1. Introduction
Natural killer (NK) cells are innate lymphocytes that account for 5–15% of the mononuclear cells in peripheral blood. They play an important role in immune reactions against cancer and infections by killing transformed or infected cells [
1]. NK cell cytotoxicity is induced via the engagement of activating receptors such as CD16, NKp30, NKG2D or 2B4 [
2]. In addition, NK cells can produce cytokines and chemokines such as Interferon (IFN)-γ, tumor necrosis factor (TNF)-α or Interleukin (IL)-10, and they have an immunoregulatory function, whereby they can regulate cells of the innate and adaptive immune system [
3]. In recent years it became clear that the activity of immune cells is greatly influenced by metabolism, as activities such as proliferation and effector functions are energy-demanding processes. Two major metabolic pathways are known to be involved in NK cell activation and proliferation: oxidative phosphorylation (OxPhos) and glycolysis [
4]. Previous studies have shown that resting NK cells mostly use OxPhos, while upon activation they increase glycolysis and upregulate OxPhos [
5]. This increases energy production and synthesis of building blocks required for biosynthetic processes to support proliferation and the production of effector molecules in NK cells [
6,
7]. For example, stimulation of NK cells by cytokines such as IL-12 and IL-15 induces their proliferation and leads to upregulation of OxPhos and glycolysis [
5]. Similarly, Keppel et al. showed that stimulation by activating receptors is dependent on both glycolysis and OxPhos [
8]. A major source of energy is glucose, but glutamine and fatty acids are also known to be important for cellular metabolism [
9]. However, it is not yet known to what extent glutamine and fatty acids play a role in NK cells.
Glucose transporters (GLUTs) are 12 membrane-spanning receptors that allow glucose to enter the cell. In humans, 14 different GLUTs are known, and NK cells predominantly express GLUT-1 (
SLC2A1) [
10,
11]. GLUT-1 and GLUT-3 are not only expressed by immune cells, but also in tumor cells due to their high metabolic demand and requirement of glucose for rapid proliferation. This makes glucose transporters a promising target for cancer therapies. Based on this fact, we have recently developed the GLUT inhibitor Glutor, which may be a promising candidate for potential anti-tumor therapies [
12]. Glutor selectively inhibits GLUT-1, -2 and -3 and inhibits GLUT-3 more potently compared to GLUT-1. Treatment with Glutor can reduce proliferation and induces cell death in various tumor cell lines with an IC
50 below 100 nM [
12].
However, as activated NK cells may also rely on GLUT function for their anti-tumor activity, we explored whether Glutor affects the ability of NK cells to proliferate, kill target cells and secrete cytokines and chemokines, and whether NK cells change their phenotype in the absence of glucose availability. Therefore, we treated primary human NK cells with Glutor in short- and long-term experiments and analyzed their functions, phenotype and proliferation. Our results show that short-term treatment with Glutor has no effect on NK cell activities, whereas long-term treatment blocks NK cell proliferation and changes their phenotype, but only has a minor impact on NK cell cytotoxicity.
2. Methods
2.1. Isolation of NK Cells and Cell Culture
NK cells were isolated and cultured as described [
13]. In short, human NK cells were isolated from peripheral blood mononuclear cells with the Dynabeads
® Untouched
TM Human NK Cell-Kit according to the manufacturer’s instructions (Invitrogen
TM, Waltham, MA, USA). For experiments with resting NK cells, isolated NK cells were rested in IMDM medium (with GlutaMAX
TM by Gibco (Waltham, MA, USA), 10% FCS, 1% penicillin/streptomycin) overnight and then used for experiments. To generate pre-activated NK cells, isolated NK cells were seeded in 96-well round-bottom plates (Nunc, Thermo Fisher Scientific, Waltham, MA, USA) at a density of 1.5–2 × 10
6 mL
−1 with irradiated feeder cells (K562-mbIL15-41BBL) in medium with 200 U/mL IL-2 (National Institutes of Health Cytokine Repositor) and 100 ng/mL IL-21 (Miltenyi Biotec, Bergisch Gladbach, Germany). On day 8, NK cells were re-stimulated with fresh feeder cells. In the next weeks, NK cells were split on a density of 1.5–2 × 10
6 mL
−1 in the presence of 100 U/mL IL-2. On day 14 recombinant 2.5 ng/mL IL-15 (PAN Biotech, Aidenbach, Germany) was added, and after three weeks NK cells could be used as pre-activated NK cells. In case of long-term treatment, Glutor (100 nM) or DMSO (0.1%) were added to the culture and every 72 h new substance was added.
