoni, J.; Oliveira-Filho, R.M.; Câmara, N.O.; Marcourakis, T.; Tavares-de-Lima, W. Formaldehyde induces lung inflammation by an oxidant and antioxidant enzymes mediated mechanism in the lung tissue. Toxicol. Lett. 2011, 207, 278–285. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; Molino, A.; Carone, M.; Ruggeri, P.; Caramori, G.; Motta, A. Differential diagnosis between newly diagnosed asthma and COPD using exhaled breath condensate metabolomics: A pilot study. Eur. Respir. J. 2018, 51, 1701825. [Google Scholar] [CrossRef]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bolton, C.E.; Broekhuizen, R.; Ionescu, A.A.; Nixon, L.S.; Wouters, E.F.; Shale, D.J.; Schols, A.M. Cellular protein breakdown and systemic inflammation are unaffected by pulmonary rehabilitation in COPD. Thorax 2007, 62, 109–114. [Google Scholar] [CrossRef][Green Version]
- Ratjen, F.; Saiman, L.; Mayer-Hamblett, N.; Lands, L.C.; Kloster, M.; Thompson, V.; Emmett, P.; Marshall, B.; Accurso, F.; Sagel, S.; et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest 2012, 142, 1259–1266. [Google Scholar] [CrossRef]
- Halper-Stromberg, E.; Gillenwater, L.; Cruickshank-Quinn, C.; O’Neal, W.K.; Reisdorph, N.; Petrache, I.; Zhuang, Y.; Labaki, W.W.; Curtis, J.L.; Wells, J.; et al. Bronchoalveolar lavage fluid from COPD patients reveals more compounds associated with disease than matched plasma. Metabolites 