Toward Establishing an Ideal Adjuvant for Non-Inflammatory Immune Enhancement
Abstract
1. Introduction
2. Innate and Acquired Immunity
3. Antibodies and T Cells in Viral Infections
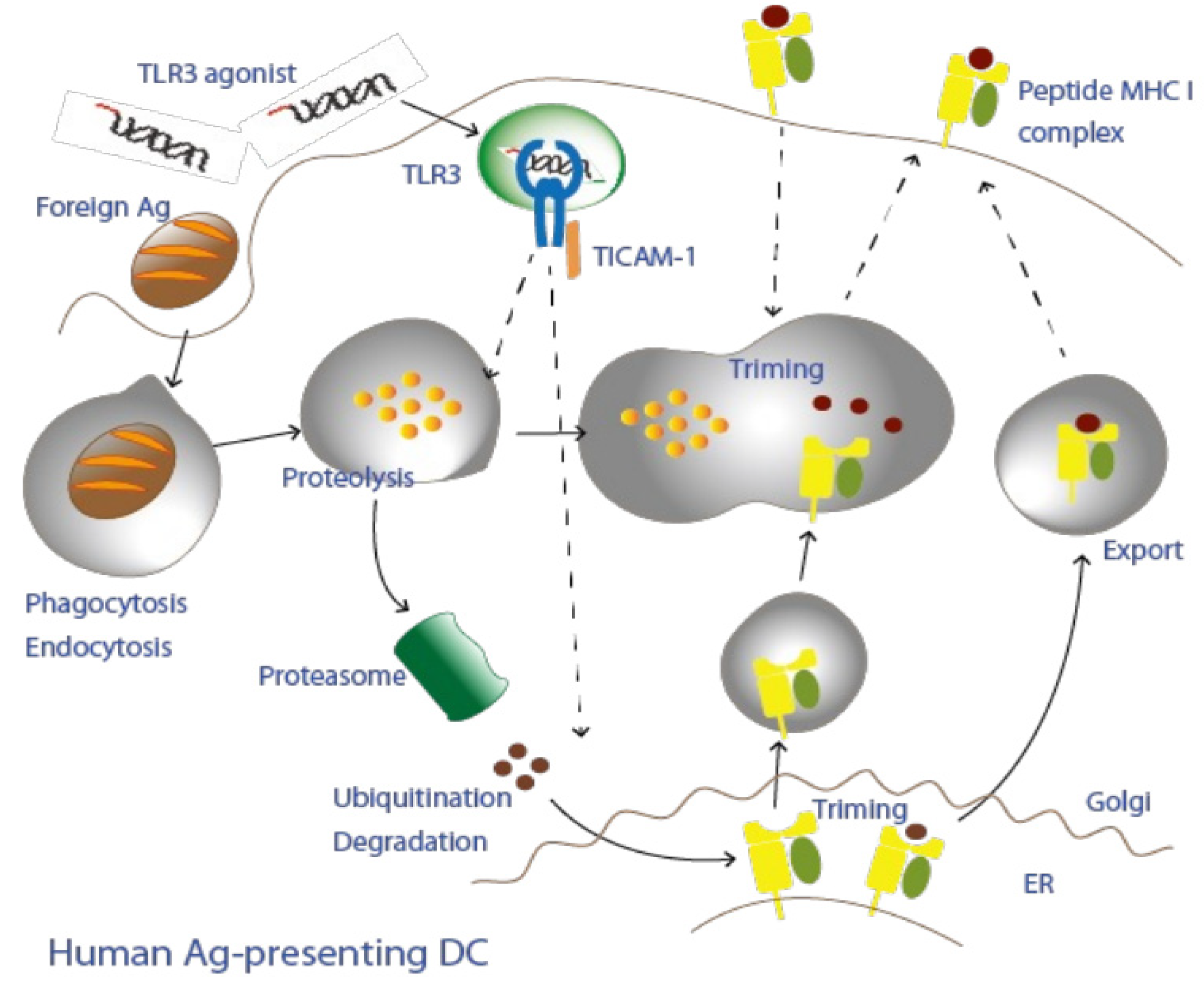
4. Cross-Antigen Presentation of Extrinsic Antigens
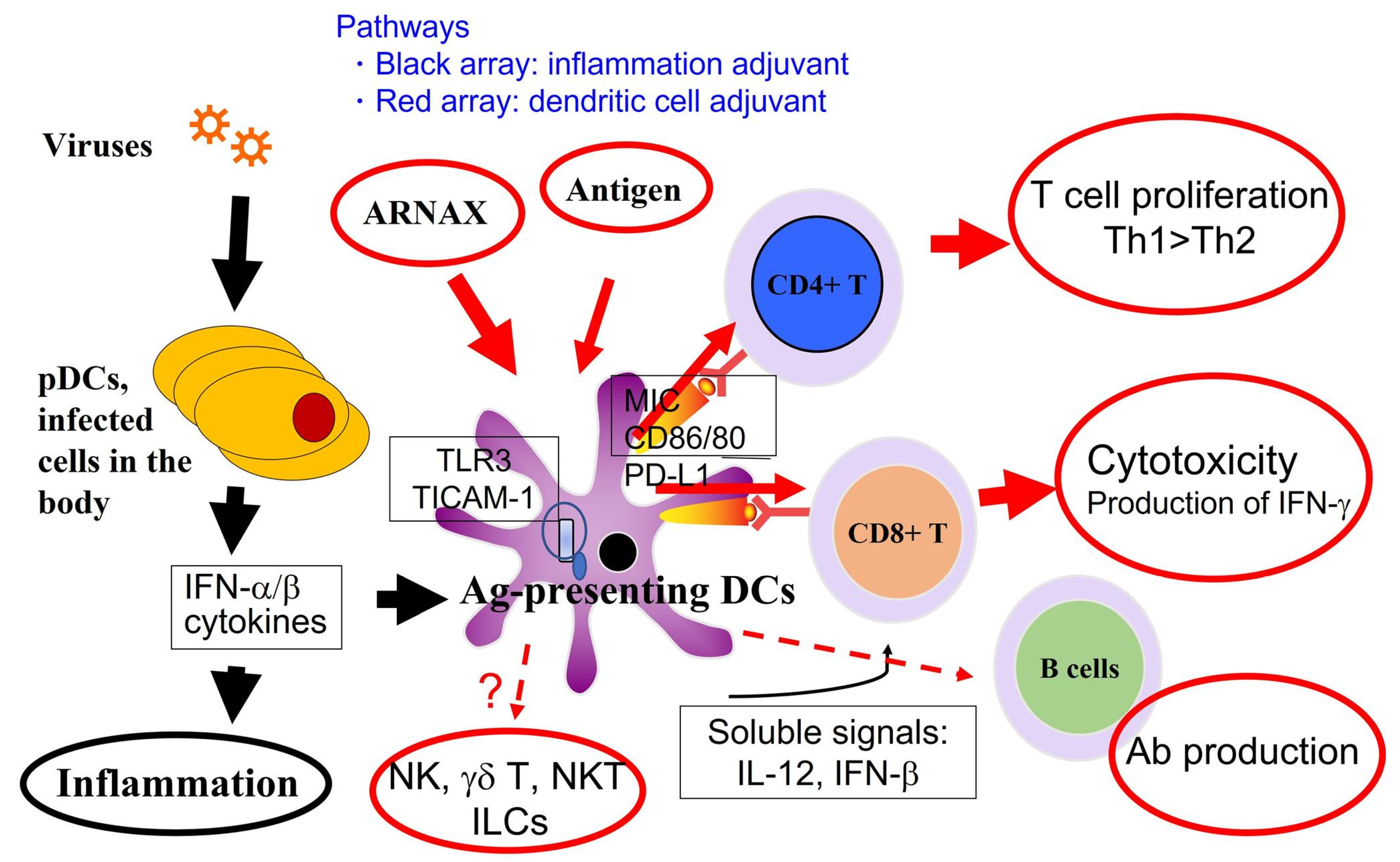
5. Recognition of Subthreshold Antigens by Dendritic Cells with Adjuvant
6. TICAM-1 Pathway in Dendritic Cells
7. Cross-Presentation Induced by the TICAM-1 Pathway in Dendritic Cells
8. Adjuvants for Cancer Vaccines
9. Adjuvant in Prophylactic Vaccine against Infectious Diseases
10. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Laupèze, B.; Hervé, C.; Di Pasquale, A.; Tavares Da Silva, F. Adjuvant Systems for vaccines: 13 years of post-licensure experience in diverse populations have progressed the way adjuvanted vaccine safety is investigated and understood. Vaccine 2019, 37, 5670–5680. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tatematsu, M.; Nishikawa, F.; Azuma, M.; Ishii, N.; Morii-Sakai, A.; Shime, H.; Seya, T. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat. Commun. 2015, 18, 6280. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Kataoka, K.; Yamagishi, J.; Ogawa, S.; Seya, T.; Matsumoto, M. A TLR3-Specific Adjuvant Relieves Innate Resistance to PD-L1 Blockade without Cytokine Toxicity in Tumor Vaccine Immunotherapy. Cell Rep. 2017, 19, 1874–1887. [Google Scholar] [CrossRef]
- Inaba, K.; Turley, S.; Iyoda, T.; Yamaide, F.; Shimoyama, S.; e Sousa, C.R.; Germain, R.N.; Mellman, I.; Steinman, R.M. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 2000, 191, 927–936. [Google Scholar] [CrossRef]
- Cho, K.J.; Roche, P.A. Regulation of MHC Class II-Peptide Complex Expression by Ubiquitination. Front. Immunol. 2013, 4, 369. [Google Scholar] [CrossRef]
- Wu, J.; Meng, Z.; Jiang, M.; Zhang, E.; Trippler, M.; Broering, R.; Bucchi, A.; Krux, F.; Dittmer, U.; Yang, D.; et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 2010, 129, 363–374. [Google Scholar] [CrossRef]
- Takaki, H.; Ichimiya, S.; Matsumoto, M.; Seya, T. Mucosal Immune Response in Nasal-Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants. J. Innate Immun. 2018, 10, 515–521. [Google Scholar] [CrossRef]
- Schenten, D.; Medzhitov, R. The control of adaptive immune responses by the innate immune system. Adv. Immunol. 2011, 109, 87–124. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsuo, A.; Matsumoto, M.; Seya, T. Pan-vertebrate toll-like receptors during evolution. Curr. Genom. 2008, 9, 488–493. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Emonet, A.; Dénervaud Tendon, V.; Marhavy, P.; Wu, D.; Lahaye, T.; Geldner, N. Co-incidence of Damage and Microbial Patterns Controls Localized Immune Responses in Roots. Cell 2020, 180, 440–453.e18. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Tena, G.; Ballestar, E. Epigenetics of Dendritic Cells in Tumor Immunology. Cancers 2022, 14, 1179. [Google Scholar] [CrossRef] [PubMed]