2.2. Cell Lines
K562 cells were cultured in DMEM (Gibco) with 10% FCS and 1% penicillin/streptomycin. MCF7 cells were cultured in DMEM (Gibco) with 10% FCS and 1% penicillin/streptomycin, 1% Sodium Pyruvat, 0.1% NeAA and 0.1% Insulin. HepG2 cells were cultured in DMEM (Gibco) with 10% FCS and 1% penicillin/streptomycin
2.3. RTCA Analysis
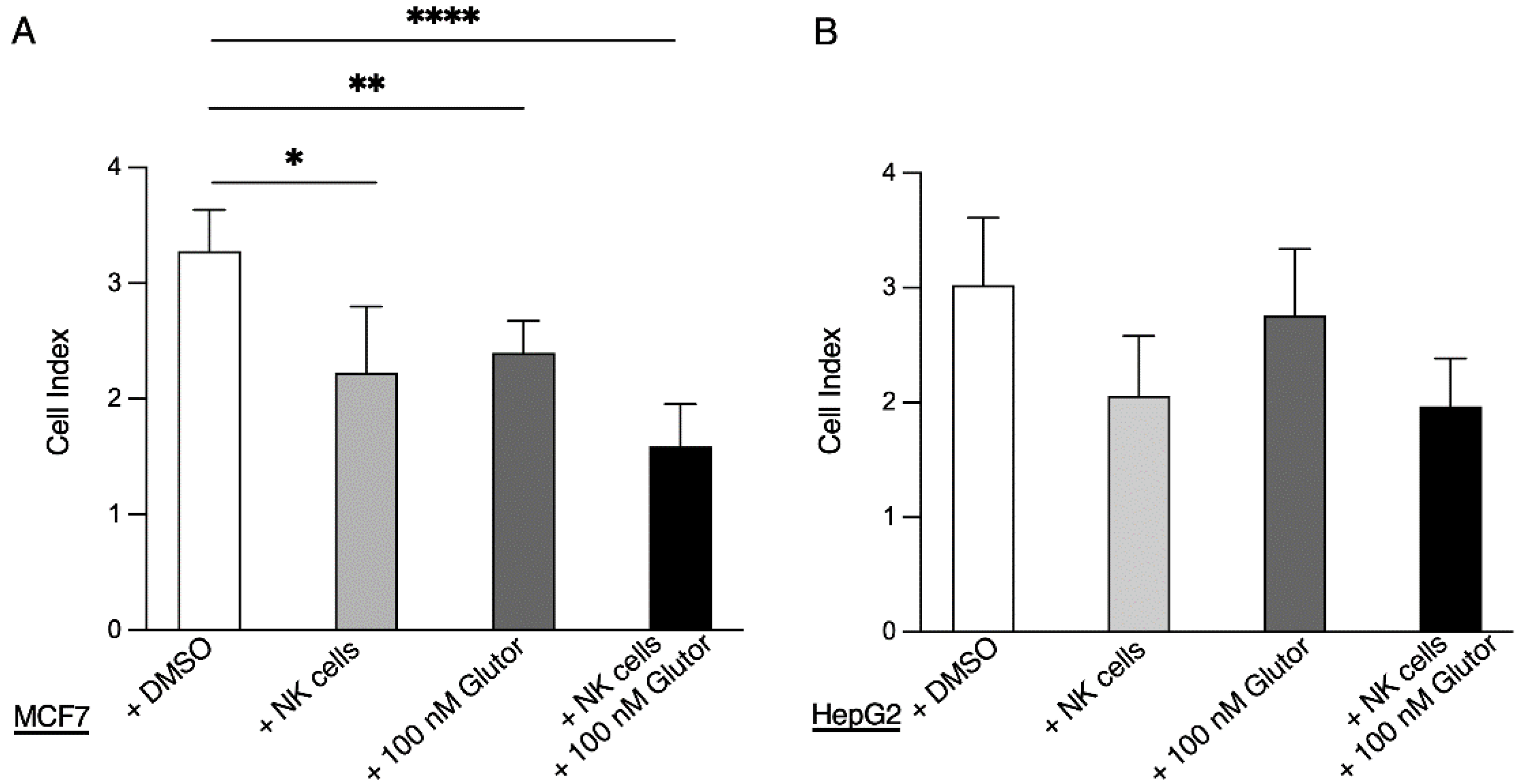
An amount of 50 µL of medium was added to the e-plates and background measurement was performed. Next, 3 × 104 MCF7 cells or HepG2 cells in 100 µL medium per well were seeded and placed into the xCELLigence. Measurement was started for about 16 h, followed by the addition of pre-activated NK cells (3.75 × 103) plus medium with DMSO (0.1%) or Glutor (100 nM) in 50 µL. Analysis was started and every 10 min for 72 h current was measured.
