A Phase II Study on the Effect of Taurisolo® Administered via AEROsol in Hospitalized Patients with Mild to Moderate COVID-19 Pneumonia: The TAEROVID-19 Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Taurisolo Preparation
2.3. Safety Analysis
2.4. Statistical Analysis
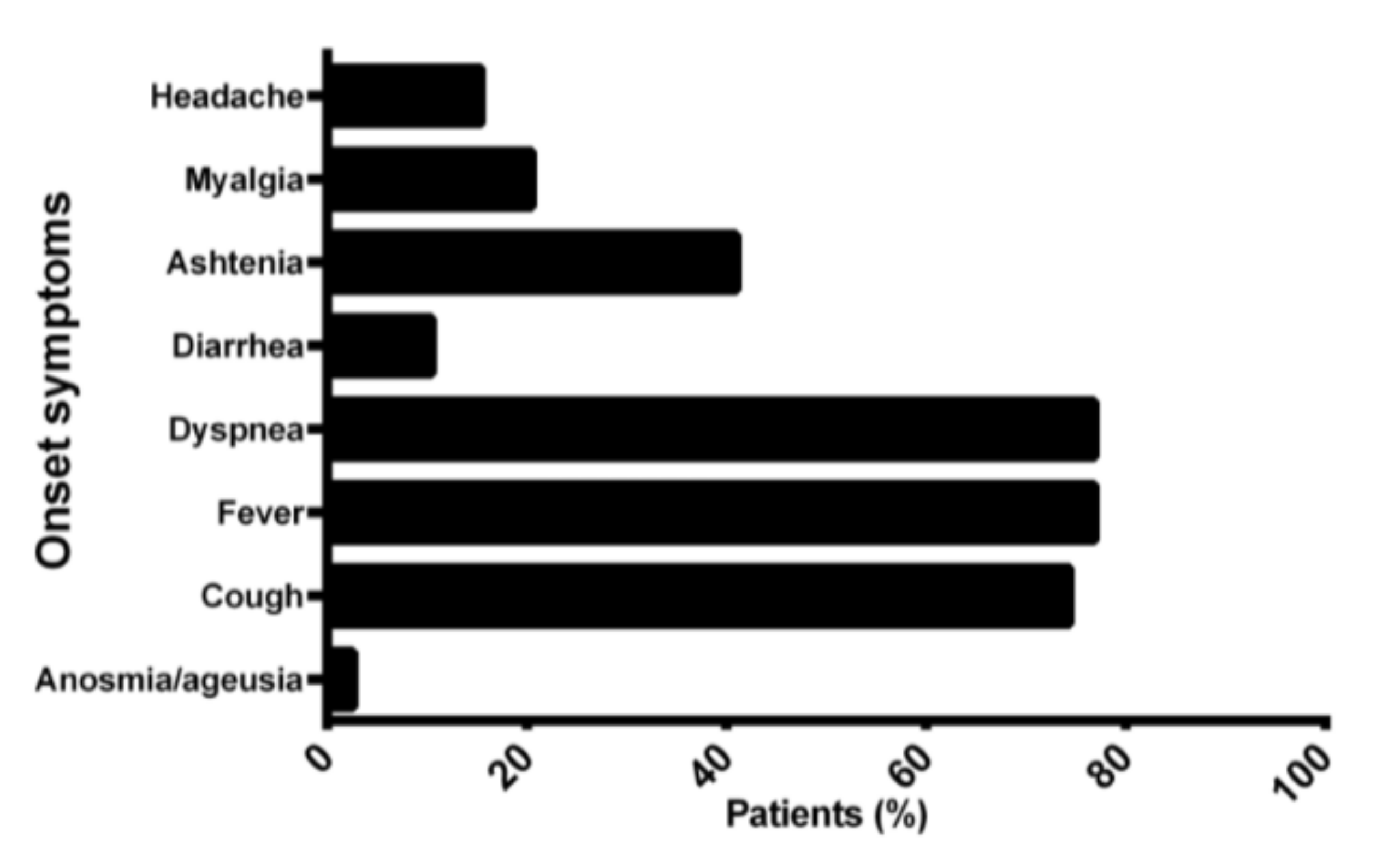
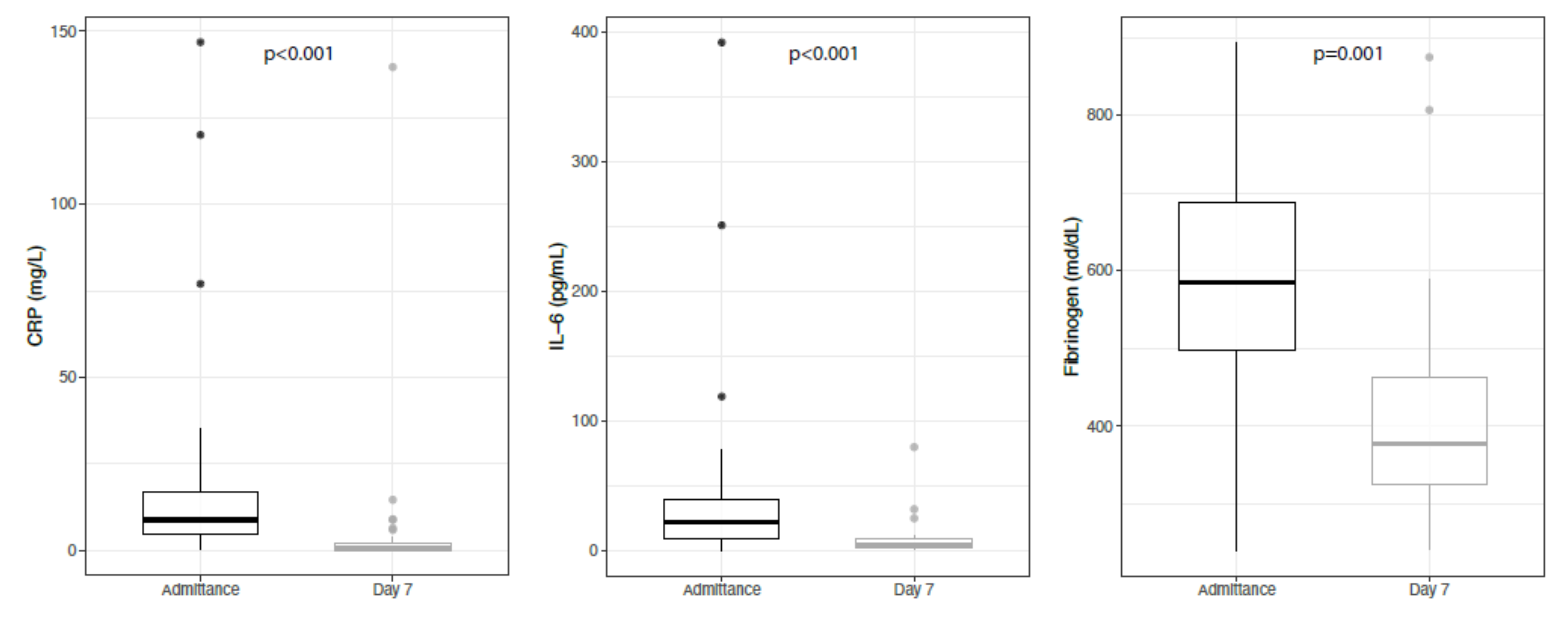
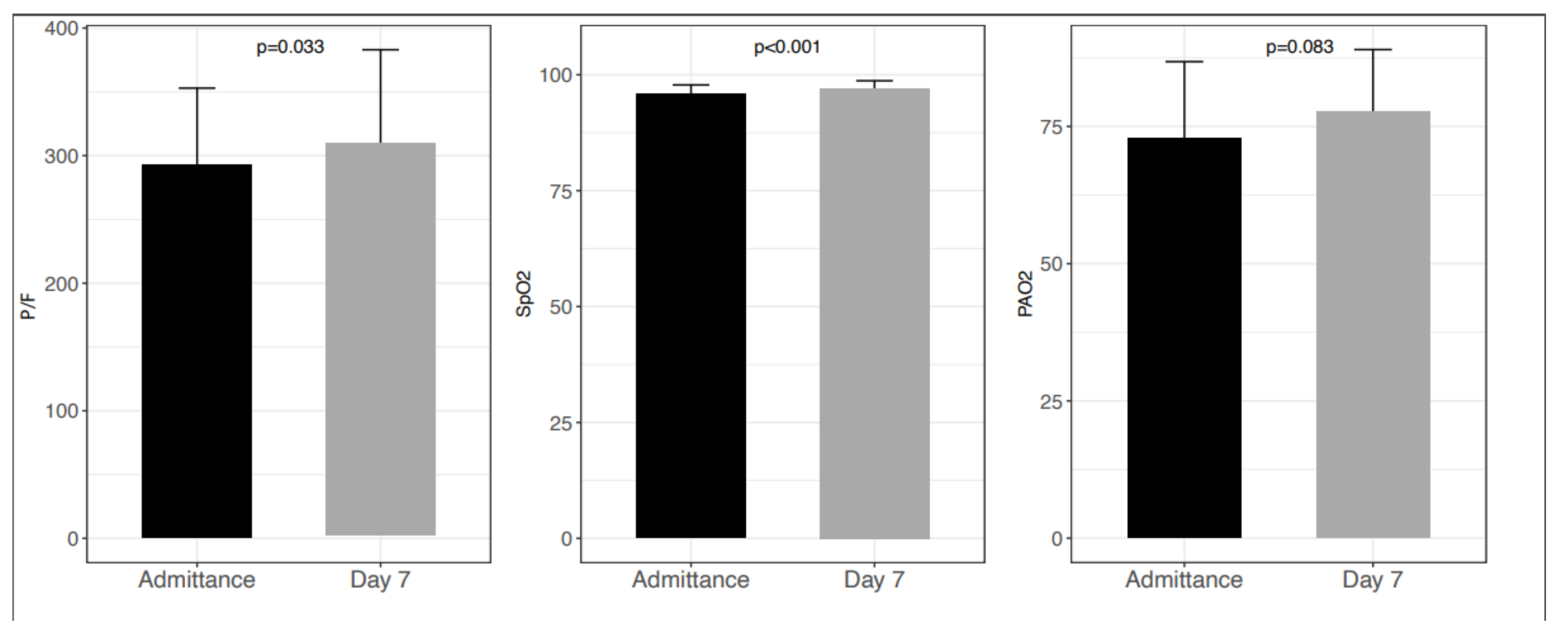
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Collaborators
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID 19); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pan, Y.; Guan, H.; Zhou, S.; Wang, Y.; Li, Q.; Zhu, T.; Hu, Q.; Xia, L. Initial CT Findings and Temporal Changes in Patients with the Novel Coronavirus Pneumonia (2019-nCoV): A Study of 63 Patients in Wuhan, China. Eur. Radiol. 2020, 30, 3306–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, L.B.; Matthay, M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.M.; Hui, P.-K.; Ma, T.K.F.; Lo, A.; To, K.-F.; Chan, W.Y.; Chow, L.T.; Ng, H.-K. Sputum Cytology of Patients with Severe Acute Respiratory Syndrome (SARS). J. Clin. Pathol. 2004, 57, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients With SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 with Convalescent Plasma. JAMA J. Am. Med. Assoc. 2020, 323, 1582. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Adaptive COVID-19 Treatment Trial (ACTT)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/Show/NCT04280705 (accessed on 19 January 2022).