- Papalexi, E.; Satija, R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 2018, 18, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.R. Viruses: Biology, Application and Control; Garland Press: New York, NY, USA, 2012; pp. 65–130. [Google Scholar]
- Pancer, Z.; Amemiya, C.T.; Ehrhardt, G.R.; Ceitlin, J.; Gartland, G.L.; Cooper, M.D. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature 2004, 430, 174–180. [Google Scholar] [CrossRef]
- Teixeira, S.M.; El-Sayed, N.M.; Araújo, P.R. The genome and its implications. Adv. Parasitol. 2011, 75, 209–230. [Google Scholar] [CrossRef]
- Fitzgerald-Bocarsly, P.; Feng, D. The role of type I interferon production by dendritic cells in host defense. Biochimie 2007, 89, 843–855. [Google Scholar] [CrossRef]
- Seya, T.; Matsumoto, M.; Ebihara, T.; Oshiumi, H. Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing. Immunol. Rev. 2009, 227, 44–53. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Burnet, F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970, 13, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.L.; Khan, N.; Basu, S.; Soni, V. B-Cell-Based Immunotherapy: A Promising New Alternative. Vaccines 2022, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Kure, S.; Oshiumi, H.; Sakoda, Y.; Suzuki, T.; Ainai, A.; Hasegawa, H.; Matsumoto, M.; Seya, T. Toll-like receptor 3 in nasal CD103+ dendritic cells is involved in immunoglobulin A production. Mucosal Immunol. 2018, 11, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef]
- Monroy-Iglesias, M.J.; Crescioli, S.; Beckmann, K.; Le, N.; Karagiannis, S.N.; Van Hemelrijck, M.; Santaolalla, A. Antibodies as biomarkers for cancer risk: A systematic review. Clin. Exp. Immunol. 2022, 209, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Azuma, M.; Oshiumi, H.; Kasamatsu, J.; Iwabuchi, K.; Matsumoto, K.; Saito, H.; Taniguchi, T.; Matsumoto, M.; Seya, T. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J. Exp. Med. 2015, 212, 1337. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, J.A.; Ogasawara, K.; Lanier, L.L. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J. Immunol. 2004, 172, 2001–2005. [Google Scholar] [CrossRef]
- Ebihara, T. Dichotomous Regulation of Acquired Immunity by Innate Lymphoid Cells. Cells 2020, 9, 1193. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Campillo-Davo, D.; Roex, G.; Versteven, M.; Smits, E.L.; Van Tendeloo, V.F. The role of the common gamma-chain family cytokines in γδ T cell-based anti-cancer immunotherapy. Cytokine Growth Factor Rev. 2018, 41, 54–64. [Google Scholar] [CrossRef]
- Gustafsson, K.; Herrmann, T.; Dieli, F. Editorial: Understanding Gamma Delta T Cell Multifunctionality-Towards Immunotherapeutic Applications. Front. Immunol. 2020, 11, 921. [Google Scholar] [CrossRef]
- Yamazaki, S.; Inaba, K.; Tarbell, K.V.; Steinman, R.M. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol. Rev. 2006, 212, 314–329. [Google Scholar] [CrossRef]