2.4. 51Chromium-Release Assay
Cytotoxicity was analyzed using a standard 4 h chromium-release assay as already described [
13,
14]. In short, target cells were labeled for 1 h at 37 °C and 5% CO
2 on a Rotator with
51Cr. Afterwards, target cells were washed twice and added to the NK cells in a 96 v-well plate. NK cells were serial diluted (1:2) starting at an E:T 2:1. After 4 h incubation time, supernatant was collected and analyzed with the Wizard
2 (Perkin Elmer, Waltham, MA, USA) gamma counter. The percentage of specific lysis was determined as follows:
2.5. Flow Cytometry
Degranulation assay: 0.1 × 106 resting or pre-activated NK cells were pre-treated with Glutor (100 nM) or DMSO (0.1%) for 30 min and afterwards stimulated via plate-bound CD16-mAb (2 µg/mL), NKp30 (2 µg/mL), NKG2D + 2B4 (2 µg/mL) or control IgG (2 µg/mL) in the presence of anti-CD107a PE-Cy5 (1:50; Clone:H4A3; BioLegend, San Diego, CA, USA) for 2 h. CD107a- expression was measured on a BD LSRFortessa flow cytometer. Data were analyzed using FlowJo software (FlowJo, Ashland, OR, USA).
Intracellular staining: 0.2 × 106 NK cells were stained for live/dead (zombie NIR 1:700; BioLegend) and CD56 using anti-CD56 BV421 (1:100; Clone:NCAM16.2; BD Biosciences, San Jose, CA, USA) for 15 min at RT. Intracellular staining was performed using 2% paraformaldehyde for fixation, Permeabilizing Solution 2 (BD Bioscience) for permeabilization and anti-GLUT1 (PE; 1:100; Clone:202915; R&D Systems, Minneapolis, MN, USA)), anti-pmTOR (PE-Cy7; 1:50; Clone:MRRBY; Thermo Fisher Scientific, Waltham, MA, USA) and anti-HK1 (AF647; 1:100; Clone: EPR10134(B); abcam) or Granzyme B (AF647; 1:200; Clone:GB11; BioLegend) and Perforin (FITC; 1:100; Clone:dG9; BioLegend) for intracellular staining (30 min, RT). Cells were measured on a BD LSRFortessa flow cytometer. Data were analyzed using FlowJo software (FlowJo).
Phenotype: Surface staining was performed using two different panels:
Panel 1: Zombie NIR (1:700; BioLegend), CD56 (BUV805; 1:4000; Clone:B159; BD Biosciences), CD3 (BUV563; 1:200; Clone:UCHT1; BD Biosciences), NKp46 (BV421; 1:50; Clone:9E2; BioLegend), 2B4 (FITC; 1:200; Clone:C1.7; BioLegend), NKp44 (PerCP-Cy5.5; 1:100; Clone:P44-8; BioLegend), DNAM-1 (AF647; 1:100; Clone:DX11; BD Biosciences), NKp30 (APC-Fire750; 1:100; Clone:P30-15; BioLegend), CD16 (PE-Dazzle; 1:100; Clone:3G8; BioLegend) and NKG2D (AF700; 1:100; Clone:FAB139N; R&D Systems).
Panel 2: CD38 (BUV395; 1:100; Clone:HB7; BD Biosciences), NKG2C (BUV496; 1:50; Clone:134591; BD Biosciences), CD3 (BUV563; 1:200; Clone:UCHT1; BD Biosciences), KLRB1 (BUV661; 1:100; Clone:NKR-3G10; BD Biosciences), CD56 (BUV805; 1:4000; Clone:B159; BD Biosciences), 4-1BB (BV421; 1:50; Clone:4B4-1; BioLegend), KLRG1 (BV510; 1:50; Clone:2F1; BioLegend), HLA-DR (BV605; 1:100; Clone:L243; BioLegend), FasL (BV650; 1:100; Clone:NOK-1, BD Biosciences), TIGIT (BV786; 1:100; Clone:741182; BD Biosciences), CD11a (AF488; 1:2000; Clone:HI111; BioLegend), CD8 (AF532;1:200; Clone:RPA-T8; Thermo Fisher Scientific), CD27 (PerCP-Cy5.5; 1:50; Clone:M-T271; BD Biosciences), NKG2A (PE; 1:100; Clone:Z199; Beckmann Coulter, Brea, CA, USA), CTLA-4 (PE-Dazzle; 1:100; Clone:BNI3; BioLegend), TRAIL (PE-Cy7; 1:50; Clone:N2B2; BioLegend), PD-1 (AF647; 1:400; Clone:EH12.1; BD Biosciences), CD18 (AF700; 1:800; Clone:TS1/18; BioLegend), Tim-3 (APC-Fire 750; 1:50; Clone:F38-2E2; BioLegend) and Zombie NIR (1:700, BioLegend). Cells were measured on a Cytek Aurora flow cytometer. Data were analyzed using FlowJo software (FlowJo).
