STING Targeting in Lung Diseases
Abstract
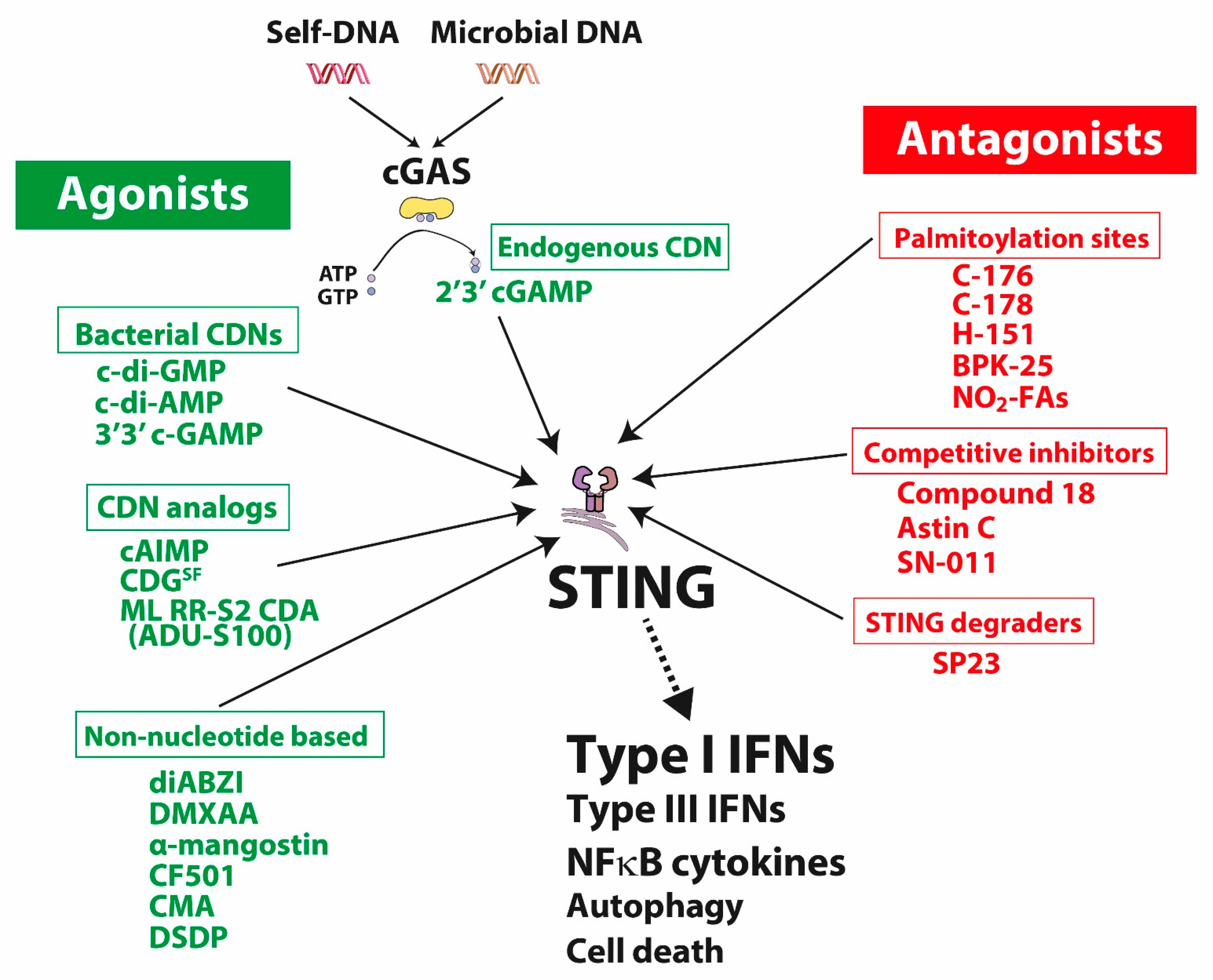
1. Introduction
2. STING Agonists
2.1. Cyclic Dinucleotides-Based STING Agonists
2.2. Non-Nucleotide-Based STING Agonists
3. STING Antagonists
3.1. STING Antagonists Targeting the Palmitoylation Sites
3.2. STING Antagonists Targeting the CDN-Binding Site
3.3. Other STING Antagonists
4. STING and Lung Diseases
4.1. Autoimmunity
4.1.1. SAVI
4.1.2. COPA
4.2. Infectious Diseases
4.2.1. Coronaviruses
4.2.2. Influenza
4.2.3. Tuberculosis
4.2.4. Streptococcus Pneumonia
4.2.5. Non-Typeable Haemophilus Influenzae (NTHI)
4.2.6. Legionella Pneumophila
4.3. Inflammatory and Allergic Diseases
4.3.1. Allergic Diseases
4.3.2. Chronic Obstructive Pulmonary Disease (COPD)
4.3.3. Fibrosis
4.4. Cancer
5. STING in Lung Vaccine/Adjuvant Formulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| c-di-AMP | Bis-(3′,5′)-cyclic diadenosine monophosphate |
| c-di-GMP | Bis-(3′,5′)-cyclic diguanosine monophosphate |
| CDN | Cyclic dinucleotide |
| CF | Cystic fibrosis |
| cGAMP | 2′3′-cyclic GMP-AMP |
| COPA | Coatomer protein subunit alpha |
| COPD | Chronic obstructive pulmonary disease |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| CTT | C-terminal tail |
| DDR | DNA damage response |
| ER | Endoplasmic reticulum |
| ERGIC | ER-Golgi intermediate compartment |
| GMWCNT | graphitized multi-walled carbon nanotubes |
| HDM | House dust mite |
| IDO | Indoleamine 2,3 dioxygenase |
| IFN | Interferon |
| ILC2 | Type 2 innate lymphoid cells |
| ILD | Interstitial lung disease |
| IPF | Idiopathic pulmonary fibrosis |
| IRF3 | Interferon regulatory factor 3 |
| ISG | Interferon-stimulated gene |
| JAK | Janus kinase |
| LLC | Lewis lung carcinoma |
| MAVS | Mitochondrial antiviral-signaling protein |
| NF-κB | Nuclear factor κB |
| NO2-Fas | Nitro-fatty acids |
| NSCLC | Non-small cell lung cancer |
| NTHI | Nontypeable Haemophilus influenzae |
| OVA | Ovalbumin |
| PARP | Poly(ADP-ribose) polymerase |
| PD-1 | Programmed death-1 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus-2 |
| SAVI | STING-associated vasculopathy with onset in infancy |
| SCID | severe combined immunodeficiency disease |
| SCLC | Small cell lung carcinoma |
| STAT | Signal transducer and activator of transcription |
| TBK1 | TANK-binding kinase 1 |
References
