Abstract
Dimethyl fumarate (DMF) is a small molecule currently approved and used in the treatment of psoriasis and multiple sclerosis due to its immuno-modulatory, anti-inflammatory, and antioxidant properties. As an Nrf2 activator through Keap1 protein inhibition, DMF unveils a potential therapeutical use that is much broader than expected so far. In this comprehensive review we discuss the state-of-art and future perspectives regarding the potential repositioning of this molecule in the panorama of eye pathologies, including Age-related Macular Degeneration (AMD). The DMF’s mechanism of action, an extensive analysis of the in vitro and in vivo evidence of its beneficial effects, together with a search of the current clinical trials, are here reported. Altogether, this evidence gives an overview of the new potential applications of this molecule in the context of ophthalmological diseases characterized by inflammation and oxidative stress, with a special focus on AMD, for which our gene–disease (KEAP1-AMD) database search, followed by a protein–protein interaction analysis, further supports the rationale of DMF use. The necessity to find a topical route of DMF administration to the eye is also discussed. In conclusion, the challenge of DMF repurposing in eye pathologies is feasible and worth scientific attention and well-focused research efforts.
Keywords:
dimethyl fumarate; DMF; Nrf2; Keap1; eye; retina; age-related macular degeneration; AMD; inflammation; repurposing; ocular disorders 1. Dimethyl Fumarate: Chemical Properties and Mechanisms of Action
Dimethyl fumarate (DMF; IUPAC name: dimethyl (E)-but-2enedioate) is a methyl ester of fumaric acid (FA) belonging to the family of fumaric acid esters (FAEs), molecules identified in nature in the Fumaria officinalis. This small molecule (MW: 144.13 g/mol) is an α,β-unsaturated carboxylic ester derived through the reaction of FA with methanol in the presence of sulfuric acid [1]. DMF and its active metabolite monomethyl fumarate (MMF) are compounds that exhibit a plethora of biological effects, ranging from anti-inflammatory and immunomodulatory properties to radio-sensitizing activities [2,3,4,5,6,7,8]. Oral administration of DMF leads to its rapidly hydrolyzation by the esterases present in the gastrointestinal (GI) tract, thus leading to the formation of MMF, whose half-life is 36 h. Systemic levels of MMF reach the peak after 2–2.5 h; conversely, DMF is not found in circulation [9,10].
The central mechanism of action of DMF is represented by the activation at low concentrations of the nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2, Nfe2l2) and its subsequent translocation in the nucleus. Specifically, the induction of this pathway is mediated through the reaction of DMF with Kelch-like ECH-associated protein 1 (Keap1), a cytoplasmic repressor of Nrf2. At basal condition, Keap1 forms an inactive complex with Nrf2, promoting its ubiquitination and proteasomal degradation through its E3 ubiquitin ligase activity [11]. As a cysteine-rich protein, Keap1 is one of the main targets of the reaction promoted by DMF (i.e., succination), which leads to the Keap1 conformational change responsible for Nrf2 release [12,13]. The consequent translocation of Nrf2 into the nucleus, leads to the binding of this transcriptional factor to antioxidants response elements (ARE) in the genome, thus promoting the expression of a variety of antioxidant and detoxifying genes, such as Heme Oxygenase-1 (HO-1), NAD(P)H Quinone Dehydrogenase 1 (HQO1), and γ-Glutamylcysteine Synthetase (GCLC) [14,15,16]. Conversely, at a high concentration, DMF induces apoptosis in different cell lines [17].
Notably, increasing interest has raised in the last years regarding the crosstalk between Nrf2 and autophagy. Specifically, the autophagy adaptor protein SQSTM1/p62 can promote the autophagy degradation of Keap1, thus inducing promoting the nuclear translocation of Nrf2 [18]. It has been suggested that the SQSTM1/p62-mediated degradation of Keap1 may be also promoted by sestrins and/or mTORC1 [19,20]. Furthermore, Nrf2 can induce the transcription of TP53, which is a well-known autophagy inducer in the presence of DNA damage [21,22]. Furthermore, a positive feedback loop was described, since Nrf2 can induce autophagy upon oxidative stress responses [23]. This finely regulated crosstalk can also explain the DMF-mediated autophagy induction observed in microglial cells [24].
According to the tissue and the cellular context, DMF is also able to induce a variety of cellular responses in a Nrf2-independent mechanisms. Particularly, it has been demonstrated that DMF can perturb cellular metabolism leading to different outcomes: for example, succination and consequent inactivation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibit aerobic glycolysis in myeloid and lymphoid cells, thus mediating anti-inflammatory effects [25]. A metabolic crisis in pancreatic carcinoma cells was described after DMF treatment through the down-regulation of methylenetetrahydrofolate dehydrogenase (MTHFD1) and methylenetetrahydrofolate dehydrogenase (NADP+ Dependent) 1 Like (MTHFD1L), two enzymes implicated in folate metabolism [26]. The anti-inflammatory properties of DMF are also exerted through the inhibition of the nuclear translocation of both the canonical and non-canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) protein [27,28]. This inhibition seems to be mediated by direct interaction and modulation of hydroxycarboxylic acid receptor 2 (HCAR2) [29]. Furthermore, both the NF-κB and Erk1/2 pathway can be down-regulated by DMF treatment through the inhibition of K63 and M1 polyubiquitin chain formation, which is essential to link Toll-like receptor (TLR) and to its down-stream effectors (i.e., NF-κB and Erk1/2) [30]. Another target of DMF is the hypoxia-inducible factor-1α (HIF-1α), leading to the disruption of its complex with the heat shock protein 90 (Hsp90) and affecting its folding and maturation [31]. DMF can also modulate glutathione metabolism in both Nrf2-dependent and independent ways [32,33,34]. Notably, as described by proteomic analysis, other essential cellular responses are mediated by the reaction of succination involving enzymes like thioredoxin-1 (TRX1), inhibitor of κβ kinase (IKKβ), and protein kinase C Ө (PKCӨ) [35,36].
2. Current Clinical Applications and Clinical Trials
Considering the role of Nrf2 in the oxidative stress response and aging [37,38], neurodegenerative disorders [39], and inflammation [40], this pathway became a candidate target in many diseases and DMF a candidate drug. Nowadays, several electrophilic and non-electrophilic Nrf2 activators are under investigation in clinical trials to assess their therapeutic efficacy in different pathological contexts [41]. Besides DMF, other compounds, such as cyanidin-3-O-glucoside (C3G), have been studied in the last decade for their capability to induce the Nrf2 pathway [42,43,44,45].
DMF/MMF, as well as other fumaric acid esters (FAEs), are molecules of great clinical interest. Specifically, DMF is a relatively well-tolerated molecule whose mechanisms of action are well characterized [46,47,48,49]. The therapeutic efficacy of DMF is primarily mediated by the activation of the Nrf2 pathway [12,13] and by the modulation of HCAR2 [29]. Nowadays, approved indications for DMF include psoriasis and multiple sclerosis (MS).
2.1. Psoriasis
Psoriasis (Psoriasis vulgaris, PV) is a chronic inflammatory disorder of the derma, with a mean frequency of ~2% worldwide, affecting men and women equally. The disease, whose onset is generally between 15 and 30 years of age, is characterized by variable expressivity, in which symptoms may be mild or severe [50]. The etiology of psoriasis is determined by both genetics and environmental factors. Genome-wide association studies (GWASs) identified several genes (more than 60) that are generally involved in T-helper 17 (Th17) cells activation [51]. Patients show red and squamous/flaky patches on the skin usually localized on the scalp, lower back, and joints. These lesions are caused by abnormal proliferation of keratinocytes and infiltration of immune cells: specifically, dendritic cells (DCs) and T-cells in the dermis and neutrophils in the epidermis [52].
As reported in literature, the skin lesions of psoriatic patients show increased DCs, which induce T-cells as well as the production of Th1 cytokines, Tumor Necrosis Factor-α (TNF-α), and inducible nitric oxide synthase (iNOS) [53,54]. Additionally, Th17 cells are involved in psoriasis pathogenesis, through the release of some interleukins, such as interleukin-17 (IL-17) and IL-22 [55].
DMF systemic treatment was identified as a potential therapy for psoriasis according to previous evidence collected on FA treatment of psoriatic patients in the late 1950s. DMF, in combination with monoethyl fumarate salts, was first licensed for psoriasis treatment in 1994 in Germany with the commercial name of Fumaderm® [56]. In vitro and in vivo studies disclosed the beneficial molecular actions exerted by DMF in the context of psoriasis. Systemic DMF treatment leads to a decrease in IL-6, IL-17, IL-22, granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming growth factor-α (TGF-α), and interferon-γ (IFN-γ) and, conversely, induces an increase in the levels of IL-10. Furthermore, DMF enhances the activation of T regulatory (Treg) cells and inhibits Th17 cells [57,58]. Notably, DMF’s beneficial effect in psoriasis is mediated by the depletion of glutathione-S-transferase [33] and by the reduction in the pro-inflammatory neutrophil population [59].
Recent in vivo findings demonstrated that the Nrf2-dependent mechanism of action of DMF plays an important role in psoriasis treatment. Specifically, DMF administration to Nrf2+/+ mice model of psoriasis leads to attenuation of the symptoms and rescues epidermal differentiation; conversely, no effects are detectable in Nrf2−/− mice models [60].
2.2. Multiple Sclerosis
Multiple sclerosis (MS) is a chronic immune-mediated disorder which affects the central nervous systems (CNS), leading to demyelination and consequent neuronal degeneration. MS primarily affects young adults, predominantly women. The etiology is mediated by both genetic and environmental factors [61]. The majority of patients (~85%) present the relapsing-remitting form of the disease (RRMS), whereas the remaining patients (~15%) show a continue progression of the disorder, defined as primary progressive MS (PPMS) [62]. The principal histopathological hallmark of MS is the presence of focal lesions in grey and white matter, characterized by neuronal demyelination and astrocytic scars. Despite the original dogma, the underlying inflammatory process is mediated by both T- and B-lymphocytes. Specifically, T-cells belong to the Human Leukocyte Antigen class I (HLA-I) CD8+ class whereas B-cells show either the CD20+ or plasma cell phenotype, according to the stage of the disorder. Generally, immune cells in MS patients invade the perivascular space and the parenchyma [63].