- Dolfi, D.V.; Duttagupta, P.A.; Boesteanu, A.C.; Mueller, Y.M.; Oliai, C.H.; Borowski, A.B.; Katsikis, P.D. Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo. J. Immunol. 2011, 186, 4599–4608. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Zheng, X.; Lin-Schmidt, X.; Chen, W.; Carpenter, T.J.; Zong, A.; Wang, W.; Heald, D.L. Development of a quantitative relationship between CAR-affinity, antigen abundance, tumor cell depletion and CAR-T cell expansion using a multiscale systems PK-PD model. MAbs 2020, 12, 1688616. [Google Scholar] [CrossRef] [PubMed]
- Ghiotto-Ragueneau, M.; Battifora, M.; Truneh, A.; Waterfield, M.D.; Olive, D. Comparison of CD28-B7.1 and B7.2 functional interaction in resting human T cells: Phosphatidylinositol 3-kinase association to CD28 and cytokine production. Eur. J. Immunol. 1996, 26, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Collins, M. The B7 family of ligands and its receptors: New pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002, 20, 29–53. [Google Scholar] [CrossRef]
- Ward, J.P.; Gubin, M.M.; Schreiber, R.D. The Role of Neoantigens in Naturally Occurring and Therapeutically Induced Immune Responses to Cancer. Adv. Immunol. 2016, 130, 25–74. [Google Scholar] [CrossRef] [PubMed]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-Associated Enhanced Disease and Pathogenic Human Coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Nagata, N.; Takaki, H.; Matsumoto, M.; Suzuki, T.; Hasegawa, H.; Seya, T. Prophylactic Vaccine Targeting TLR3 on Dendritic Cells Ameliorates Eosinophilic Pneumonia in a Mouse SARS-CoV Infection Model. Immunohorizons 2022, 6, 275–282. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Shiwa, N.; Sekizuka, T.; Sano, K.; Ainai, A.; Hemmi, T.; Kataoka, M.; Kuroda, M.; Hasegawa, H.; Suzuki, T.; et al. A lethal mouse model for evaluating vaccine-associated enhanced respiratory disease during SARS-CoV-2 infection. Sci. Adv. 2022, 8, eabh3827. [Google Scholar] [CrossRef]
- Ita, K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development. Arch. Med. Res. 2021, 52, 15–24. [Google Scholar] [CrossRef]
- Ascough, S.; Paterson, S.; Chiu, C. Induction and subversionof human protective immunity: Contrasting influenza and respiratory syncytial virus. Front. Immunol. 2018, 9, 323. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Uda, A.; Suzuki, T.; Tsunetsugu-Yokota, Y.; Sato, Y.; Morikawa, S.; Tashiro, M.; Sata, T.; Hasegawa, H.; Nagata, N. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J. Virol. 2014, 88, 8597–8614. [Google Scholar] [CrossRef] [PubMed]