2.6. SCENITH
An amount of 0.2 × 10
6 NK cells/well were plated in 100 µL IMDM media and incubated in the presence or absence of Oligomycin (1 µM), 2-DG (100 nM) or Oligomycin + 2-DG for 30 min at 37 °C and 5% CO
2. Next, 100 µL IMDM media containing Puromycin (10 µg/mL) was added to each well and incubated for 20 min at 37 °C and 5% CO
2. Then, cells were washed with ice-cold PBS, and Fc-Block for 5 min at 4 °C was performed followed by a surface staining using zombie NIR (1:700; BioLegend), anti-CD56 BV421 (1:100; Clone:NCAM16.2; BD Biosciences) and anti-CD16 PE-Dazzle (1:100; Clone:3G8; BioLegend) for 30 min at 4 °C. Cells were subsequently washed, fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (Invitrogen
TM, Waltham, MA, USA) and then intracellular staining using anti-Puromycin AF488 (1:200; Clone:12D10; Sigma-Aldrich, St. Louis, MO, USA) and anti-pmTOR PE-Cy7 (1:100; Clone:MRRBY; Thermo Fisher Scientific) was performed for 30 min. Lastly, cells were washed again and then measured on a Cytek Aurora flow cytometer. Gylcolytic dependency, mitochondrial dependency, glycolytic capacity and FAO (fatty acid oxidation) and AAO (amino acid oxidation) capacity were calculated using the formula as already described by Argüello et al. [
15]
2.7. ELISA
An amount of 0.1 × 10
6 resting or pre-activated NK cells were pre-treated with Glutor (100 nM) or DMSO (0.1%) for 30 min and afterwards stimulated via plate-bound CD16-mAb (2 µg/mL), NKp30 (2 µg/mL), NKG2D + 2B4 (2 µg/mL) or control IgG (2 µg/mL) or via IL-12 (0.25 ng/µL) + IL-18 (1.25 ng/µL) as previously described [
13]. After 16 h supernatants were collected. IFN-γ secretion was analyzed using the ELISA MAX™ Deluxe Set Human IFN-γ by BioLegend according to the manufacturer’s instructions. Measurement was performed using a GloMax
R instrument (Promega, Fitchburg, WI, USA).
2.8. Legendplex
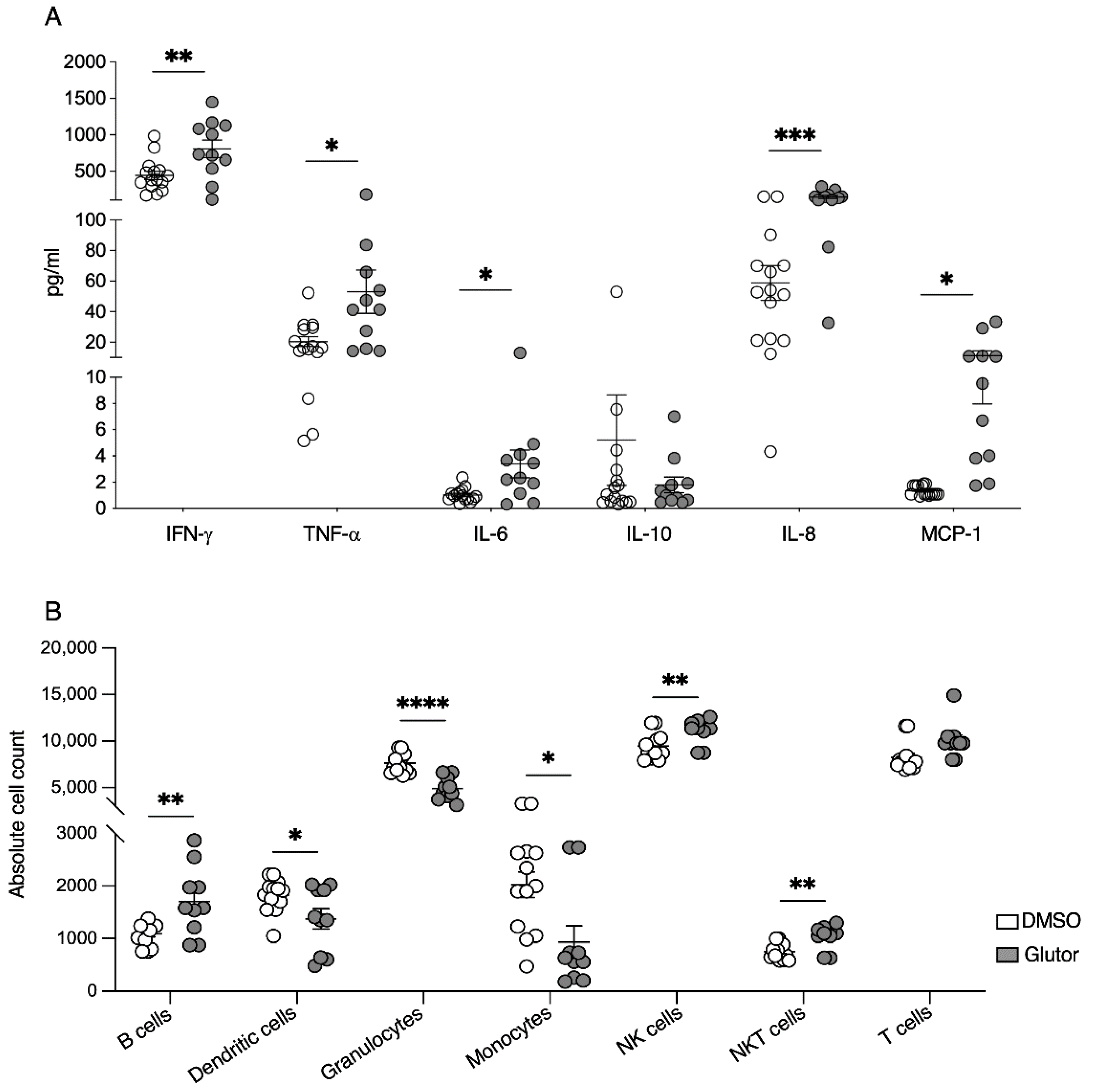
Supernatants of long-term treated NK cells were collected 24 h after medium exchange and used for multiplex-bead array using the Legendplex Inflammation Panel 1 Human (BioLegend). Assay was performed according to the manufacturer’s instructions (BioLegend).