MS therapeutic strategies are mainly based on inhibiting neuroinflammation or promote neuroprotection and remyelination. DMF exerts beneficial effects on both inflammation and neuroprotection, thus becoming an excellent reference compound in the treatment of MS. Particularly, different clinical trials showed how DMF is well-tolerated by MS patients at different doses, leading to a remission of the symptoms or to inhibition of relapses in RRMS [64,65,66,67].
DMF modulates both microglia/astrocytes and immune cells. The anti-inflammatory effect on glial cells is due to the inhibition of the release of several pro-inflammatory molecules, i.e., nitric oxide (NO), IL-1β, IL-6, and TNF-α [68]. The DMF-induced modulation of microglia is also mediated by the HCAR2 signaling pathway as well as by Nrf2, thus promoting a shift of the glial phenotype from a pro-inflammatory to a non-activated one [6,69]. Moreover, the activation of Nrf2 pathway leads to a reduction in the inflammatory levels in astrocyte through glutathione and HO-1; overall, the Nrf2-mediated beneficial effects of DMF were also demonstrated in vivo: neuroprotection after DMF treatment was observed in a model of hypoxic-ischemic damage [70,71,72]. Recently, it has been demonstrated that astrocyte protection against oxidative stress is mediated by the Nrf2-induced up-regulation of oxidative stress-induced growth inhibitor 1 (OSGIN-1), a protein implicated in the oxidative stress response [73]. Notably, DMF effects on the immune system play a key role in MS treatment. DMF can induce immunosuppression through HO-1 induction in presence of glutathione depletion [34]. Recently, it has been demonstrated that DMF can alter the phenotypic profile of B- and T-cells, thus inhibiting their activation in MS patients and promoting the release of beneficial cytokines, such as IL-4 [74,75]. Another described effect of DMF in MS patients is the inhibition of Th1/Th17 cell differentiation and the reduced expression of circulatory pro-inflammatory cytokines [76].
2.3. Ongoing Clinical Trials and New Potential Indications
To date, several clinical trials are ongoing to study the effects of DMF in the contexts of different disorders. The majority of these studies are focused on MS (95) and psoriasis (12). In Table 1, all the clinical trials involving DMF/MMF in different disorders are reported, excluding the well-known applications in psoriasis and MS (all the studies involving patients with a diagnosis of MS or psoriasis were excluded).

Table 1.
Clinical trials involving DMF/MMF in different diseases. Exclusion criteria: patients involving a diagnosis of MS or psoriasis. The terminated trials were interrupted for low recruitment.
Beneficial effects of DMF in autoimmune disorder have been widely described in the literature: an example is represented by the therapeutic effect of FAEs in the treatment of discoid lupus erythematosus [77]. Pre-clinical studies demonstrated that osteoclasts modulation by DMF plays a key role in rheumatoid arthritis (RA): particularly, DMF leads to a decrease in receptor activator of nuclear factor kappa-B ligand (RANKL) levels after ROS generation through Nrf2 activation. Moreover, DMF can alter osteoclasts differentiation by inhibiting nuclear factor of activated T-cell 1 (NFATc1) and activating the Erk1/2 and p38 MAPK pathways [78,79]. Modulation of the immune system exerted by DMF also plays an important role in the treatment of systemic sclerosis [80]. The beneficial effects of DMF were also demonstrated in cancer. Specifically, this molecule can induce cell death in different types of blood cancers [81,82,83]. Similarly, in vitro and in vivo pre-clinical studies demonstrated that in solid tumors DMF exerts a therapeutic potential both alone and in combination with other compounds [26,84,85,86,87].
The increasing number of clinical trials in cerebrovascular diseases aimed at studying the therapeutic potential of DMF is based on the increasing in vitro and in vivo pre-clinical evidence that has been raised in the last years [88]. Considering the growing and its anti-inflammatory and antioxidant properties, DMF has the potential to be considered/used more broadly in other pathologies characterized by inflammation and/or oxidative stress.
3. DMF and Eye Disorders: State of the Art
The Nrf2-dependent mechanism of action of DMF confers attractiveness from a therapeutic point of view to this molecule in the context of ocular diseases [89]. Indeed, impairment or loss of Nrf2 content or activity were described in several ocular pathologies, such as uveitis, diabetic retinopathy, retinal ganglion cells (RGCs) degeneration, AMD, cataracts, and glaucoma [90,91,92,93,94,95]. Nrf2−/− animal models display similar characteristics reminiscent of AMD. The knockout (KO) of Nrf2 also leads to an increase of RGCs death compared to wild-type (WT) mice in a model of oxidative stress-mediated RGCs degeneration as well as an increase in intercellular adhesion molecule 1 (ICAM-1), IL-6, TNF-α, cyclooxygenase 2 (COX-2), iNOS, and monocyte chemoattractant protein 1 (MCP-1) compared to WT mice after the administration of lipopolysaccharide (LPS). Interestingly, administration of Nrf2 inducers can revert these phenotypes, thus mitigating the cell death/degeneration rate and the inflammatory/innate immune response. Genetic studies have also revealed that a single nucleotide polymorphism (SNP) in NFE2L2 gene is associated with an increased risk for AMD. Furthermore, Nrf2 pathway plays a fundamental role in the maintenance of cellular redox homeostasis during aging, thus confirming its involvement in age-related ocular diseases [96].
Increasing evidence suggested that DMF has a strong potential in eye pathologies and might be used in several ophthalmological contexts. This hypothesis is based on the evidence collected on MS patients. Optic neuritis is the first demyelinating symptom that occurs in 15–20% of MS patients and almost 50% of patients experience this problem during the disease progression [37]. In vivo studies have also revealed that DMF, as well as other canonical treatment of MS, exerts a higher beneficial effect than IFN-γ on RGCs by preventing their loss in MS patients [97]. Moreover, two case reports described the beneficial effects of systemic DMF administration in counteracting the symptoms of uveitis and cystoid macular edema [98,99]. Based on this evidence, a clinical trial started in 2022 with the aim to study the effects of oral administration of DMF in AMD patients. Specifically, 30 patients between 55- to 85-years-old with central or non-central geographic atrophy (GA) in at least one eye, and visual acuity between 25/25 and 20/200 in the affected eye, will be treated with 120 mg DMF twice a day for the first week, and then with 240 mg DMF twice a day for 51 weeks (NCT04292080). The results will be compared with those collected in a cohort of AMD subjects under no specific treatment. Table 2 reports an exhaustive list of the literature evidence and relative references supporting the DMF potential use in ophthalmology. Figure 1 summarizes the main DMF beneficial effects referring to in vivo studies and evidence in human subjects in the context of eye pathologies.

Table 2.
Studies and clinical trials about DMF applications in the context of ophthalmological diseases. The symbol ↑ indicates “increase”, whereas ↓ indicates “decrease”.
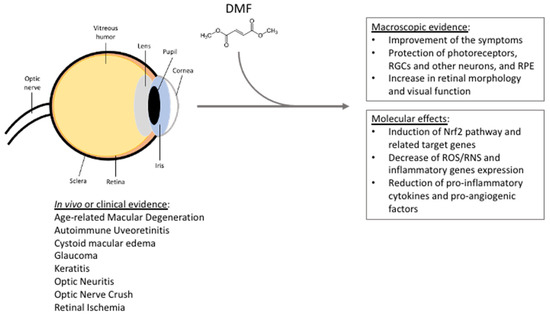
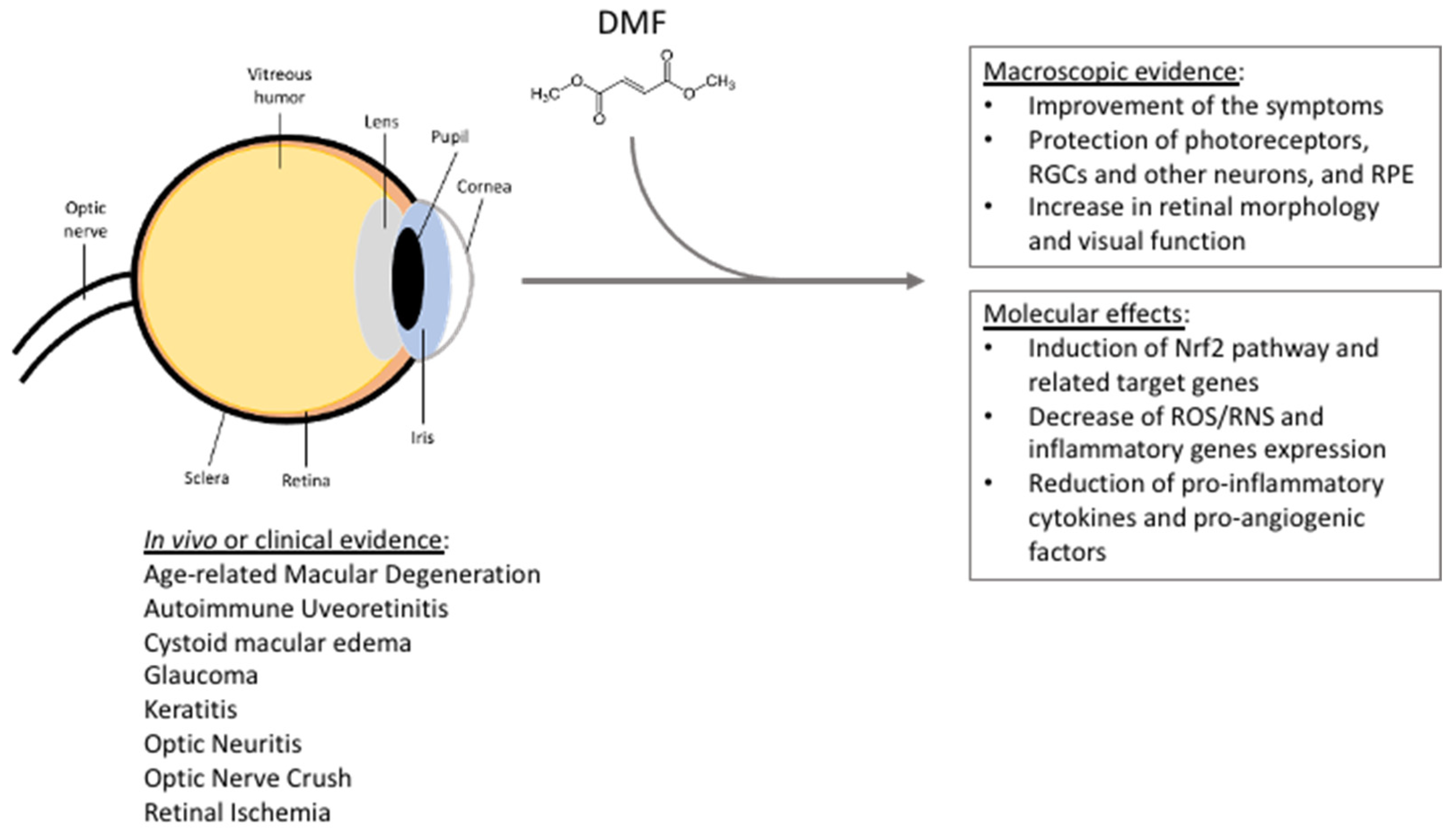
Figure 1.