- Shingai, M.; Inoue, N.; Okuno, T.; Okabe, M.; Akazawa, T.; Miyamoto, Y.; Ayata, M.; Honda, K.; Kurita-Taniguchi, M.; Matsumoto, M.; et al. Wild-type measles virus infection in human CD46/CD150-transgenic mice: CD11c-positive dendritic cells establish systemic viral infection. J. Immunol. 2005, 175, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Takeda, M.; Tahara, M.; Shingai, M.; Oshiumi, H.; Matsumoto, M.; Seya, T. The MyD88 pathway in plasmacytoid and CD4+ dendritic cells primarily triggers type I IFN production against measles virus in a mouse infection model. J. Immunol. 2013, 191, 4740–4747. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.I.; Huis In ’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Seya, T.; Akazawa, T.; Uehori, J.; Matsumoto, M.; Azuma, I.; Toyoshima, K. Role of toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res. 2003, 23, 4369–4376. [Google Scholar]
- Coban, C.; Koyama, S.; Takeshita, F.; Akira, S.; Ishii, K.J. Molecular and cellular mechanisms of DNA vaccines. Hum. Vaccin. 2008, 4, 453–456. [Google Scholar] [CrossRef]
- Seya, T.; Takeda, Y.; Takashima, K.; Yoshida, S.; Azuma, M.; Matsumoto, M. Adjuvant immunotherapy for cancer: Both dendritic cell-priming and check-point inhibitor blockade are required for immunotherapy. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 153–160. [Google Scholar] [CrossRef]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: TLR3 agonists in cancer therapy. Oncoimmunology 2020, 9, 1771143. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Takeda, Y.; Tatematsu, M.; Seya, T. Toll-Like Receptor 3 Signal in Dendritic Cells Benefits Cancer Immunotherapy. Front. Immunol. 2017, 8, 1897. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Takeda, Y.; Seya, T. Targeting Toll-like receptor 3 in dendritic cells for cancer immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 937–946. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Grouard, G.; Rissoan, M.-C.; Filgueira, L.; Durand, I.; Banchereau, J.; Liu, Y.-J. The enigmatic plasmacytoid T cells develop into dendritic cells with Interleukin 3 and CD40-ligand. J. Exp. Med. 1997, 185, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef]
- Matsumoto, M.; Oshiumi, H.; Seya, T. Antiviral responses induced by the TLR3 pathway. Rev. Med. Virol. 2011, 21, 67–77. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Segura, E. Review of Mouse and Human Dendritic Cell Subsets. Methods Mol. Biol. 2016, 1423, 3–15. [Google Scholar] [CrossRef]
- Poulin, L.F.; Salio, M.; Griessinger, E.; Anjos-Afonso, F.; Craciun, L.; Chen, J.L.; Keller, A.M.; Joffre, O.; Zelenay, S.; Nye, E.; et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J. Exp. Med. 2010, 207, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.N.; Randolph, G.J.; Murphy, K.; Gautier, E.L.; Kedl, R.M.; Lahoud, M.H.; Caminschi, I.; Shortman, K.; Henson, P.M.; Jakubzick, C.V. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 2011, 208, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Seya, T.; Takeda, Y.; Matsumoto, M. A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv. Drug Deliv. Rev. 2019, 147, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kikkawa, S.; Kohase, M.; Miyake, K.; Seya, T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 2002, 293, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Funami, K.; Tanabe, M.; Oshiumi, H.; Shingai, M.; Seto, Y.; Yamamoto, A.; Seya, T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 2003, 171, 3154–3162. [Google Scholar] [CrossRef]