2.9. Migration Assay
Transwell plates (HTS Transwell®-96 Permeable Support with 5.0 µm Pore Polycarbonate Membrane, Corning, Corning, NY, USA) were used. Supernatants of long-term treated NK cells were collected 24 h after medium exchange. Then, 235 µL of the supernatants was added to the lower chamber and 0.25 × 106 of freshly isolated PBMCs and granulocytes in 80 µL were added onto the insert. After 5 h of incubation (37 °C, 5% CO2), plates were incubated for 10 min at 4 °C, performed to induce detachment of monocytes. Cells were then removed from the lower chamber, transferred to a v-well plate, and pelleted by centrifugation. In the next step, cells were resuspended in 50 µL FACS-buffer containing an antibody mix of CD3 PerCP (1:100; Clone:SK7, BioLegend), CD56 BV421 (1:50; Clone:NCAM16.2; BD Biosciences), CD14 FITC (1:50 Clone: 61D3; Santa Cruz Biotechnology, Dallas, TX, USA), CD19 AF700 (1:50; Clone:HIB19; BioLegend), CD66b APC (1:200; Clone:G10F5; BioLegend) and CD11c PE (1:100; Clone: B-ly6; BD BioSciences) transferred to TruCount tubes (BD BioSciences) and incubated for 20 min at RT in the dark. Measurement of cells was performed using the BD LSRFortessa. Data were analyzed using FlowJo software (FlowJo) and absolute cell counts were calculated according to the manufacturer’s instructions.
2.10. Glucose Uptake Assay
An amount of 0.25 × 106 long-term treated NK cells per well was seeded and Glucose uptake-GloTM Assay (Promega, Madison, WI, USA) was performed according to the manufacturer’s instructions. Luminescence was recorded using a GloMaxR instrument (Promega).
2.11. Serial Killing Assays
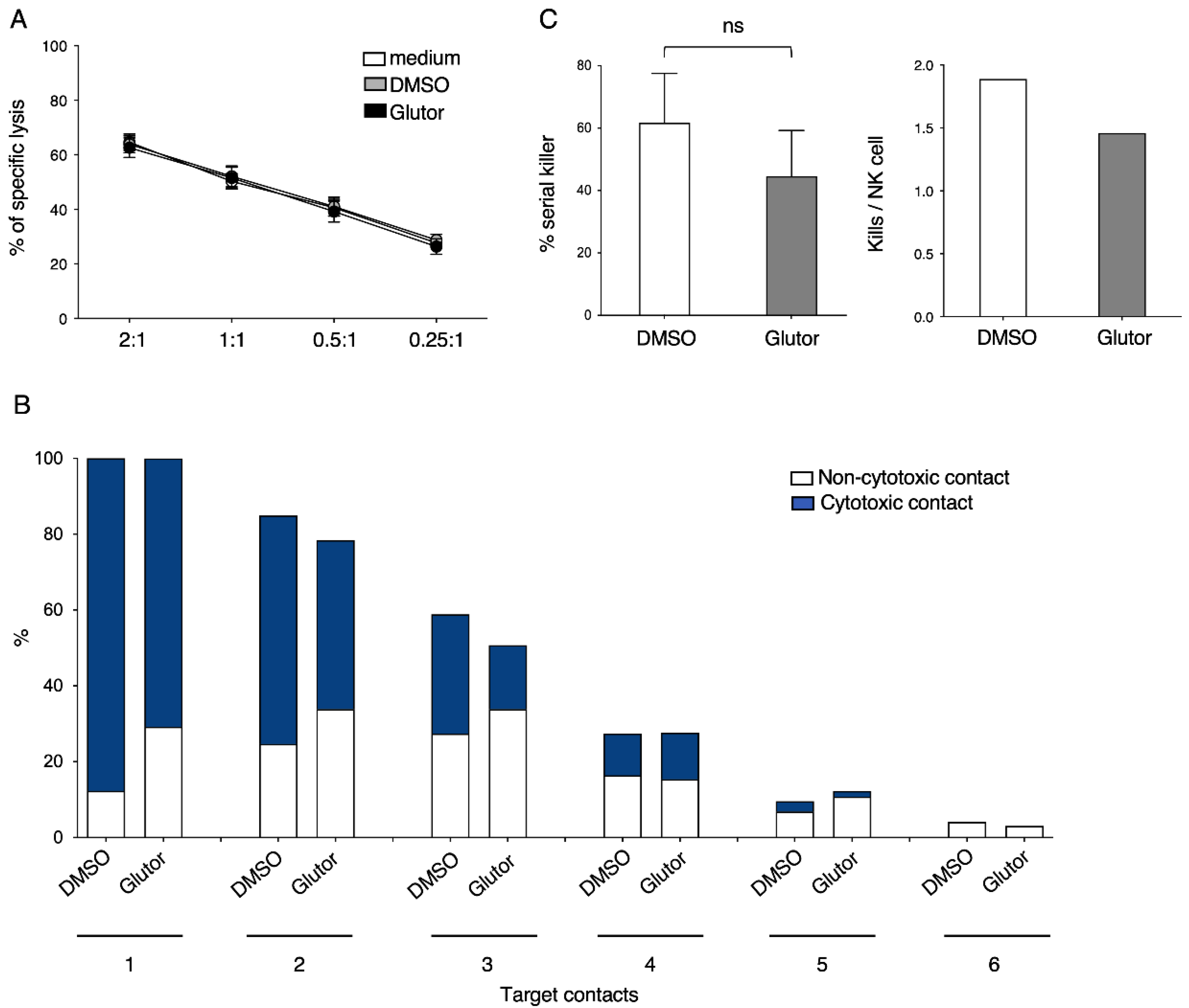
Assays were performed as described before [
16]. In brief, microchips were equilibrated with 200 µL CTL medium for 1 h at 37 °C with 5% CO
2, followed by removing the media and the addition of 200 µL CTL medium and 30 µL (30,000 cells) of resuspended MCF7 cells. After 5–10 min at 37 °C and 5% CO