Summarizing scheme reporting DMF beneficial effects in the context of eye pathologies, based on in vivo studies, clinical trials, and clinical and case studies. See the text for further details. RGCs = retinal ganglion cells; RPE = retinal pigment epithelium; ROS/RNS = reactive oxygen/nitrogen species.
In vitro studies have elucidated the protective role of DMF on retinal pigment epithelial cells (RPE), essential for photoreceptor homeostasis and participating to the constitution of the retinal–blood barrier (RBB). Specifically, DMF can induce an increase in GSH in RPE cells [111] and plays a key role in RPE cells response to several harmful stimuli. DMF inhibits apoptosis in model of high-glucose (HG) stress based on ARPE-19. Moreover, DMF treatment leads to a decrease in iNOS, COX-2, IL-1β, and vascular endothelial growth factor (VEGF) [115]. DMF-mediated anti-inflammatory effects were also described in primary human RPE cells treated with TNF-α for 5 days. Again, pre-treatment with DMF restored the normal cellular phenotype, promoting a reduction in IL-6, IL-8, MCP-1, and mitochondrial oxidative phosphorylation system (OXPHOS) protein levels. These modifications were also associated with a rescue of normal mitochondrial morphology as well as a metabolic normalization, through the regulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) and pyruvate kinase isozyme M2 (PKM2) [117]. The protective effects of DMF through the activation of Nrf2 and down-stream effectors (e.g., HO-1 and p62) was also demonstrated in ARPE-19 cells exposed to different toxic stimuli, such as oxidative stress, lipid peroxidation products, and protein misfolding [116]; in particular, under pro-oxidant stress, DMF was able to both counteract the cell mortality and decrease ROS levels.
Notably, MMF plays a protective role, even if in other cells of the eye. This molecule can regulate the proton-coupled folate transporter (PCFT) in Müller cells, thus preventing reactive gliosis, and can induce the sodium-independent cysteine/glutamate antiporter solute carrier family 7 member 11 (SCL7A11) in RPE cells, thus potentiating its antioxidant signaling [101,112].
All the evidence was corroborated in in vivo studies. Treatment with DMF of BALB/c mice infected with Herpes simplex virus 1 (HSV-1, KOS strain) showed a reduction in the onset of severe keratitis and a consistent decrease of CD3+, CD11b+, GR-1+, and F4-80+ cells [100]. Positive results were also collected after the treatment of patients with noninfectious uveitis with FAEs [98].
Other beneficial effects were described in the context of optic nerve injury. Particularly, treatment with increasing concentrations of DMF was able to ameliorate the outcome of C57BL/6J mice subjected to optic nerve crush (ONC). An increase in Tubβ3+ RGCs and in the amplitude of positive scotopic threshold response (pSTR) was detected, together with an increase in Nrf2 and HO-1 protein levels [107]. This evidence, together with the evidence collected by You and colleagues [97], suggests a potential application of DMF in the context of glaucoma. In another study, DMF was administered using a different therapeutic scheme in two mouse models based on light-induced photoreceptor loss and ONC, respectively. In this case, DMF exerted a beneficial effect through the reduction of reactive microglia and the increase in GSH levels. Conversely, no effects were detected on ONC [108]. The same beneficial effects were observed in a model of optic neuritis. This pathology occurs frequently in RRMS patients, which experience acute monocular vision loss. Additionally, in in vivo animal models based on the inoculation of myelin oligodendrocyte glycoprotein peptide (i.e., MOG35–55) in C57BL/6J mice, the DMF administration led to a decrease in the severity of the inflammation thanks to an increase in Nrf2 levels [106]. The immunomodulatory action of DMF was also demonstrated in rat models of endogenous uveitis and corneal lymphangiogenesis. In the former study it was observed that DMF impaired NO, TNF-α, CD68, CD20, CD25, and CD8 levels; the latter showed that DMF treatment induced a reduction in MCP-1, TNF-α, IL-6, IL-1β, VEGF, and macrophages infiltration through NF-κB inhibition [109,110].
Other studies strengthened the rationale of the use of DMF in the context of ocular diseases. Specifically, ocular disorders represent a common comorbidity in vascular diseases, such as diabetes. An example is diabetic retinopathy (DR), a pathological condition characterized by vascular and endothelial dysfunctions [119]. Nrf2 dysfunction is strongly associated with vascular-related eye pathologies, as reported in several in vivo studies, which demonstrates a reduced activation of Nrf2-signaling pathway in DR and low circulatory levels of this protein [92,120,121]. Considering these premises, DMF efficacy was also investigated in in vitro models of endothelial cells, such as HUVEC and HBMVEC [113,114]. These two studies showed that DMF was able to reduce the TNF-α-mediated pro-inflammatory response in HUVEC, but no effects were detected in HBMVEC. Notably, it has been recently demonstrated that DMF treatment is able to activate Nrf2/HO-1 pathway and to reduce basal ROS levels in human primary retinal endothelial cell (HREC) line, and to protect these cells from the damage of HG stress [118], thus further highlighting the beneficial effects of DMF.
The efficacy of DMF in ocular pathologies was also demonstrated by a case report of Kofler and colleagues [99]. Oral therapy with Fumaderm® was applied to an 88-year old woman affected by cystoid macular edema. The oral administration was initially 40 mg/die and then was increased to 480 mg/die. Since the patient experienced abdominal pain and diarrhea, DMF administration was reduced to 240 mg/die. After 24 months the patient showed a progressive improvement of her condition: after nine months a reduction in the number of cysts was observed; a decrease in retinal thickness was detected after 12 months until the symptoms regressed from severe to mild.
Additionally, MMF promoted similar beneficial effects. C57BL/6J Nrf2−/− mice models of ischemia/reperfusion damage showed an improvement of the eye’s health. After 48 h post-treatment, authors described an increase in Nrf2 levels and in retinal function (measured by electroretinography, ERG) and a decrease in inflammatory gene expression, in gliosis level, and neuronal loss in the ganglion cell layer (GCL) [105]. An MMF-mediated increase in HO-1 and a concomitant decrease in caspase-1, IL-1β, TNF-α, and in NLR family pyrin domain containing 3 (NLRP3) was also described in albino BABL/c mice exposed to bright white light for one hour to mimic light-induced retinopathy [104]. In in vitro and in vivo models of sickle cell disease (SCD), the treatment with MMF was able to induce γ-globin and fetal hemoglobin and to reduce the levels of oxidative stress and inflammatory markers [102,103].
4. DMF and Eye Disorders: Rationale and Comparison with Other Molecules
Several molecules have been identified as inducers of the Nrf2-pathway and some of them are already under investigation in clinical trials. As described [41,122], many Nrf2 activators showed promising therapeutic potential in various types of disorders. However, as reported, the application of some of these compounds in clinical practice may be limited by a variety of factors. For example, Michael acceptors, such as olefins, are inhibitors of the Keap1-Nrf2 binding, thus leading to the release and consequent activation of Nrf2; however, despite their therapeutic efficacy, these molecules show a classical dose-response curve in which at low concentrations they react with the desired targets, such as Keap1, whereas at higher concentrations they induce cytotoxicity due to off-target effects derived by their chemical reactivity. Another example is represented by natural compounds, such as curcumin and related agents. These molecules share a similar mechanism of action with Michael acceptors and received great attention due to their biological action. Unfortunately, the use of these molecules is limited by their instability, poor or not-evaluated in vivo bioavailability, and rapid degradation.
Different natural compounds were investigated in the context of eye pathologies. An example is sulforaphane (SFN), a molecule present in cruciferous vegetables (Brassicaceae family). Both in vitro and in vivo studies demonstrated that this compound played Nrf2-mediated beneficial effects against photooxidative damage in RPE cells and mitigated light-induced retinal damage in mice [123,124]. SFN, as already described for DMF, can protect eye cells (e.g., photoreceptors, retinal pigment epithelial cells, lens cells, retinal endothelial cells, and retinal pericytes), and it exerts anti-inflammatory effects preventing microglia activation [125,126,127,128,129,130]. Despite this evidence, SFN is not suitable for clinical practice due to some limitation. Particularly, SFN treatment in vivo highlighted the presence of unsolved challenges in its clinical applications (e.g., source selection, extraction, and not characterized protocols) [131,132,133,134]. Nowadays, no clinical trials involving SFN in eye pathologies are available.
Same considerations can be made for other natural compounds, such as flavonoids, carotenoids, and vitamins. All these compounds showed beneficial effects in vitro and in vivo models of ocular pathologies. However, issues regarding the source, bioavailability in humans, and the lack of well-designed clinical trials limit their application in clinical practice [135].
Novel interesting approaches might be represented by molecular hybrids with an Nrf2-dependent mechanism of action like that characterizing DMF. An example is represented by natural-inspired hybrids (NIHs), a family of compounds that were synthesized starting from the chemical structure of curcumin [116]. This strategy seems to be encouraging to overcome the issues that characterized the use of natural compounds, like curcumin (e.g., short half-life, low solubility, and rapid metabolism). These molecules can modulate the Nrf2 and NF-κB pathways in different cell lines, such as SH-SY5Y and THP-1 [136,137]. The efficacy of NIHs was also studied in the context of the eye. Particularly, in the last two years their chemical properties as well as their beneficial effects in vitro and in vivo model of ocular pathologies have been elucidated. In ARPE-19 cells the treatment with these compounds leads to an increase in Nrf2 protein levels, as well as in its down-stream effectors HO-1 and p62. These effects promoted cytoprotection against different toxic stimuli, such as oxidative stress and dysfunction in protein homeostasis [116,138]. Notably, the NIH lead compound was able to increase Nrf2 levels in ex vivo retinal explants, promoting its nuclear translocation and the induction of down-stream effectors, such as HO-1 and NQO1 [139]. Moreover, NIH treatment promoted a reduction in caspase-1 and GFAP levels in presence/absence of oxidative stress, thus underlying its ability to counteract reactive gliosis and promote retinal cell viability.