2019, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Lucidi, V.; Paris, D.; Montemitro, E.; Shohreh, R.; Mores, N.; Melck, D.; Santini, G.; Majo, F.; Motta, A. Metabolomic analysis by nuclear magnetic resonance spectroscopy as a new approach to understanding inflammation and monitoring of pharmacological therapy in children and young adults with cystic fibrosis. Front. Pharmacol. 2018, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Kozu, R.; Senjyu, H.; Jenkins, S.C.; Mukae, H.; Sakamoto, N.; Kohno, S. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration 2011, 81, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Spanevello, A. Heterogeneity of pulmonary rehabilitation: Like apples and oranges—both healthy fruit. Eur. Respir. J. 2014, 43, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Papa, A.; Maniscalco, M.; Di Minno, M.N.D. COVID-19 and functional disability: Current insights and rehabilitation strategies. Postgrad. Med. J. 2021, 97, 469–470. [Google Scholar] [CrossRef]
- Huffman, K.M.; Slentz, C.A.; Kraus, W.E. Control arms in exercise training studies: Transitioning from an era of intervention efficacy to one of comparative clinical effectiveness research. J. Appl. Physiol. 2011, 111, 946–948. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Dryden, S.; Casaburi, R.; Hanania, N.A. Long-term benefits of pulmonary rehabilitation in patients with COPD: A 2-year follow-up study. Chest 2021, 159, 967–974. [Google Scholar] [CrossRef]

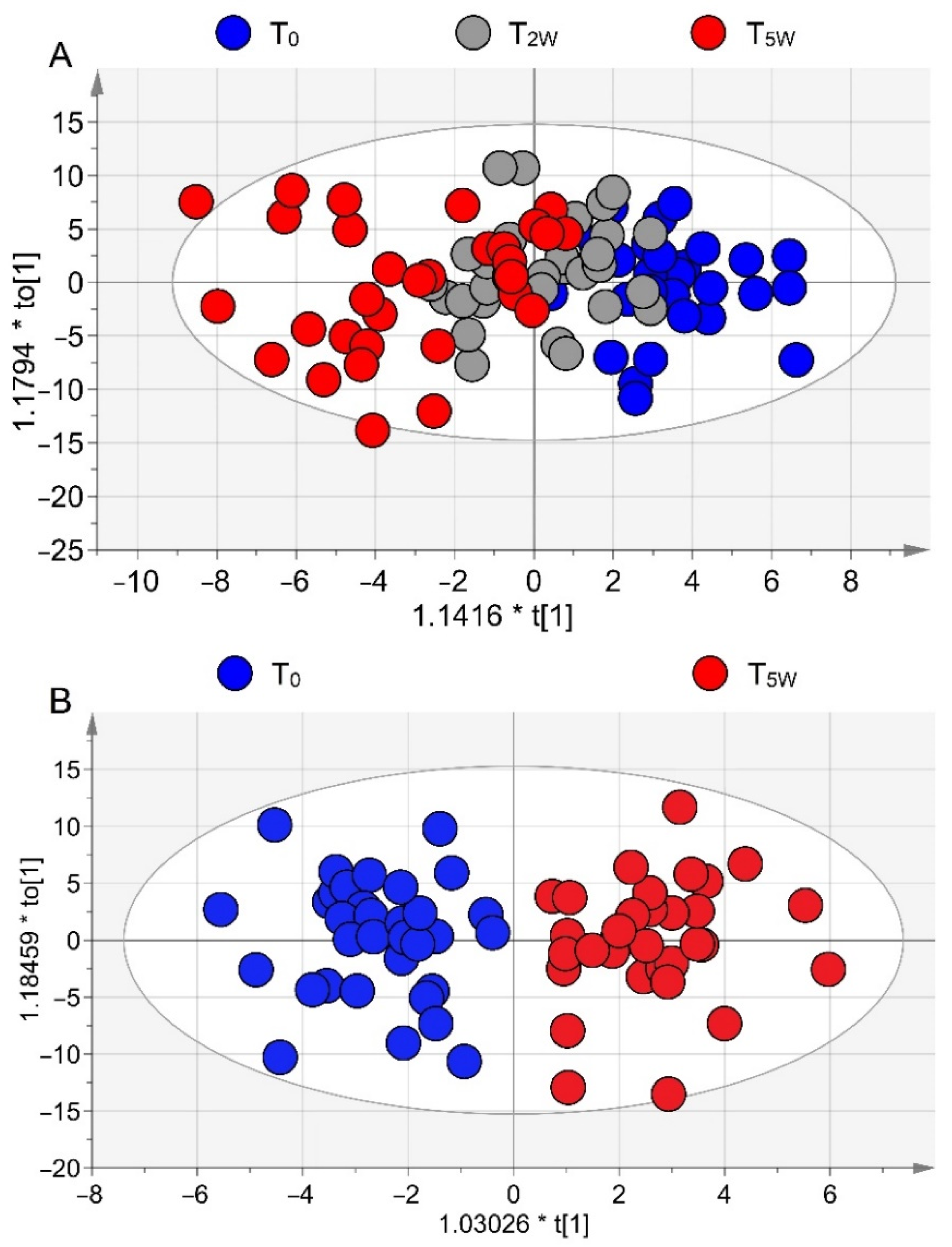