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open-Label Non-Randomized Clinical Trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo®) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-Activity Analysis of Flavonoids: Direct and Indirect Antioxidant, and Antiinflammatory Potencies and Toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and Mitochondria: An Update on Their Increasingly Emerging ROS-Scavenging Independent Actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as Antioxidants: Determination of Radical-Scavenging Efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-Inflammatory Effects of Resveratrol in Lung Epithelial Cells: Molecular Mechanisms. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [Green Version]
- Trotta, V.; Lee, W.-H.; Loo, C.-Y.; Haghi, M.; Young, P.M.; Scalia, S.; Traini, D. In Vitro Biological Activity of Resveratrol Using a Novel Inhalable Resveratrol Spray-Dried Formulation. Int. J. Pharm. 2015, 491, 190–197. [Google Scholar] [CrossRef]
- Tena, A.F.; Clarà, P.C. Deposition of Inhaled Particles in the Lungs. Arch. Bronconeumol. Engl. Ed. 2012, 48, 240–246. [Google Scholar] [CrossRef]
- Trotta, V.; Scalia, S. Pulmonary Delivery Systems for Polyphenols. Drug Dev. Ind. Pharm. 2017, 43, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y.; Li, S. CT Image Visual Quantitative Evaluation and Clinical Classification of Coronavirus Disease (COVID-19). Eur. Radiol. 2020, 30, 4407–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of Fourteen Polyphenols in Pulses by High Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and Correlation Study with Antioxidant Activity and Colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Martelli, A.; Flori, L.; Gorica, E.; Piragine, E.; Saviano, A.; Annunziata, G.; Di Minno, M.; Ciampaglia, R.; Calcaterra, I.; Maione, F.; et al. Vascular Effects of the Polyphenolic Nutraceutical Supplement Taurisolo®: Focus on the Protection of the Endothelial Function. Nutrients 2021, 13, 1540. [Google Scholar] [CrossRef]
- Annunziata, G.; Capó, X.; Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Tejada, S.; Tur, J.A.; Ciampaglia, R.; Guerra, F.; Maisto, M.; Tenore, G.C.; et al. Ex Vivo Study on the Antioxidant Activity of a Winemaking By-Product Polyphenolic Extract (Taurisolo®) on Human Neutrophils. Antioxidants 2021, 10, 1009. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled Budesonide in the Treatment of Early COVID-19 (STOIC): A Phase 2, Open-Label, Randomised Controlled Trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef]
- Singanayagam, A.; Patel, M.; Charlett, A.; Bernal, J.L.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of Infectiousness and Correlation with RT-PCR Cycle Threshold Values in Cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef]
- Hu, X.; Xing, Y.; Jia, J.; Ni, W.; Liang, J.; Zhao, D.; Song, X.; Gao, R.; Jiang, F. Factors Associated with Negative Conversion of Viral RNA in Patients Hospitalized with COVID-19. Sci. Total Environ. 2020, 728, 138812. [Google Scholar] [CrossRef]
- Cariani, L.; Orena, B.S.; Ambrogi, F.; Gambazza, S.; Maraschini, A.; Dodaro, A.; Oggioni, M.; Orlandi, A.; Pirrone, A.; Renteria, S.C.U.; et al. Time Length of Negativization and Cycle Threshold Values in 182 Healthcare Workers with Covid-19 in Milan, Italy: An Observational Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 5313. [Google Scholar] [CrossRef]
- Simonis, A.; Theobald, S.J.; Fatkenheuer, G.; Rybniker, J.; Malin, J.J. A Comparative Analysis of Remdesivir and Other Repurposed Antivirals Against SARS-CoV-2. EMBO Mol. Med. 2021, 13, E13105. [Google Scholar] [CrossRef] [PubMed]
- Overview|COVID-19 Rapid Guideline: Severe Asthma|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng166/resources/covid19-rapid-guideline-severe-asthma-pdf-66141904108741 (accessed on 22 January 2022).