5. AMD: A Paradigm for DMF Repurposing
Among ocular diseases, AMD may represent an interesting indication for DMF therapeutic applications. This neurodegenerative disorder affects elderly people worldwide and is the major cause of blindness. AMD is classified in dry (~90%) and wet (~10%) forms [140]. The hallmarks in AMD onset consist of RPE degeneration, the increase of ROS levels, and autophagy’s loss of homeostasis [141,142]. The Keap1/Nrf2 pathway plays a key role in the maintenance of RPE cellular homeostasis, as well as in ROS neutralization [143,144]. The importance of this pathway in AMD onset was also demonstrated through the generation of double knockout (dKO) mice for Nrf2 and Ppargc1a [145]. Notably, the loss of these genes led to an age-dependent RPE degeneration, accumulation of ROS, alteration in photoreceptor morphology, and visual impairment. Moreover, a single nucleotide polymorphism (SNP) in the NFE2L2 gene (rs6726395) has been identified, thus strengthening the association of Nrf2 pathway and AMD [146].
As said before, DMF reacts with Keap1 protein leading to Nrf2 activation; we thus examined the link between Keap1 and AMD by consulting the gene–disease DisGeNet database, followed by investigation of Keap1’s multiple physical associations through protein–protein interaction (PPI) analyses (Figure 2); this search revealed that DMF may exert other additional beneficial biological effects on RPE cells than the ones already mentioned.
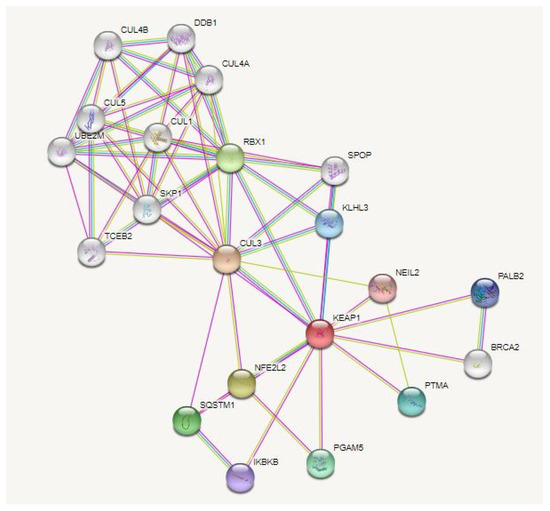
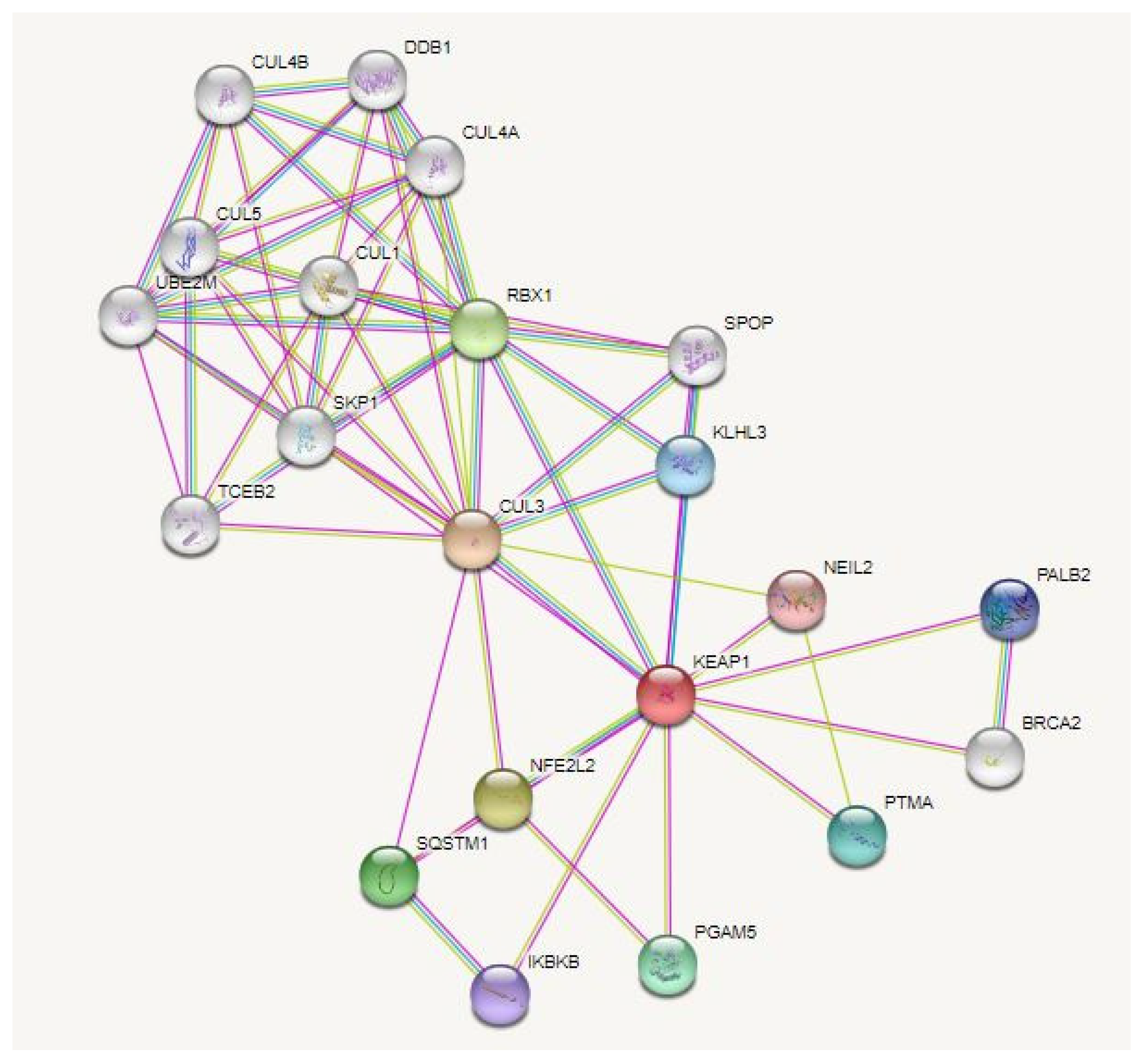
Figure 2.
PPI (protein–protein interaction) network of Keap1. In DisGeNet (https://disgenet.org/, accessed on 29 September 2022), the association of Keap1 and AMD was verified. The PPI network for Keap1 was then analyzed using the STRING database (https://string-db.org/, accessed in 29 September 2022); the interactions were analyzed in Homo sapiens, setting the confidence basis to 0.4 and selecting only the physical interactions.
Specifically, it is possible to identify an interaction between Keap1 and the enzyme NEIL2 (Nei-like 2) that, in mammalian cells, contributes to the UV-induced oxidative stress response together with YB-1 (Y-Box-binding 1) protein [147]. Another interesting interaction is that occurring with BRCA2 (Breast Cancer gene 2) and PALB2 (Partner and Localizer of BRCA2), two components of the DNA repair system mediated by homologous recombination (HR) [148]. Notably, one interactor of Keap1 is RBX1 (RING-box protein one), which is itself an interactor of DDB1 and DDB2 (DNA damage binding protein 1 and 2), two proteins involved in the nucleotide and UV-damage excision repair systems [149].
According to our analyses, as well as literature evidence, the crosstalk between Nrf2 and autophagy, together with the direct interaction between Keap1 and SQSTM1/p62, suggests possible anti-inflammatory properties of DMF mediated by autophagy induction. Indeed, the autophagic process contributes to the modulation of inflammation through different mechanisms, two of which are the degradation of inflammasome-related proteins [150], and the neutralization of oxidative stress [151]. Moreover, autophagy also plays a role in epithelial-to-mesenchymal transition (EMT), which contributes to the onset of RPE’s fibrosis observed in AMD [152] by preventing or reverting it [153,154].
Interestingly, and based on the DMF-induced beneficial effects in PR and RPE cells, we suggest that DMF treatment may also represent a therapeutic approach in the Retinitis Pigmentosa (RP), an inherited disease featured by visual loss and dramatically affecting quality of life of young and adult patients. Specifically, it has been demonstrated that MMF administration (50 mg/kg, intraperitoneally every other day, P14-42) to Pde6βrd10/J (rd10) mice, a model of RP, led to a sustained up-regulation of antioxidant genes through Nrf2 pathway activation [155]. However, since these effects in MMF-treated rd10 mice were not accompanied by significative improvements in the retinal morphology and function compared with untreated diseased controls, the authors concluded that, in their experimental conditions, MMF may not be sufficient to delay the catastrophic PR damage characteristic of the rd10 mouse, and suggested that MMF may be able to prevent, but not restore, PR degeneration [155]. Among others, these findings and related observations suggest that MMF, but more pertinently DMF, which boosts a more robust response than its metabolite MMF [156], may take advantage of different times, concentrations, and/or routes of administrations, as discussed in the next paragraph.
6. Adverse Events of DMF Systemic Treatment and Its Potential Topical Application
DMF is currently used as an oral treatment in clinical practice and shows effectiveness, tolerability, and good bioavailability. However, adverse effects (AEs) have been described in this route of administration, particularly affecting the GI tract. The most common AEs, principally observed in females, are diarrhea, stomachache, nausea, vomiting, and cramps [157]. It has been demonstrated that DMF crosses the blood–brain barrier [158] and it may be the same for the blood–retinal barrier; however, for the treatment of eye pathologies, a topical administration—if possible and available—might be more preferable than the systemic route for various pharmacokinetic and pharmacodynamic aspects. For example, compared to the oral route, topical administration of a drug usually allows for: using a lower therapeutic dosage, since systemic distribution is reduced or absent; avoiding or minimizing systemic side-effects; treating patients with alterations in ADME (e.g., low gastrointestinal absorption, liver dysfunction, renal failure), which would be otherwise excluded/damaged by the systemic treatment; easily delivering the drug with no or low off-target effects on other organs/tissues.