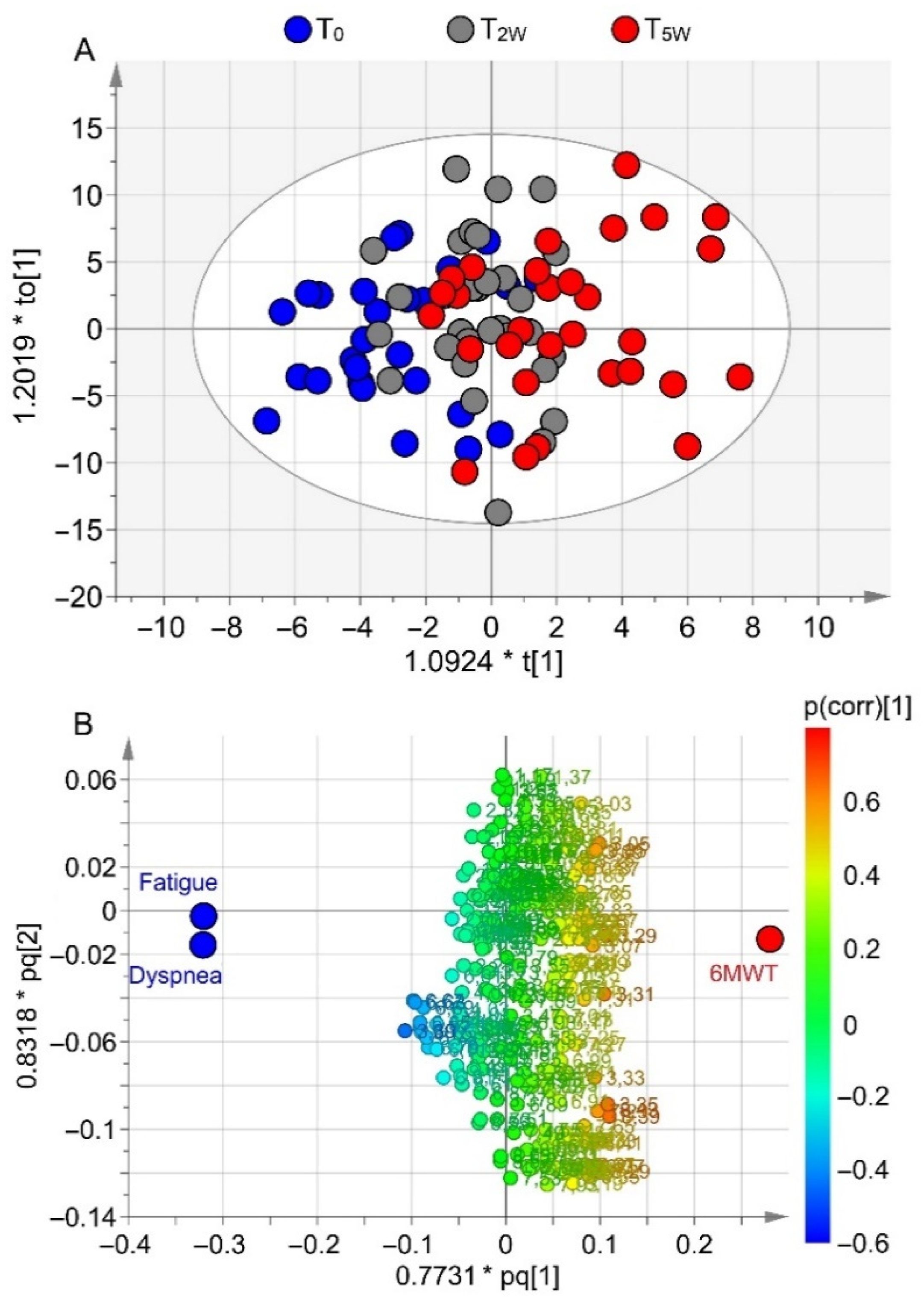


| Rehabilitation (n = 35) | Controls (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| T0 | T5W | p Value (1–2) | T0 | T5W | p Value (1–3) | p Value (2–4) | p Value (3–4) | |
| Age (year) | 70.7 ± 7.7 | - | - | 70.7 ± 5.7 | - | 0.90 | - | - |
| Sex (M/F) | 31/4 | - | - | 15/0 | - | 0.82 | - | - |
| BMI (kg/m2) | 29.8 ± 6.04 | - | - | 26.75 ± 2.28 | - | 0.060 | - | - |
| Smoke | 31 × 10−4 S | - | - | 24 × 10−2 S | - | 0.99 | - | - |
| GOLD C (n) | 2 | - | - | 1 | - | 0.99 | - | - |
| GOLD D (n) | 33 | - | - | 14 | - | 0.99 | - | - |
| FEV1 (L) | 1.19 ± 0.40 | 1.24 ± 0.43 | 0.29 | 1.22 ± 0.39 | 1.21 ± 0.31 | 0.80 | 0.81 | 0.61 |
| FEV1 (%) | 48.03 ± 13.66 | 50.35 ± 15.39 | 0.21 | 42.27 ± 8.63 | 42.17 ± 7.53 | 0.12 | 0.056 | 0.63 |
| FVC (L) | 2.59 ± 0.72 | 2.50 ± 0.80 | 0.56 | 2.48 ± 0.52 | 2.47 ± 0.45 | 0.56 | 0.030 | 0.82 |
| FVC (%) | 80.57 ± 16.14 | 78.44 ± 20.51 | 0.37 | 68.67 ± 11.24 | 69.17 ± 10.24 | 0.010 | 0.073 | 0.52 |
| FEV1/FVC | 47.4 ± 15.45 | 50.68 ± 13. 96 | 0.01 | 47.40 ± 10.26 | 47.55 ± 10.19 | 0.99 | 0.44 | 0.66 |
| FEF25-75 (L/s) | 1.99 ± 1.68 | 2.14 ± 1.6 | 0.04 | 1.08 ± 0.52 | 1.18 ± 0.51 | 0.040 | 0.028 | 0.21 |
| SaO2 (%) | 92.51 ± 5.06 | 93.42 ± 5.12 | 0.03 | 91.52 ± 5.41 | 92.12 ± 5.01 | 0.80 | 0.41 | 0.45 |
| 6MWT (m) | 191.11 ± 50.26 | 298.54 ± 66.74 | <0.001 | 211.23 ± 48.36 | 210.24 ± 55.71 | 0.31 | <0.0001 | 0.62 |