- Colaneri, M.; Sacchi, P.; Zuccaro, V.; Biscarini, S.; Sachs, M.; Roda, S.; Pieri, T.C.; Valsecchi, P.; Piralla, A.; Seminari, E.; et al. Clinical Characteristics of Coronavirus Disease (COVID-19) Early Findings from a Teaching Hospital in Pavia, North Italy, 21 to 28 February 2020. Eurosurveillance 2020, 25, 2000460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, M.; Zhao, M.; Guo, S.; Xu, Y.; Ye, J.; Ding, W.; Wang, Z.; Ye, D.; Pan, W.; et al. The Clinical Characteristics and Prognosis Factors of Mild-Moderate Patients With COVID-19 in a Mobile Cabin Hospital: A Retrospective, Single-Center Study. Front. Public Health 2020, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Mahat, R.K.; Panda, S.; Rathore, V.; Swain, S.; Yadav, L.; Sah, S.P. The Dynamics of Inflammatory Markers in Coronavirus Disease-2019 (COVID-19) Patients: A Systematic Review and Meta-Analysis. Clin. Epidemiol. Glob. Health 2021, 11, 100727. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-J.; Tan, L.; Ren, L.; Shao, Y.; Tao, W.; Wang, Y. COVID-19 Risk Appears to Vary Across Different Alcoholic Beverages. Front. Nutr. 2022, 8, 1146. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Zamparelli, M.S.; Santoro, C.; Ciampaglia, R.; Stornaiuolo, M.; Tenore, G.C.; Sanduzzi, A.; Novellino, E. May Polyphenols Have a Role Against Coronavirus Infection? An Overview of in Vitro Evidence. Front. Med. 2020, 7, 240. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness Against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2021, 28, 202–221. [Google Scholar] [CrossRef]
| Compound | Mean Value (µg/Taurisolo® 100 mg + Polygonum Cuspidatum 4.75 mg sin gle do se) ± SD | |
|---|---|---|
| Pre-Aerosolization | After Aerosolization | |
| Ferulic acid | 1.459 ± 0.098 | n.d. |
| Resveratrol | 4656.25 ± 21.00 | 3597 ± 38.00 |
| Caffeic acid | 3.50 ± 0.300 | 3.273 ± 0.214 |
| p-cumaric acid | 12.275 ± 0.277 | 0.47 ± 0.122 |
| Rutin | 9.881 ± 0.731 | n.d. |
| Quercetin | 13.541 ± 0.469 | 11.192 ± 0.234 |
| Procyanidin B1 dimer | 94.633 ± 5.520 | 30.553 ± 1.842 |
| Procyanidin B2 dimer | 64.589 ± 5.917 | 18.155 ± 2.500 |
| Syringic acid | 31.095 ± 0.001 | 29.101 ± 1.842 |
| Epicatechin | 169.655 ± 10.960 | 26.157 ± 0.443 |
| Gallic acid | 19.946 ± 0.459 | n.d. |
| Catechin | 249.904 ± 30.741 | 61.662 ± 0.353 |
| Clinical Data at Admission (43 Patients) | |
|---|---|
| Mean HRCT Chung score | 12/20 [10/20; 16/20] |
| Mean P/F | 290 [206; 434] |
| Mean lactates (mmol/L) | 1.17 [0.4; 2.8] |
| Mean Procalcitonin (ng/mL) | 0.13 [0.03; 1.6] |
| Mean CRP (mg/L) | 17.54 [0.5; 143] |
| Mean IL-6 (pg/mL) | 44.8 [2.5; 390] |
| Mean KL-6 | 341.16 [203; 711] |
| Mean white cells | 207.5 [133.2; 325.2] |
| Mean Dimer | 585 [498; 687] |
| Mean Fibrinogen | 6.2 [4.2; 9.9] |
| Baseline Characteristics of Patients | |
|---|---|
| Mean age | 61.8±12.4 years |
| Mean BMI | 27.9 ± 4.4Kg/m2 |
| Sex | |
| Female | 11 (25.5%) |
| Male | 32 (74.5%) |
| Smoke | |
| Actual smokers | 6 (14%) |
| Former smokers | 7(16.3%) |
| Never smokers | 30 (69.7%) |
| Comorbidities | |
| Immunosuppression | 3 (7.5%) |
| COPD | 3 (7.5%) |
| Asthma | 3 (7.5%) |
| Interstitial lung disease | 1 (2.5%) |