2, the distribution and amount of cells/well was checked, and if there were wells with 60–80 tumor cells, media were exchanged to stop sinking of further tumor cells. Microchip was incubated for 16 h at 37 °C and 5% CO
2 to allow attachment of the tumor cells. After 16 h, a medium exchange was performed and 200 µL CTL medium containing the dead cell stain SYTOX Blue (1:1000, Thermo Fisher scientific) was added. Afterward, the microchip was placed into the incubation chamber of the ApoTome System with Axio Observer 7 microscope (Zeiss, Jena, Germany) equipped with a 20×/0.8 Plan-Apochromat objective and an incubation chamber with environmental control (37 °C, 5% CO
2, humidity device PM S1). Next, long-term treated NK cells were added so that an optimal distribution was achieved with about 10–20 NK cells per well. Time-lapse microscopy was started and every 3 min for 16 h a picture was taken using an Axiocam 506 mono camera. SYTOX Blue was excited using the Colibri 7 LED-module 475 (filter set 38 HE LED) and brightfield was acquired using the TL LED module.
2.12. Seahorse Analysis
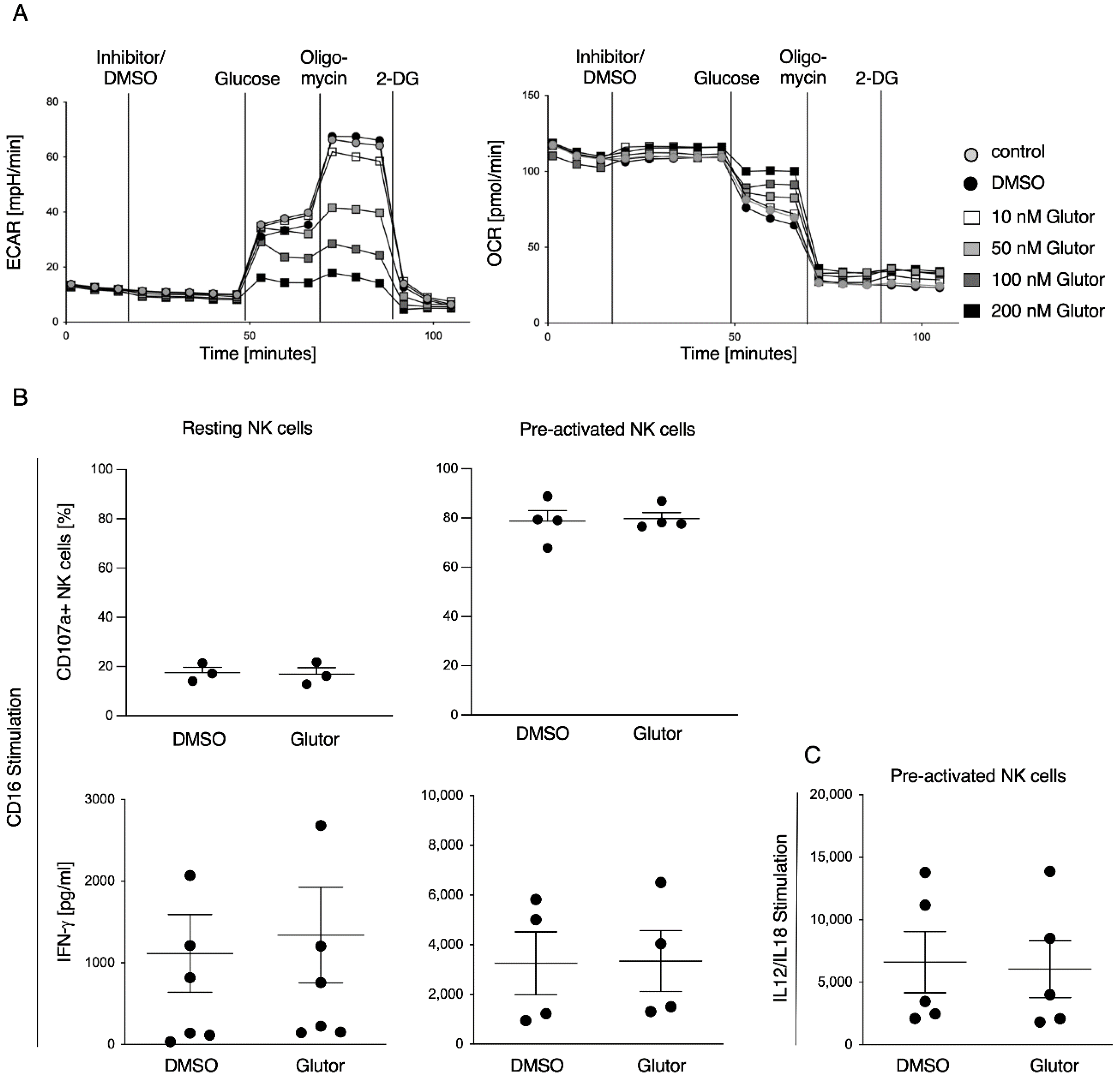
Energetic phenotype of pre-activated NK cells was analyzed using the glycolysis stress test kit (Agilent, Santa Clara, CA, USA). Experiment was performed according to the manufacturer’s instructions (Agilent). In brief, culture plates were coated with poly-l-lysine, followed by the addition of 0.25 × 106 pre-activated NK cells in 80 µL RPMI medium without glucose per well. Cells were incubated for 30 min at 37 °C without CO2. In the meantime, injection ports were loaded with 10× concentrated substance (final concentration after injection: Port A: 100 nM Glutor/0.1% DMSO/medium; Port B: 10 mM Glucose; Port C: Oligomycin; Port D: 50 mM 2-DG) and subsequent calibration of the sensors was performed. Lastly, 100 µL RPMI medium without glucose was added to the cells and measurement was started.
2.13. RNA-Seq