| Fatigue | 8.31 ± 2.23 | 2.71 ± 2.08 | <0.001 | 7.81 ± 2.13 | 7.73 ± 2.18 | 0.52 | <0.0001 | 0.34 |
| Dyspnea | 9.06 ± 1.61 | 2.86 ± 1.97 | <0.001 | 8.76 ± 1.82 | 8.73 ± 1.57 | 0.36 | <0.0001 | 0.71 |
| T0 | T2W | T5W | Members | Correct (%) | |
|---|---|---|---|---|---|
| T0 | 27 | 2 | 0 | 29 | 93.10 |
| T2W | 12 | 9 | 10 | 31 | 29.03 |
| T5W | 0 | 6 | 25 | 31 | 80.65 |
| No Class | 0 | 0 | 0 | 0 | |
| Total | 39 | 17 | 35 | 91 | 67.03 |
| Fisher’s Prob. | 6.7×10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniscalco, M.; Paris, D.; Cuomo, P.; Fuschillo, S.; Ambrosino, P.; Tramice, A.; Palomba, L.; Motta, A. Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate. Cells 2022, 11, 344. https://doi.org/10.3390/cells11030344
Maniscalco M, Paris D, Cuomo P, Fuschillo S, Ambrosino P, Tramice A, Palomba L, Motta A. Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate. Cells. 2022; 11(3):344. https://doi.org/10.3390/cells11030344
Chicago/Turabian StyleManiscalco, Mauro, Debora Paris, Paola Cuomo, Salvatore Fuschillo, Pasquale Ambrosino, Annabella Tramice, Letizia Palomba, and Andrea Motta. 2022. "Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate" Cells 11, no. 3: 344. https://doi.org/10.3390/cells11030344
APA StyleManiscalco, M., Paris, D., Cuomo, P., Fuschillo, S., Ambrosino, P., Tramice, A., Palomba, L., & Motta, A. (2022). Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate. Cells, 11(3), 344. https://doi.org/10.3390/cells11030344