- Heidegger, S.; Kreppel, D.; Bscheider, M.; Stritzke, F.; Nedelko, T.; Wintges, A.; Bek, S.; Fischer, J.C.; Graalmann, T.; Kalinke, U.; et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade. EBioMedicine 2019, 41, 146–155. [Google Scholar] [CrossRef]
- Ruzicka, M.; Koenig, L.M.; Formisano, S.; Boehmer, D.F.R.; Vick, B.; Heuer, E.M.; Meinl, H.; Kocheise, L.; Zeitlhöfler, M.; Ahlfeld, J.; et al. RIG-I-based immunotherapy enhances survival in preclinical AML models and sensitizes AML cells to checkpoint blockade. Leukemia 2020, 34, 1017–1026. [Google Scholar] [CrossRef]
- Akira, S. Toll-like receptor signaling. J. Biol. Chem. 2003, 278, 38105–38108. [Google Scholar] [CrossRef]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef]
- Garcon, N.; Chomez, P.; van Mechelen, M. Glaxo- SmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Kassianos, A.J.; Hardy, M.Y.; Ju, X.; Vijayan, D.; Ding, Y.; Vulink, A.J.; McDonald, K.J.; Jongbloed, S.L.; Wadley, R.B.; Wells, C.; et al. Human CD1c (BDCA-1)+ myeloid dendritic cells secrete IL-10 and display an immuno-regulatory phenotype and function in response to Escherichia coli. Eur. J. Immunol. 2012, 42, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Oshiumi, H.; Matsumoto, M.; Seya, T. TICAM-1 is dispensable in STING-mediated innate immune responses in myeloid immune cells. Biochem. Biophys. Res. Commun. 2018, 499, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Majumdar, T.; Kessler, P.; Ozhegov, E.; Zhang, Y.; Chattopadhyay, S.; Barik, S.; Sen, G.C. STING Requires the Adaptor TRIF to Trigger Innate Immune Responses to Microbial Infection. Cell Host Microbe. 2016, 20, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Smith, A.P.; Guiducci, C.; Wonderlich, E.R.; Normolle, D.; Watkins, S.C.; Barrat, F.J.; Barratt-Boyes, S.M. Blocking TLR7- and TLR9-mediated IFN-α production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog. 2013, 9, e1003530. [Google Scholar] [CrossRef]
- Hoeffel, G.; Ripoche, A.C.; Matheoud, D.; Nascimbeni, M.; Escriou, N.; Lebon, P.; Heshmati, F.; Guillet, J.G.; Gannagé, M.; Caillat-Zucman, S.; et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity 2007, 27, 481–492. [Google Scholar] [CrossRef]
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Seya, T.; Shime, H.; Takaki, H.; Azuma, M.; Oshiumi, H.; Matsumoto, M. TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. Oncoimmunology 2012, 1, 917–923. [Google Scholar] [CrossRef]
- Hanson, B. Necroptosis: A new way of dying? Cancer Biol. Ther. 2016, 17, 899–910. [Google Scholar] [CrossRef]
- Yoneyama, M.; Suhara, W.; Fukuhara, Y.; Fukuda, M.; Nishida, E.; Fujita, T. Direct triggering of the type I interferon system by virus infection: Activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998, 17, 1087–1095. [Google Scholar] [CrossRef]