The 10 × 106 long-term treated NK cells (21 days) were used for RNA Isolation. RNA Isolation was performed using RNAeasy Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions with an additional step of DNAse treatment. Further analysis regarding purity and RNA sequencing was performed at the Genomics and Transcriptomics Facility (GTF) at the University Hospital Essen. In brief, QuantSeq 3′ mRNA-Seq Library Prep Kit FWD was used for library preparation. Sequencing was performed on the Illumina NextSeq500. Reads were processed with standard Illumina (San Diego, CA, USA) Basecalling, then trimmed with TrimGalore v0.6.0 and standard settings. Alignment was performed with hisat2 v2.2.1 to grch38 and standard settings. Raw counts were summed up with summarizeOverlaps from R-Package GenomicAlignments v1.8.4. RNA-Seq data were stored in the Gene expression omnibus archive (accession: GSE216156).
2.14. Statistics
Statistical analyses were performed using GraphPad Prism version 9 (Dotmatics, Boston, MA, USA).
4. Discussion
Here, we characterized the effect of the GLUT inhibitor Glutor on the functional activities of human NK cells. Upon blocking glucose uptake by Glutor, NK cells immediately showed reduced glycolysis and increased OxPhos. However, these acute changes in their metabolism did not affect their functions, as NK cell cytotoxicity and IFN-γ production were not altered upon acute Glutor treatment. This could be due to the intracellular storage of glucose and possibly the use of alternative energy sources such as glutamine or fatty acids. Additionally, NK cell cytotoxicity relies mostly on pre-formed effector proteins that are stored within the lytic granules and that just need to be mobilized upon NK cell activation [
17]. Therefore, only a little energy is needed for their immediate effector functions, and it has been described that the low basal metabolic rate of resting NK cells is sufficient to promote acute NK cell effector responses [
8].