- Kranzusch, P.J.; Wilson, S.C.; Lee, A.S.; Berger, J.M.; Doudna, J.A.; Vance, R.E. Ancient Origin of cGAS-STING Reveals Mechanism of Universal 2′,3′ cGAMP Signaling. Mol. Cell 2015, 59, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Zhang, C.; Chen, Z.J.; Bai, X.C.; Zhang, X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 2019, 567, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Chen, Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal 2012, 5, ra20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019, 567, 394–398. [Google Scholar] [CrossRef]
- Mukai, K.; Konno, H.; Akiba, T.; Uemura, T.; Waguri, S.; Kobayashi, T.; Barber, G.N.; Arai, H.; Taguchi, T. Activation of STING requires palmitoylation at the Golgi. Nat. Commun. 2016, 7, 11932. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef]
- Dobbs, N.; Burnaevskiy, N.; Chen, D.; Gonugunta, V.K.; Alto, N.M.; Yan, N. STING Activation by Translocation from the ER Is Associated with Infection and Autoinflammatory Disease. Cell Host Microbe 2015, 18, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jiang, Q.; Guan, Y.; Gao, P.; Zhang, R.; Zhao, Z.; Jiang, Z. Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING. Immunity 2021, 54, 962–975.e968. [Google Scholar] [CrossRef]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jonsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-kappaB Signaling after Nuclear DNA Damage. Mol. Cell 2018, 71, 745–760.e745. [Google Scholar] [CrossRef]
- Danilchanka, O.; Mekalanos, J.J. Cyclic dinucleotides and the innate immune response. Cell 2013, 154, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Diner, E.J.; Burdette, D.L.; Wilson, S.C.; Monroe, K.M.; Kellenberger, C.A.; Hyodo, M.; Hayakawa, Y.; Hammond, M.C.; Vance, R.E. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013, 3, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Slavik, K.M.; Morehouse, B.R.; Ragucci, A.E.; Zhou, W.; Ai, X.; Chen, Y.; Li, L.; Wei, Z.; Bähre, H.; König, M.; et al. cGAS-like receptors sense RNA and control 3′2′-cGAMP signalling in Drosophila. Nature 2021, 597, 109–113. [Google Scholar] [CrossRef]
- Holleufer, A.; Winther, K.G.; Gad, H.H.; Ai, X.; Chen, Y.; Li, L.; Wei, Z.; Deng, H.; Liu, J.; Frederiksen, N.A.; et al. Two cGAS-like receptors induce antiviral immunity in Drosophila. Nature 2021, 597, 114–118. [Google Scholar] [CrossRef]
- Cohen, D.; Melamed, S.; Millman, A.; Shulman, G.; Oppenheimer-Shaanan, Y.; Kacen, A.; Doron, S.; Amitai, G.; Sorek, R. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature 2019, 574, 691–695. [Google Scholar] [CrossRef]