- Murphy, T.L.; Tussiwand, R.; Murphy, K.M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 2013, 13, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Sampath, P.; Bonilla, B.; Ashley, A.; Hou, W.; Byrd, D.; Thorne, S.H. Manipulating TLR Signaling Increases the Anti-tumor T Cell Response Induced by Viral Cancer Therapies. Cell Rep. 2016, 15, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Taniguchi, T. Toll-like receptor signaling and IRF transcription factors. IUBMB Life 2006, 58, 290–295. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Uenaka, A.; Ono, T.; Akisawa, T.; Wada, H.; Yasuda, T.; Nakayama, E. Identification of a unique antigen peptide pRL1 on BALB/c RL male 1 leukemia recognized by cytotoxic T lymphocytes and its relation to the Akt oncogene. J. Exp. Med. 1994, 180, 1599–1607. [Google Scholar] [CrossRef]
- Boon, T. Tumor antigens recognized by cytolytic T lymphocytes: Present perspectives for specific immunotherapy. Int. J. Cancer 1993, 54, 177–180. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermot, D.F. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Mestrallet, G.; Sone, K.; Bhardwaj, N. Strategies to overcome DC dysregulation in the tumor microenvironment. Front. Immunol. 2022, 13, 980709. [Google Scholar] [CrossRef]
- Seya, T.; Shime, H.; Matsumoto, M. Functional Alteration of Tumor-infiltrating Myeloid Cells in RNA Adjuvant Therapy. Anticancer Res. 2015, 35, 4385–4392. [Google Scholar] [PubMed]
- Tatematsu, M.; Funami, K.; Seya, T.; Matsumoto, M. Extracellular RNA Sensing by Pattern Recognition Receptors. J. Innate Immun. 2018, 10, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Iwakabe, K.; Sekimoto, M.; Ohmi, Y.; Yahata, T.; Nakui, M.; Sato, T.; Habu, S.; Tashiro, H.; Sato, M.; et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J. Exp. Med. 1999, 190, 617–627. [Google Scholar] [CrossRef]
- Franchi, L.; Nunez, G. The NALP2 inflammasome is critical for Alum-mediated IL-1b secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008, 38, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.M.; Conacer, M.; Hunter, C.A.; Mohrs, M.; Brombacher, F.; Alexander, J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 1999, 163, 6448–6454. [Google Scholar] [PubMed]
- Nishimura, T.; Nakui, M.; Sato, M.; Iwakabe, K.; Kitamura, H.; Sekimoto, M.; Ohta, A.; Koda, T.; Nishimura, S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000, 46, S52–S61. [Google Scholar] [CrossRef] [PubMed]
- Denton, A.E.; Roberts, E.W.; Fearon, D.T. Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018, 1060, 99–114. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Brito, L.A.; Malyala, P.; O’Hagan, D.T. Vaccine adjuvant formulations: A pharmaceutical perspective. Semin. Immunol. 2013, 25, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Souayah, N.; Michas-Martin, P.A.; Nasar, A.; Krivitskaya, N.; Yacoub, H.A.; Khan, H.; Qureshi, A.I. Guillain–Barré syndrome after Gardasil vaccination: Data from Vaccine Adverse Event Reporting System 2006–2009. Vaccine 2011, 29, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Abe, R.T.; Hineno, A.; Tsunekawa, K.; Nakane, S.; Ikeda, S. Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine. Intern. Med. 2014, 53, 2185–2200. [Google Scholar] [CrossRef]