The potential DMF topical application has been limited by contact dermatitis evidenced almost four decades ago in patients with psoriasis following a cutaneous DMF treatment [159]. However, the DMF-induced cutaneous toxicity may distress anatomical structures that are phenotypically different, or even absent in the eye; in addition, this reaction may be mediated by other inflammatory factors than the ones present at ocular level. Indeed, the eye is characterized by an “immune privilege”, and its reactions may differ from other body districts [160]. Beyond these hypotheses and considerations, relevant efforts in the discovery of novel delivery systems of DMF have been made, with the aim to improve the tolerability of this molecule and/or its biodistribution to selected body districts. For instance, studies on a cutaneous treatment with a sensitization-free DMF prodrug (isosorbide DMF) [161] on DMF-loaded transethosomes [162] and on vitamin-derived nanolipoidal carriers for brain delivery of DMF [163] showed encouraging in vitro, ex vivo, and preclinical evidence.
Although not specifically designed for the ocular treatment, these observations indicate that nanotechnological delivery systems of DMF will be likely available for topical administration in eye pathologies soon. Of course, it will be also relevant to find the most suitable formulation to deliver DMF specifically to the retinal cells affected by a given pathology; accordingly, administrations by intraocular injection and eye drops represent feasible, although antithetic routes with respective limitations [164]. Considering that various liposomes, nanoparticles, and microspheres are currently useful delivery systems overcoming the limitations of topic ocular drug administration [165,166], more than one of these approaches may represent a feasible strategy for DMF ophthalmic delivery.
Last, but not least, in virtue of the proven benefits of DMF, its topical treatments may have the potential to prevent or counteract more than one eye pathology, as this drug may display either protective or rescue/therapeutic effects; however, additional preclinical studies will be needed to clarify these points, and to better tailor the DMF use in the panorama of ocular diseases.
7. Conclusions
DMF is an effective treatment that has been used for almost 60 years in Europe and is approved for psoriasis and RRMS. The rising evidence of the beneficial effects of DMF in in vitro and in vivo models of eye disorders encourages its repurposing for the treatment of ocular pathologies. The reasons for DMF attractiveness rely on its mechanism of action, which is mainly mediated by Nrf2 activation. This transcriptional factor is a therapeutic target in eye disorders, such as AMD, in virtue of Nrf2-pathway’s role in the pathogenesis of several ocular affections. Notably, Nrf2 reduced activity is associated with the hallmarks of various ocular disorders: oxidative stress, inflammation, and vascular dysfunction.
In conclusion, the challenge of DMF repurposing in eye pathologies has just begun, and it is already worth further scientific attention and research efforts.
Author Contributions
Conceptualization, M.A., S.G. and F.M.; writing—original draft preparation, F.M.; writing—review and editing, M.A. and S.G.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saidu, N.; Kavian, N.; Leroy, K.; Jacob, C.; Nicco, C.; Batteux, F.; Alexandre, J. Dimethyl fumarate, a two-edged drug: Current status and future directions. Med. Res. Rev. 2019, 39, 1923–1952. [Google Scholar] [CrossRef] [PubMed]
- Seidel, P.; Hostettler, K.E.; Hughes, J.M.; Tamm, M.; Roth, M. Dimethylfumarate inhibits CXCL10 via haem oxygenase-1 in airway smooth muscle. Eur. Respir. J. 2013, 41, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Seidel, P.; Roth, M. Anti-inflammatory dimethylfumarate: A potential new therapy for asthma? . Mediat. Inflamm. 2013, 2013, 875403. [Google Scholar] [CrossRef]
- Kees, F. Dimethyl fumarate: A Janus-faced substance? Expert Opin. Pharmacother. 2013, 14, 1559–1567. [Google Scholar] [CrossRef]
- Schulze-Topphoff, U.; Varrin-Doyer, M.; Pekarek, K.; Spencer, C.M.; Shetty, A.; Sagan, S.A.; Cree, B.A.; Sobel, R.A.; Wipke, B.T.; Steinman, L.; et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc. Natl. Acad. Sci. USA 2016, 113, 4777–4782. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Held, K.D.; Epp, E.R.; Clark, E.P.; Biaglow, J.E. Effect of dimethyl fumarate on the radiation sensitivity of mammalian cells in vitro. Radiat. Res. 1988, 115, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Held, K.D.; Epp, E.R.; Awad, S.; Biaglow, J.E. Postirradiation sensitization of mammalian cells by the thiol-depleting agent dimethyl fumarate. Radiat. Res. 1991, 127, 75–80. [Google Scholar] [CrossRef]
- Linker, R.A.; Haghikia, A. Dimethyl fumarate in multiple sclerosis: Latest developments, evidence and place in therapy. Ther. Adv. Chronic Dis. 2016, 7, 198–207. [Google Scholar] [CrossRef]
- Brück, J.; Dringen, R.; Amasuno, A.; Pau-Charles, I.; Ghoreschi, K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp. Dermatol. 2018, 27, 611–624. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Suzuki, T.; Motohashi, H.; Onodera, K.; Satomi, S.; Kensler, T.W.; Yamamoto, M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012, 53, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.; Deshmukh, P.; Krishnappa, G.; Kommu, P.; Padmanabhan, B. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1. FEBS J. 2021, 288, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Saidu, N.E.; Noé, G.; Cerles, O.; Cabel, L.; Kavian-Tessler, N.; Chouzenoux, S.; Bahuaud, M.; Chéreau, C.; Nicco, C.; Leroy, K.; et al. Dimethyl Fumarate Controls the NRF2/DJ-1 Axis in Cancer Cells: Therapeutic Applications. Mol. Cancer Ther. 2017, 16, 529–539. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Manning, B.D. mTORC1 signaling activates NRF1 to increase cellular proteasome levels. Cell Cycle 2015, 14, 2011–2017. [Google Scholar] [CrossRef]
- Kim, S.B.; Pandita, R.K.; Eskiocak, U.; Ly, P.; Kaisani, A.; Kumar, R.; Cornelius, C.; Wright, W.E.; Pandita, T.K.; Shay, J.W. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. USA 2012, 109, E2949–E2955. [Google Scholar] [CrossRef] [PubMed]
- Jin, S. p53, Autophagy and tumor suppression. Autophagy 2005, 1, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Kapuy, O.; Papp, D.; Vellai, T.; Bánhegyi, G.; Korcsmáros, T. Systems-Level Feedbacks of NRF2 Controlling Autophagy upon Oxidative Stress Response. Antioxidants 2018, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Gupta, D.P.; Park, S.H.; Yang, H.J.; Song, G.J. Anti-Inflammatory Effects of Dimethyl Fumarate in Microglia via an Autophagy Dependent Pathway. Front. Pharmacol. 2021, 12, 612981. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef]
- Chen, K.; Wu, S.; Ye, S.; Huang, H.; Zhou, Y.; Zhou, H.; Wu, S.; Mao, Y.; Shangguan, F.; Lan, L.; et al. Dimethyl Fumarate Induces Metabolic Crisie to Suppress Pancreatic Carcinoma. Front. Pharmacol. 2021, 12, 617714. [Google Scholar] [CrossRef]
- Gillard, G.O.; Collette, B.; Anderson, J.; Chao, J.; Scannevin, R.H.; Huss, D.J.; Fontenot, J.D. DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J. Neuroimmunol. 2015, 283, 74–85. [Google Scholar] [CrossRef]
- Loewe, R.; Holnthoner, W.; Gröger, M.; Pillinger, M.; Gruber, F.; Mechtcheriakova, D.; Hofer, E.; Wolff, K.; Petzelbauer, P. Dimethylfumarate Inhibits TNF-Induced Nuclear Entry of NF-κB/p65 in Human Endothelial Cells. J. Immunol. 2002, 168, 4781–4787. [Google Scholar] [CrossRef]
- Chen, H.; Assmann, J.; Krenz, A.; Rahman, M.; Grimm, M.; Karsten, C.M.; Koehl, J.; Offermanns, S.; Wettschureck, N.; Schwaninger, M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J. Clin. Investig. 2014, 124, 2188–2192. [Google Scholar] [CrossRef]
- McGuire, V.A.; Ruiz-Zorrilla Diez, T.; Emmerich, C.H.; Strickson, S.; Ritorto, M.S.; Sutavani, R.V.; Weiβ, A.; Houslay, K.F.; Knebel, A.; Meakin, P.J.; et al. Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci. Rep. 2016, 6, 31159. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Fang, J.; Chen, Y.; Li, H.; Gao, K. Dimethyl fumarate inhibits the expression and function of hypoxia-inducible factor-1α (HIF-1α). Biochem. Biophys. Res. Commun. 2014, 448, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Dietrich, M.; Herrmann, A.K.; Schacht, T.; Albrecht, P.; Methner, A. Dimethyl Fumarate Induces Glutathione Recycling by Upregulation of Glutathione Reductase. Oxidative Med. Cell. Longev. 2017, 2017, 6093903. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Mazzilli, S.; Di Prete, M.; Dattola, A.; Cosio, T.; Lettieri Barbato, D.; Costanza, G.; Lanna, C.; Manfreda, V.; Gaeta Schumak, R.; et al. The Role of Glutathione-S Transferase in Psoriasis and Associated Comorbidities and the Effect of Dimethyl Fumarate in This Pathway. Front. Med. 2022, 9, 760852. [Google Scholar] [CrossRef]
- Lehmann, J.C.; Listopad, J.J.; Rentzsch, C.U.; Igney, F.H.; von Bonin, A.; Hennekes, H.H.; Asadullah, K.; Docke, W.D. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J. Investig. Dermat. 2007, 127, 835–845. [Google Scholar] [CrossRef]