| Bronchiectasis | 2 (5%) |
| Hepatopathy | 1 (2.5%) |
| Arterial hypertension | 24 (58.5%) |
| Dyslipidemia | 4 (10.3%) |
| Myocardial infarction | 5 (12.8%) |
| Hypertensive heart disease | 2 (5%) |
| Ischemic heart disease | 5 (12.8%) |
| Atrial fibrillation | 3 (7.7%) |
| Arterial vasculopathy | 2 (5.1%) |
| Active solid neoplasia | 3 (7.7%) |
| Diabetes | 7 (17.1%) |
| Previous home therapy | |
| Oral Steroids | 13 (33.3%) |
| PPI (proton pump inhibitors) | 12 (31.6%) |
| ICS/LABA | 2 (5.3%) |
| ICS/LABA/LAMA | 1 (2.6%) |
| LAMA | 2 (5.1%) |
| Oxygen therapy | 2 (5.1%) |
| ACEi (ACE inhibitors) | 9 (23.1%) |
| Angiotensin receptor antagonists | 9 (23.1%) |
| ASA (acetylsalicylic acid) | 6 (15.4%) |
| Other antiplatelets | 1 (2.6%) |
| DOAC (direct oral anticoagulants) | 4 (10.3%) |
| Insulin | 2 (5.1%) |
| Oral hypoglycemics | 2 (5.1%) |
| COVID-19 Pneumonia Treatment | |
|---|---|
| Remdesivir | 9 (20%) |
| High-dose corticosteroids | 19 (44%) |
| Low-flow oxygen therapy | 8 (18.6%) |
| High-flow oxygen therapy | 5 (11.6%) |
| Low molecular weight heparin as prophylaxis (LMWH) | 14 (32.6%) |
| Low molecular weight heparin as therapy (LMWH) | 1 (2.3%) |
| Other anticoagulants | 1 (2.3%) |
| Antibiotics | 10 (23.3%) |
| Day 0 | Day 1 | Day 3 | Day 5 | |
|---|---|---|---|---|
| Taurisolo® | 29.1 (43 pt) | 32.8 (37 pt) | 34.3 (28 pt) | 35.4 (18 pt) |
| Tauri with Remde | 30.2 (9 pt) | 32.5 (9 pt) | 34.9 (9 pt) | 35.4 (9 pt) |
| Tauri without Remde | 30.2 (34 pt) | 26.7 (28 pt) | 29.7 (17 pt) | 29.9 (9 pt) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanduzzi Zamparelli, S.; Capitelli, L.; Coppola, N.; Venditto, C.; Santoro, C.; Annunziata, G.; Bruzzese, D.; Cuomo, N.; Gentile, I.; Bocchino, M.; et al. A Phase II Study on the Effect of Taurisolo® Administered via AEROsol in Hospitalized Patients with Mild to Moderate COVID-19 Pneumonia: The TAEROVID-19 Study. Cells 2022, 11, 1499. https://doi.org/10.3390/cells11091499
Sanduzzi Zamparelli S, Capitelli L, Coppola N, Venditto C, Santoro C, Annunziata G, Bruzzese D, Cuomo N, Gentile I, Bocchino M, et al. A Phase II Study on the Effect of Taurisolo® Administered via AEROsol in Hospitalized Patients with Mild to Moderate COVID-19 Pneumonia: The TAEROVID-19 Study. Cells. 2022; 11(9):1499. https://doi.org/10.3390/cells11091499
Chicago/Turabian StyleSanduzzi Zamparelli, Stefano, Ludovica Capitelli, Nicola Coppola, Claudia Venditto, Ciro Santoro, Giuseppe Annunziata, Dario Bruzzese, Nunzia Cuomo, Ivan Gentile, Marialuisa Bocchino, and et al. 2022. "A Phase II Study on the Effect of Taurisolo® Administered via AEROsol in Hospitalized Patients with Mild to Moderate COVID-19 Pneumonia: The TAEROVID-19 Study" Cells 11, no. 9: 1499. https://doi.org/10.3390/cells11091499
APA StyleSanduzzi Zamparelli, S., Capitelli, L., Coppola, N., Venditto, C., Santoro, C., Annunziata, G., Bruzzese, D., Cuomo, N., Gentile, I., Bocchino, M., & Sanduzzi Zamparelli, A. (2022). A Phase II Study on the Effect of Taurisolo® Administered via AEROsol in Hospitalized Patients with Mild to Moderate COVID-19 Pneumonia: The TAEROVID-19 Study. Cells, 11(9), 1499. https://doi.org/10.3390/cells11091499