The stimulation of resting NK cells with feeder cells and cytokines induces NK cell proliferation and activation. This is accompanied by a strong increase in glycolytic activity to provide sufficient energy and precursors for anabolic processes [
6,
7]. In the presence of Glutor, glucose uptake and therefore glycolysis were severely inhibited in NK cells. This resulted in a lack of proliferation and was accompanied by a delayed induction of mTOR phosphorylation. Cytokines such as IL-2, IL-15 and IL-21 are known to relay their signals via mTOR, and mTOR plays an important role in the maturation, activation and metabolism of NK cells [
18,
19]. A previous study also showed a direct interaction between mTOR and HK1 [
20], which could explain why we observed a delayed upregulation of HK1. In line with our previous results showing that Glutor treatment can induce an upregulation of GLUT-3 in tumor cells [
12], our transcriptional analysis demonstrates that NK cells try to counteract the inhibited uptake of glucose by increasing the expression of
GLUT-3 (SLC2A3),
GLUT-13 (
SLC2A13) and
GLUT-14 (SLC2A14), as well as other genes related to glycolysis. GLUT1 protein levels were also increased on Glutor-treated cells. Additionally, our metabolic SCENITH analysis also showed that Glutor-treated NK cells had significantly reduced glycolytic capacity, while showing higher mitochondrial dependency than control NK cells. The increase in hypoxia-related genes is likely a result of the lack of glucose in Glutor-treated NK cells, and the upregulation of genes related to the p53 pathway indicate that the NK cells are in cell cycle arrest, which would explain their lack of proliferation. Additionally, the upregulation of
CTLA-4 could play a role in the impaired IFN-γ production by long-term Glutor-treated NK cells [
21]. While Glutor-treated NK cells did not proliferate, they still survived in culture and did not undergo apoptosis. This could be connected to the increase in
IL7R expression, as IL-7 can promote the survival of NK cells [
22]. Additionally, this may be because Glutor did not block glucose uptake completely or because the NK cells utilized alternative energy sources such as glutamine. In support of this, we observed that NK cells did not proliferate but underwent apoptosis when cultured in glucose-free medium or when we treated them with Glutor in combination with a glutaminase inhibitor (
Figure S4).
This drastic change in metabolism of long-term Glutor-treated NK cells clearly had an impact on the phenotype of the cells. We observed a decreased expression of the activating receptors CD16, CD8, NKG2C and 2B4. In addition, CD38, which is known to play a role during activation and adhesion of NK cells, was downregulated [
23]. Decreased expression could be observed for the adhesion molecule CD18 [
24]. The reduced expression of these activating and adhesion receptors could also explain the reduced degranulation and IFN-γ production of the cells. The induction of the inhibitory receptor TIGIT [
25] was diminished, which may also be a result of the impaired activation of Glutor-treated NK cells. The expressions of KLRB1, KLRG1 and HLA-DR were increased. KLRG1 is a marker for exhausted NK cells [
26] and KLRB1 is a marker for NK cell function and primarily labels pro-inflammatory NK cells [
27]. This is consistent with the changes in constitutive cytokine and chemokine secretion we observed in the Glutor-treated NK cells. The production of the pro-inflammatory cytokines IFN-γ, TNF-α and IL-6 was significantly increased, whereas the anti-inflammatory cytokine IL-10 showed reduced levels. For the chemokines MCP-1 and IL-8, we observed a marked increase in spontaneous secretion of both chemokines. Therefore, the inhibition of glucose uptake and glycolysis also changed the regulatory function of NK cells. This suggests that the increase in glycolysis is important for reducing the inflammatory phenotype of activated NK cells.
While the block in glycolysis completely prevented NK cell proliferation, the cytotoxic activity was only slightly reduced. Stimulation via CD16 or NKp30 showed a significant reduction in degranulation as well as IFN-γ secretion in Glutor-treated NK cells. However, stimulation via NKG2D + 2B4 did not show these functional impairments. This could be due to a different metabolic dependence of ITAM-based signaling which is mediated by CD16 and NKp30 versus the ITT and ITSM-based signaling that is initiated by NKG2D and 2B4 [
2,
28]. Another explanation could be the decreased expression of CD16 on the long-term treated NK cells; however, in the case of NKp30 we could not detect a significantly decreased expression. However, the reduced degranulation had no effect on the cytotoxic activity of NK cells in a 4h
51Cr release assay, and the slight reduction in the serial killing activity of these NK cells was not significant. Therefore, the NK cell cytotoxic machinery seems to be rather robust and only little affected by the altered metabolism upon prolonged inhibition of glucose uptake. Alternative energy sources such as glutamine could be sufficient to maintain the cytotoxic activity of NK cells. Additionally, the reliance on pre-formed effector molecules may explain the intact killing activity of Glutor-treated NK cells.
In summary, blocking glucose uptake by Glutor has a large impact on NK cell proliferation. The phenotype of the NK cells is changed by the lack of glycolysis, whereas the cytotoxic machinery seems to be rather robust and only shows a limited impairment upon long-term inhibition of glucose transporters. However, for tumor surveillance the lack of NK cell proliferation would have a large impact on NK-cell-mediated tumor control. Therefore, it is important to consider the effect on immune cells when targeting the metabolism of tumor cells.