- Schroeder, A.; Warnken, U.; Röth, D.; Klika, K.D.; Vobis, D.; Barnert, A.; Bujupi, F.; Oberacker, T.; Schnölzer, M.; Nicolay, J.P.; et al. Targeting Thioredoxin-1 by dimethyl fumarate induces ripoptosome-mediated cell death. Sci. Rep. 2017, 7, 43168. [Google Scholar] [CrossRef]
- Blewett, M.M.; Xie, J.; Zaro, B.W.; Backus, K.M.; Altman, A.; Teijaro, J.R.; Cravatt, B.F. Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells. Sci. Signal 2016, 9, rs10. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyúl-Tóth, Á.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Speciale, A.; Ferrari, D.; Cristani, M.; Saija, A.; Cimino, F. Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-κB pathways. Toxicol. Lett. 2015, 239, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lian, Y.; Hu, C.; Yang, S.; Pei, B.; Yao, M.; Zhu, X.; Shang, L.; Li, Z. Cyanidin-3-glucoside protects against high glucose-induced injury in human nucleus pulposus cells by regulating the Nrf2/HO-1 signaling. J. Appl. Toxicol. 2022, 42, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhovtis Ryerson, L.; Green, R.; Confident, G.; Pandey, K.; Richter, B.; Bacon, T.; Sammarco, C.; Laing, L.; Kalina, J.; Kister, I. Efficacy and tolerability of dimethyl fumarate in White-, African- and Hispanic- Americans with multiple sclerosis. Ther. Adv. Neurol. Disord. 2016, 9, 454–461. [Google Scholar] [CrossRef]
- Ochi, H.; Niino, M.; Onizuka, Y.; Hiramatsu, K.; Hase, M.; Yun, J.; Matta, A.; Torii, S. 72-Week Safety and Tolerability of Dimethyl Fumarate in Japanese Patients with Relapsing-remitting Multiple Sclerosis: Analysis of the Randomised, Double Blind, Placebo-Controlled, Phase III APEX Study and its Open-Label Extension. Adv. Ther. 2018, 35, 1598–1611. [Google Scholar] [CrossRef]
- Sabin, J.; Urtiaga, S.; Pilo, B.; Thuissard, I.; Galan, V.; Sainz de la Maza, S.; Costa-Frossard, L.; Gómez-Moreno, M.; Díaz-Díaz, J.; Oreja-Guevara, C.; et al. Tolerability and safety of dimethyl fumarate in relapsing multiple sclerosis: A prospective observational multicenter study in a real-life Spanish population. J. Neurol. 2020, 267, 2362–2371. [Google Scholar] [CrossRef]
- Mallucci, G.; Annovazzi, P.; Miante, S.; Torri-Clerici, V.; Matta, M.; La Gioia, S.; Cavarretta, R.; Mantero, V.; Costantini, G.; D’Ambrosio, V.; et al. Two-year real-life efficacy, tolerability and safety of dimethyl fumarate in an Italian multicentre study. J. Neurol. 2018, 265, 1850–1859. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis--epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef]
- Capon, F. The Genetic Basis of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2526. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.E.; Callis, K.P.; Papenfuss, J.; Hoffman, M.S.; Hansen, C.B.; Wong, B.; Panko, J.M.; Krueger, G.G. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J. Investig. Dermatol. 2006, 126, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Turka, L.A.; Nickoloff, B.J. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J. Clin. Investig. 1994, 94, 202–209. [Google Scholar] [PubMed]
- Lowes, M.A.; Chamian, F.; Abello, M.V.; Fuentes-Duculan, J.; Lin, S.L.; Nussbaum, R.; Novitskaya, I.; Carbonaro, H.; Cardinale, I.; Kikuchi, T.; et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl. Acad. Sci. USA 2005, 102, 19057–19062. [Google Scholar] [CrossRef]
- Zaba, L.C.; Cardinale, I.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Novitskaya, I.; Khatcherian, A.; Bluth, M.J.; Lowes, M.A.; et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007, 204, 3183–3194. [Google Scholar] [CrossRef]
- Walker, F.; Adamczyk, A.; Kellerer, C.; Belge, K.; Brück, J.; Berner, T.; Merten, K.; Núnez Gómez, N.; Neureither, M.; Röcken, M.; et al. Fumaderm® in daily practice for psoriasis: Dosing, efficacy and quality of life. Br. J. Dermatol. 2014, 171, 1197–1205. [Google Scholar] [CrossRef]
- Ockenfels, H.M.; Schultewolter, T.; Ockenfels, G.; Funk, R.; Goos, M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br. J. Dermatol. 1998, 139, 390–395. [Google Scholar] [CrossRef]
- Sulaimani, J.; Cluxton, D.; Clowry, J.; Petrasca, A.; Molloy, O.E.; Moran, B.; Sweeney, C.M.; Malara, A.; McNicholas, N.; McGuigan, C.; et al. Dimethyl fumarate modulates the Treg-Th17 cell axis in patients with psoriasis. Br. J. Dermatol. 2021, 184, 495–503. [Google Scholar] [CrossRef]
- Morrison, P.J.; Suhrkamp, I.; Gerdes, S.; Mrowietz, U. Oral dimethyl fumarate induces changes within the peripheral neutrophil compartment of patients with psoriasis that are linked with skin improvement. Br. J. Dermatol. 2021, 185, 605–615. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishitsuka, Y.; Inoue, S.; Nakamura, Y.; Saito, A.; Okiyama, N.; Fujisawa, Y.; Furuta, J.; Watanabe, R.; Fujimoto, M. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Regulates Epidermal Keratinization under Psoriatic Skin Inflammation. Am. J. Pathol. 2020, 190, 577–585. [Google Scholar] [CrossRef]
- Dyment, D.A.; Ebers, G.C.; Sadovnick, A.D. Genetics of multiple sclerosis. Lancet Neurol. 2004, 3, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, A.; Frisell, T.; Burman, J.; Salzer, J.; Fink, K.; Hallberg, S.; Hambraeus, J.; Axelsson, M.; Nimer, F.A.; Sundström, P.; et al. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing-remitting multiple sclerosis or clinically isolated syndrome in Sweden: A rater-blinded, phase 3, randomised controlled trial. Lancet Neurol. 2022, 21, 693–703. [Google Scholar] [CrossRef]
- Kappos, L.; Gold, R.; Miller, D.H.; Macmanus, D.G.; Havrdova, E.; Limmroth, V.; Polman, C.H.; Schmierer, K.; Yousry, T.A.; Yang, M.; et al. BG-12 Phase IIb Study Investigators. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: A multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008, 372, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Sievers, J.; Rickert, U.; Rostami-Yazdi, M.; Mrowietz, U.; Lucius, R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflamm. 2010, 7, 30. [Google Scholar] [CrossRef]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef]
- Liu, L.; Vollmer, M.K.; Ahmad, A.S.; Fernandez, V.M.; Kim, H.; Doré, S. Pretreatment with Korean red ginseng or dimethyl fumarate attenuates reactive gliosis and confers sustained neuroprotection against cerebral hypoxic-ischemic damage by an Nrf2-dependent mechanism. Free Radic. Biol. Med. 2019, 131, 98–114. [Google Scholar] [CrossRef]
- Liu, L.; Vollmer, M.K.; Kelly, M.G.; Fernandez, V.M.; Fernandez, T.G.; Kim, H.; Doré, S. Reactive Gliosis Contributes to Nrf2-Dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng Against Hypoxic-Ischemia: Focus on Hippocampal Injury. Mol. Neurobiol. 2020, 57, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.X.; Lisi, L.; Dello Russo, C.; Polak, P.E.; Sharp, A.; Weinberg, G.; Kalinin, S.; Feinstein, D.L. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and heme oxygenase-1. ASN Neuro 2011, 3, e00055. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.S.; Matos, M.F.; Richter, K.E.; Li, B.; Scannevin, R.H. The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. Sci. Rep. 2017, 7, 42054. [Google Scholar] [CrossRef] [PubMed]
- Najjar, E.; Staun-Ram, E.; Volkowich, A.; Miller, A. Dimethyl fumarate promotes B cell-mediated anti-inflammatory cytokine profile in B and T cells, and inhibits immune cell migration in patients with MS. J. Neuroimmunol. 2020, 343, 577230. [Google Scholar] [CrossRef]
- Zoghi, S.; Amirghofran, Z.; Nikseresht, A.; Ashjazadeh, N.; Kamali-Sarvestani, E.; Rezaei, N. Cytokine secretion pattern in treatment of lymphocytes of multiple sclerosis patients with fumaric acid esters. Immunol. Investig. 2011, 40, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Schimrigk, S.; Brune, N.; Hellwig, K.; Lukas, C.; Bellenberg, B.; Rieks, M.; Hoffmann, V.; Pöhlau, D.; Przuntek, H. Oral fumaric acid esters for the treatment of active multiple sclerosis: An open-label, baseline-controlled pilot study. Eur. J. Neurol. 2006, 13, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Tsianakas, A.; Herzog, S.; Landmann, A.; Patsinakidis, N.; Perusquía Ortiz, A.M.; Bonsmann, G.; Luger, T.A.; Kuhn, A. Successful treatment of discoid lupus erythematosus with fumaric acid esters. J. Am. Acad Dermatol. 2014, 71, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kanzaki, H.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; Nakamura, Y. Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J. Cell. Mol. Med. 2018, 22, 1138–1147. [Google Scholar]
- Nishioku, T.; Kawamoto, M.; Okizono, R.; Sakai, E.; Okamoto, K.; Tsukuba, T. Dimethyl fumarate prevents osteoclastogenesis by decreasing NFATc1 expression, inhibiting of erk and p38 MAPK phosphorylation, and suppressing of HMGB1 release. Biochem. Biophys. Res. Commun. 2020, 530, 455–461. [Google Scholar] [CrossRef]
- Toyama, T.; Looney, A.P.; Baker, B.M.; Stawski, L.; Haines, P.; Simms, R.; Szymaniak, A.D.; Varelas, X.; Trojanowska, M. Therapeutic Targeting of TAZ and YAP by Dimethyl Fumarate in Systemic Sclerosis Fibrosis. J. Investig. Dermatol. 2018, 138, 78–88. [Google Scholar] [CrossRef]