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, S.; O’Callaghan, K.P.; Offit, P.A. Vaccine Safety: Myths and Misinformation. Front. Microbiol. 2020, 11, 372. [Google Scholar] [CrossRef]
- Guidance for Cancer Immuno-Therapy in Japanese. Available online: https://www.mhlw.go.jp/web/t_doc?dataId=00tc3979&dataType=1&pageNo=1 (accessed on 10 November 2022).
- Jain, A.; Mittal, S.; Tripathi, L.P.; Nussinov, R.; Ahmad, S. Host-pathogen protein-nucleic acid interactions: A comprehensive review. Comp. Struc. Biotechnol. J. 2022, 20, 4415–4436. [Google Scholar] [CrossRef]
- Rostamizadeh, L.; Molavi, O.; Rashid, M.; Ramazani, F.; Baradaran, B.; Lavasanaifar, A.; Lai, R. Recent advances in cancer immunotherapy: Modulation of tumor microenvironment by Toll-like receptor ligands. BioImpact 2022, 12, 261–290. Available online: http://bi.tbzmed.ac.ir/ (accessed on 2 December 2022). [CrossRef]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef]
| Presentation | Class I | Class II | Cross-Present |
|---|---|---|---|
| Origin of the peptide | Intracellular | Extracellular | Vacuolar/Cytosolic |
| Localization | Proteasome | Endosome | Phagosome |
| Expressing cells | All host cells | Immune cells | DCs |
| Invariant chain | No | Yes | No |
| T cell binding Response to ARNAX | CD8 in CTL Unknown | CD4 in helper T Unknown | CD8 in CTL Up-regulated |
| Ligand | Receptor | Adapter | Target Cells | Outcome | |||
|---|---|---|---|---|---|---|---|
| Mouse DC | Human DC | Skewing | Cross-Present | Cytokine Storm | |||
| ARNAX | TLR3 | TICAM-1 | CD8α DC CD103 DC | CD141 DC | Th1 | +++ | - |
| Ampligen | TLR3/MDA5 | TICAM-1 > MAVS | Not restricted | Th1 | ++(?) * | +(?) * | |
| PolyI:C | MDA5/TLR3 | TICAM-1 < MAVS | Not restricted | Th1 | +++ | +++ | |
| MPLA | TLR4 | TICAM-1 > MyD88 | CD8a DC CD103 DC | CD1c DC | Th1 | ++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seya, T.; Tatematsu, M.; Matsumoto, M. Toward Establishing an Ideal Adjuvant for Non-Inflammatory Immune Enhancement. Cells 2022, 11, 4006. https://doi.org/10.3390/cells11244006
Seya T, Tatematsu M, Matsumoto M. Toward Establishing an Ideal Adjuvant for Non-Inflammatory Immune Enhancement. Cells. 2022; 11(24):4006. https://doi.org/10.3390/cells11244006
Chicago/Turabian StyleSeya, Tsukasa, Megumi Tatematsu, and Misako Matsumoto. 2022. "Toward Establishing an Ideal Adjuvant for Non-Inflammatory Immune Enhancement" Cells 11, no. 24: 4006. https://doi.org/10.3390/cells11244006
APA StyleSeya, T., Tatematsu, M., & Matsumoto, M. (2022). Toward Establishing an Ideal Adjuvant for Non-Inflammatory Immune Enhancement. Cells, 11(24), 4006. https://doi.org/10.3390/cells11244006