- Schmitt, A.; Xu, W.; Bucher, P.; Grimm, M.; Konantz, M.; Horn, H.; Zapukhlyak, M.; Berning, P.; Brändle, M.; Jarboui, M.A.; et al. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 2021, 138, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, J.P.; Albrecht, J.D.; Assaf, C.; Dippel, E.; Stadler, R.; Wehkamp, U.; Wobser, M.; Guelow, K.; Goerdt, S.; Krammer, P.H. Dimethyl fumarate (DMF) therapy in CTCL: Results from a clinical phase II study. Eur. J. Cancer 2021, 156 (Suppl. S1), S21–S22. [Google Scholar] [CrossRef]
- Maeta, T.; Sato, T.; Asano, K.; Ito, S. Dimethyl Fumarate Induces Apoptosis via. Inhibiting NF-κB and STAT3 Signaling in Adult T-cell Leukemia/Lymphoma Cells. Anticancer Res. 2022, 42, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Debasly, S.; Genest, L.; Froget, G.; Castagné, V. Therapeutic Potential of Fingolimod and Dimethyl Fumarate in Non-Small Cell Lung Cancer Preclinical Models. Int. J. Mol. Sci. 2022, 23, 8192. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Su, R.; Jia, Y.; Lai, X.; Su, H.; Fan, Y.; Wang, Y.; Xing, W.; Qin, J. Dimethyl Fumarate Combined With Vemurafenib Enhances Anti-Melanoma Efficacy via. Inhibiting the Hippo/YAP, NRF2-ARE, and AKT/mTOR/ERK Pathways in A375 Melanoma Cells. Front. Oncol. 2022, 12, 794216. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Asano, R.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Dimethyl fumarate suppresses metastasis and growth of melanoma cells by inhibiting the nuclear translocation of NF-κB. J. Dermatol. Sci. 2020, 99, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Shafer, D.; Tombes, M.B.; Shrader, E.; Ryan, A.; Bandyopadhyay, D.; Dent, P.; Malkin, M. Phase I trial of dimethyl fumarate, temozolomide, and radiation therapy in glioblastoma. Neurooncol. Adv. 2020, 2, vdz052. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jha, N.K.; Sadek, B.; Ojha, S. Repurposing Dimethyl Fumarate for Cardiovascular Diseases: Pharmacological Effects, Molecular Mechanisms, and Therapeutic Promise. Pharmaceuticals 2022, 15, 497. [Google Scholar] [CrossRef]
- Nakagami, Y. Nrf2 Is an Attractive Therapeutic Target for Retinal Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7469326. [Google Scholar] [CrossRef]
- Nagai, N.; Thimmulappa, R.K.; Cano, M.; Fujihara, M.; Izumi-Nagai, K.; Kong, X.; Sporn, M.B.; Kensler, T.W.; Biswal, S.; Handa, J.T. Nrf2 is a critical modulator of the innate immune response in a model of uveitis. Free Radic. Biol. Med. 2009, 47, 300–306. [Google Scholar] [CrossRef]
- Himori, N.; Yamamoto, K.; Maruyama, K.; Ryu, M.; Taguchi, K.; Yamamoto, M.; Nakazawa, T. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J. Neurochem. 2013, 127, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T.; et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, J.; Yoshida, T.; Eberhart, C.G.; Xu, Z.; Kombairaju, P.; Sporn, M.B.; Handa, J.T.; Duh, E.J. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2011, 51, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.H. Involvement of Nrf2 in Ocular Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef]
- Pietrucha-Dutczak, M.; Amadio, M.; Govoni, S.; Lewin-Kowalik, J.; Smedowski, A. The Role of Endogenous Neuroprotective Mechanisms in the Prevention of Retinal Ganglion Cells Degeneration. Front. Neurosci. 2018, 12, 834. [Google Scholar] [CrossRef]
- Arnold, A.C. Evolving management of optic neuritis and multiple sclerosis. Am. J. Ophthalmol. 2005, 139, 1101–1108. [Google Scholar] [CrossRef]
- You, Y.; Barnett, M.H.; Yiannikas, C.; Parratt, J.; Matthews, J.G.; Graham, S.L.; Klistorner, A. Interferon-β Is Less Effective Than Other Drugs in Controlling the Rate of Retinal Ganglion Cell Loss in MS. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e971. [Google Scholar] [CrossRef]
- Heinz, C.; Heiligenhaus, A. Improvement of noninfectious uveitis with fumaric acid esters: Results of a pilot study. Arch. Ophthalmol. 2007, 125, 569–571. [Google Scholar] [CrossRef][Green Version]
- Kofler, L.; Kathrein-Schneider, S.; Schweinzer, K.; Kofler, H. Fumaric acid: A possible new therapy for macular edema? Int. Ophthalmol. 2019, 39, 1627–1631. [Google Scholar] [CrossRef]
- Heiligenhaus, A.; Li, H.; Wasmuth, S.; Bauer, D. Influence of dimethylfumarate on experimental HSV-1 necrotizing keratitis. Graefes. Arch. Clin. Exp. Ophthalmol. 2004, 242, 870–877. [Google Scholar] [CrossRef]
- Ananth, S.; Babu, E.; Veeranan-Karmegam, R.; Bozard Baldowski, B.R.; Boettger, T.; Martin, P.M. Induction of the cystine/glutamate exchanger SLC7A11 in retinal pigment epithelial cells by the antipsoriatic drug monomethylfumarate. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Promsote, W.; Makala, L.; Li, B.; Smith, S.B.; Singh, N.; Ganapathy, V.; Pace, B.S.; Martin, P.M. Monomethylfumarate induces γ-globin expression and fetal hemoglobin production in cultured human retinal pigment epithelial (RPE) and erythroid cells, and in intact retina. Investig. Ohpthalmol. Vis. Sci. 2014, 55, 5382–5393. [Google Scholar] [CrossRef] [PubMed]
- Promsote, W.; Powell, F.L.; Veean, S.; Thounaojam, M.; Markand, S.; Saul, A.; Gutsaeva, D.; Bartoli, M.; Smith, S.B.; Ganapathy, V.; et al. Oral Monomethyl Fumarate Therapy Ameliorates Retinopathy in a Humanized Mouse Model of Sickle Cell Disease. Antioxid. Redox Signal 2016, 25, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ryals, R.C.; Huang, S.J.; Weller, K.K.; Titus, H.E.; Robb, B.M.; Saad, F.W.; Salam, R.A.; Hammad, H.; Yang, P.; et al. Monomethyl Fumarate Protects the Retina From Light-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1275–1285. [Google Scholar] [CrossRef]
- Cho, H.; Hartsock, M.J.; Xu, Z.; He, M.; Duh, E.J. Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion. J. Neuroinflamm. 2015, 12, 239. [Google Scholar] [CrossRef]
- Zyla, K.; Larabee, C.M.; Georgescu, C.; Berkley, C.; Reyna, T.; Plafker, S.M. Dimethyl fumarate mitigates optic neuritis. Mol. Vis. 2019, 25, 446–461. [Google Scholar]
- Mori, S.; Kurimoto, T.; Maeda, H.; Nakamura, M. Dimethyl Fumarate Promotes the Survival of Retinal Ganglion Cells after Optic Nerve Injury, Possibly through the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2021, 22, 297. [Google Scholar] [CrossRef]
- Dietrich, M.; Hecker, C.; Nasiri, M.; Samsam, S.; Issberner, A.; Kohne, Z.; Hartung, H.P.; Albrecht, P. Neuroprotective Properties of Dimethyl Fumarate Measured by Optical Coherence Tomography in Non-inflammatory Animal Models. Front. Neurol. 2021, 11, 601628. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Li, Z.; Li, H.; Chen, Y.; Chen, X.; Su, W.; Liang, D. Subconjunctival injections of dimethyl fumarate inhibit lymphangiogenesis and allograft rejection in the rat cornea. Int. Immunopharmacol. 2021, 96, 107580. [Google Scholar] [CrossRef]
- Labsi, M.; Soufli, I.; Belguendouz, H.; Djebbara, S.; Hannachi, L.; Amir, Z.C.; Touil-Boukoffa, C. Beneficial effect of dimethyl fumarate on experimental autoimmune uveitis is dependent of pro-inflammatory markers immunomodulation. Inflammopharmacology 2021, 29, 1389–1398. [Google Scholar] [CrossRef]
- Nelson, K.C.; Carlson, J.L.; Newman, M.L.; Sternberg, P.; Jones, D.P., Jr.; Kavanagh, T.J.; Diaz, D.; Cai, J.; Wu, M. Effect of dietary inducer dimethylfumarate on glutathione in cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1927–1935. [Google Scholar]
- Bozard, B.R.; Chothe, P.P.; Tawfik, A.; Williams, C.; Fulzele, S.; Prasad, P.D.; Martin, P.M.; Ganapathy, V.; Smith, S.B. Regulation of proton-coupled folate transporter in retinal Müller cells by the antipsoriatic drug monomethylfumarate. Glia 2012, 60, 333–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haarmann, A.; Nehen, M.; Deiß, A.; Buttmann, M. Fumaric Acid Esters Do Not Reduce Inflammatory NF-κB/p65 Nuclear Translocation, ICAM-1 Expression and T-Cell Adhesiveness of Human Brain Microvascular Endothelial Cells. Int. J. Mol. Sci. 2015, 16, 19086–19095. [Google Scholar] [CrossRef]
- Gerhardt, S.; König, V.; Doll, M.; Hailemariam-Jahn, T.; Hrgovic, I.; Zöller, N.; Kaufmann, R.; Kippenberger, S.; Meissner, M. Dimethylfumarate protects against TNF-α-induced secretion of inflammatory cytokines in human endothelial cells. J. Inflamm. 2015, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; Bucolo, C.; Drago, F.; Rossi, S.; Di Rosa, M.; Imbesi, R.; D’Agata, V.; Giunta, S. Attenuation of High Glucose-Induced Damage in RPE Cells through p38 MAPK Signaling Pathway Inhibition. Front. Pharmacol. 2021, 12, 684680. [Google Scholar] [CrossRef]
- Catanzaro, M.; Lanni, C.; Basagni, F.; Rosini, M.; Govoni, S.; Amadio, M. Eye.-Light on Age-Related Macular Degeneration: Targeting Nrf2-Pathway as a Novel Therapeutic Strategy for Retinal Pigment Epithelium. Front. Pharmacol. 2020, 11, 844. [Google Scholar]
- Shu, D.Y.; Frank, S.I.; Fitch, T.C.; Karg, M.M.; Butcher, E.R.; Nnuji-John, E.; Kim, L.A.; Saint-Geniez, M. Dimethyl Fumarate Blocks Tumor Necrosis Factor-Alpha-Driven Inflammation and Metabolic Rewiring in the Retinal Pigment Epithelium. Front. Mol. Sci. 2022, 15, 896786. [Google Scholar] [CrossRef]
- Manai, F.; Amadio, M. Dimethyl Fumarate Triggers the Antioxidant Defense System in Human Retinal Endothelial Cells through Nrf2 Activation. Antioxidants 2022, 11, 1924. [Google Scholar] [CrossRef]
- Sorrentino, F.S.; Matteini, S.; Bonifazzi, C.; Sebastiani, A.; Parmeggiani, F. Diabetic retinopathy and endothelin system: Microangiopathy versus endothelial dysfunction. Eye 2018, 32, 1157–1163. [Google Scholar] [CrossRef]
- Zhong, Q.; Mishra, M.; Kowluru, R.A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Investig. OphThalmol. Vis. Sci. 2013, 54, 3941–3948. [Google Scholar] [CrossRef]
- Sireesh, D.; Dhamodharan, U.; Ezhilarasi, K.; Vijay, V.; Ramkumar, K.M. Association of NF-E2 Related Factor 2 (Nrf2) and inflammatory cytokines in recent onset Type 2 Diabetes Mellitus. Sci. Rep. 2018, 8, 5126. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Talalay, P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc. Natl. Acad. Sci. USA 2004, 101, 10446–10451. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Masutani, H.; Kim, Y.C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 979–987. [Google Scholar] [CrossRef]
- Kong, L.; Tanito, M.; Huang, Z.; Li, F.; Zhou, X.; Zaharia, A.; Yodoi, J.; McGinnis, J.F.; Cao, W. Delay of photoreceptor degeneration in tubby mouse by sulforaphane. J. Neurochem. 2007, 101, 1041–1052. [Google Scholar] [CrossRef]
- del V Cano, M.; Reyes, J.M.; Park, C.Y.; Gao, X.; Mori, K.; Chuck, R.S.; Gehlbach, P.L. Demonstration by redox fluorometry that sulforaphane protects retinal pigment epithelial cells against oxidative stress. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2606–2612. [Google Scholar] [CrossRef]
- Liu, H.; Smith, A.J.; Lott, M.C.; Bao, Y.; Bowater, R.P.; Reddan, J.R.; Wormstone, I.M. Sulforaphane can protect lens cells against oxidative stress: Implications for cataract prevention. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5236–5248. [Google Scholar] [CrossRef]
- Ziaei, A.; Schmedt, T.; Chen, Y.; Jurkunas, U.V. Sulforaphane decreases endothelial cell apoptosis in fuchs endothelial corneal dystrophy: A novel treatment. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6724–6734. [Google Scholar] [CrossRef]
- Maeda, S.; Matsui, T.; Ojima, A.; Takeuchi, M.; Yamagishi, S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr. Res. 2014, 34, 807–813. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, X.A.; Tso, M.O. Minocycline and sulforaphane inhibited lipopolysaccharide-mediated retinal microglial activation. Mol. Vis. 2007, 13, 1083–1093. [Google Scholar]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Kensler, T.W. The Challenges of Designing and Implementing Clinical Trials With Broccoli Sprouts… and Turning Evidence Into Public Health Action. Front. Nutr. 2021, 8, 648788. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Nam, H.Y.; Lew, S.Y.; Naidu, M.; David, P.; Kamalden, T.A.; Hadie, S.; Lim, L.W. Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review. Pharmaceuticals 2022, 15, 101. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M.; et al. Modulation of Keap1/Nrf2/ARE Signaling Pathway by Curcuma- and Garlic-Derived Hybrids. Front. Pharmacol. 2020, 10, 1597. [Google Scholar] [CrossRef]
- Fagiani, F.; Catanzaro, M.; Buoso, E.; Basagni, F.; Di Marino, D.; Raniolo, S.; Amadio, M.; Frost, E.H.; Corsini, E.; Racchi, M.; et al. Targeting Cytokine Release Through the Differential Modulation of Nrf2 and NF-κB Pathways by Electrophilic/Non-Electrophilic Compounds. Front. Pharmacol. 2020, 11, 1256. [Google Scholar] [CrossRef]
- Koskela, A.; Manai, F.; Basagni, F.; Liukkonen, M.; Rosini, M.; Govoni, S.; Monte, M.D.; Smedowski, A.; Kaarniranta, K.; Amadio, M. Nature-Inspired Hybrids (NIH) Improve Proteostasis by Activating Nrf2-Mediated Protective Pathways in Retinal Pigment Epithelial Cells. Antioxidants 2022, 11, 1385. [Google Scholar] [CrossRef]
- Rossino, M.G.; Amato, R.; Amadio, M.; Rosini, M.; Basagni, F.; Cammalleri, M.; Dal Monte, M.; Casini, G. A Nature-Inspired Nrf2 Activator Protects Retinal Explants from Oxidative Stress and Neurodegeneration. Antioxidants 2021, 10, 1296. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death. Dis. 2017, 8, e2537. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.L.; Plafker, S.M. Oxidative Stress and the Nrf2 Anti-Oxidant Transcription Factor in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, E.; Sliwinski, T.; Danisz, K.; Blasiak, J.; Sklodowska, A.; Romaniuk, D.; Watala, C.; Szaflik, J.; Szaflik, J.P. Association between polymorphism of the NQO1, NOS3 and NFE2L2 genes and AMD. Front. Biosci. 2013, 18, 80–90. [Google Scholar]
- Das, S.; Chattopadhyay, R.; Bhakat, K.K.; Boldogh, I.; Kohno, K.; Prasad, R.; Wilson, S.H.; Hazra, T.K. Stimulation of NEIL2-mediated oxidized base excision repair via YB-1 interaction during oxidative stress. J. Biol. Chem. 2007, 282, 28474–28484. [Google Scholar] [CrossRef]
- Hanenberg, H.; Andreassen, P.R. PALB2 (partner and localizer of BRCA2). Atlas Genet. Cytogenet. Oncol. Haematol. 2018, 22, 484–490. [Google Scholar] [CrossRef]
- Scrima, A.; Konícková, R.; Czyzewski, B.K.; Kawasaki, Y.; Jeffrey, P.D.; Groisman, R.; Nakatani, Y.; Iwai, S.; Pavletich, N.P.; Thomä, N.H. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 2008, 135, 1213–1223. [Google Scholar] [CrossRef]
- Netea-Maier, R.T.; Plantinga, T.S.; van de Veerdonk, F.L.; Smit, J.W.; Netea, M.G. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy 2016, 12, 245–260. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.; Kannan, R.; Felszeghy, S.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. The Regulation of NFE2L2 (NRF2) Signalling and Epithelial-to-Mesenchymal Transition in Age-Related Macular Degeneration Pathology. Int. J. Mol. Sci. 2019, 20, 5800. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wang, W.; Xue, J.; Hua, F.; Mu, R.; Lin, H.; Yan, J.; Lv, X.; Chen, X.; Hu, Z.W. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 2012, 72, 3238–3250. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; D’Alessandro, G.; Lepore, F.; Corazzari, M.; Caldarola, S.; Valacca, C.; Faienza, F.; Esposito, V.; Limatola, C.; Cecconi, F.; et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 2015, 9, 1612–1625. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, J.; Saul, A.; Smith, S.B. Comparison of Neuroprotective Effects of Monomethylfumarate to the Sigma 1 Receptor Ligand (+)-Pentazocine in a Murine Model of Retinitis Pigmentosa. Investig. Ophthal. Vis. Sci. 2020, 61, 5. [Google Scholar] [CrossRef]
- Brennan, M.; Allaire, N.; Huss, D.; Cullen, P.; Thai, A.; Szak, S.; Thomas, A.; Gianni, D.; Carulli, J.; Fontenot, J.; et al. Dimethyl Fumarate and Monomethyl Fumarate are Distinguished by Non-Overlapping Pharmacodynamic Effects In Vivo. Neurology 2014, 82 (Suppl. S10), 206. [Google Scholar]
- Landeck, L.; Asadullah, K.; Amasuno, A.; Pau-Charles, I.; Mrowietz, U. Dimethyl fumarate (DMF) vs. monoethyl fumarate (MEF) salts for the treatment of plaque psoriasis: A review of clinical data. Arch. Dermatol. Res. 2018, 310, 475–483. [Google Scholar] [CrossRef]
- Brennan, M.S.; Patel, H.; Allaire, N.; Thai, A.; Cullen, P.; Ryan, S.; Lukashev, M.; Bista, P.; Huang, R.; Rhodes, K.J.; et al. Pharmacodynamics of Dimethyl Fumarate Are Tissue Specific and Involve NRF2-Dependent and -Independent Mechanisms. Antioxid. Redox Signal 2016, 24, 1058–1071. [Google Scholar] [CrossRef]
- Schäfer, G.N. Fumarsäure lindert die Schuppenflechte. Selecta 1984, 15, 1260–1261. [Google Scholar]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef]
- Bojanowski, K.; Ibeji, C.U.; Singh, P.; Swindell, W.R.; Chaudhuri, R.K. A Sensitization-Free Dimethyl Fumarate Prodrug, Isosorbide Di-(Methyl Fumarate), Provides a Topical Treatment Candidate for Psoriasis. JID. Innov. 2021, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Benedusi, M.; Cervellati, F.; Sguizzato, M.; Montesi, L.; Bondi, A.; Drechsler, M.; Pula, W.; Valacchi, G.; Esposito, E. Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. Int. J. Mol. Sci. 2022, 23, 8756. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, G.; Kumar, R.; Malik, R.; Singh, B.; Katare, O.P.; Raza, K. Vitamin-Derived Nanolipoidal Carriers for Brain Delivery of Dimethyl Fumarate: A Novel Approach with Preclinical Evidence. ACS Chem. Neurosci. 2017, 8, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E.; Rittenhouse, K.D.; Wilson, C.G.; Weber, D.A.; Kuppermann, B.D.; et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: Basic research to clinical applications. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5403–5420. [Google Scholar] [CrossRef] [PubMed]
- Jóhannesson, G.; Stefánsson, E.; Loftsson, T. Microspheres and Nanotechnology for Drug Delivery. Dev. Ophthalmol. 2016, 55, 93–103. [Google Scholar] [PubMed]
- Thareja, A.; Hughes, H.; Alvarez-Lorenzo, C.; Hakkarainen, J.J.; Ahmed, Z. Penetration Enhancers for Topical Drug Delivery to the Ocular Posterior Segment-A Systematic Review. Pharmaceutics 2021, 13, 276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).