- Morehouse, B.R.; Govande, A.A.; Millman, A.; Keszei, A.F.A.; Lowey, B.; Ofir, G.; Shao, S.; Sorek, R.; Kranzusch, P.J. STING cyclic dinucleotide sensing originated in bacteria. Nature 2020, 586, 429–433. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef]
- Jin, L.; Getahun, A.; Knowles, H.M.; Mogan, J.; Akerlund, L.J.; Packard, T.A.; Perraud, A.L.; Cambier, J.C. STING/MPYS mediates host defense against Listeria monocytogenes infection by regulating Ly6C(hi) monocyte migration. J. Immunol. 2013, 190, 2835–2843. [Google Scholar] [CrossRef] [PubMed]
- Archer, K.A.; Durack, J.; Portnoy, D.A. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014, 10, e1003861. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Martin, F.J.; Soong, G.; Harfenist, B.S.; Aguilar, J.L.; Ratner, A.J.; Fitzgerald, K.A.; Schindler, C.; Prince, A. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio 2011, 2, e00016-11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, Y. STING or Sting: cGAS-STING-Mediated Immune Response to Protozoan Parasites. Trends Parasitol. 2020, 36, 773–784. [Google Scholar] [CrossRef]
- Ahn, J.; Barber, G.N. STING signaling and host defense against microbial infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Couillin, I.; Riteau, N. STING Signaling and Sterile Inflammation. Front Immunol. 2021, 12, 753789. [Google Scholar] [CrossRef]
- Mackenzie, K.J.; Carroll, P.; Martin, C.A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A.; et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017, 548, 461–465. [Google Scholar] [CrossRef]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 466–470. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Cordova, A.F.; Hess, G.T.; Bassik, M.C.; Li, L. SLC19A1 Is an Importer of the Immunotransmitter cGAMP. Mol. Cell 2019, 75, 372–381.e375. [Google Scholar] [CrossRef] [PubMed]
- Luteijn, R.D.; Zaver, S.A.; Gowen, B.G.; Wyman, S.K.; Garelis, N.E.; Onia, L.; McWhirter, S.M.; Katibah, G.E.; Corn, J.E.; Woodward, J.J.; et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 2019, 573, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Lahey, L.J.; Mardjuki, R.E.; Wen, X.; Hess, G.T.; Ritchie, C.; Carozza, J.A.; Böhnert, V.; Maduke, M.; Bassik, M.C.; Li, L. LRRC8A:C/E Heteromeric Channels Are Ubiquitous Transporters of cGAMP. Mol. Cell 2020, 80, 578–591.e575. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, X.; Planells-Cases, R.; Chu, J.; Wang, L.; Cao, L.; Li, Z.; López-Cayuqueo, K.I.; Xie, Y.; Ye, S.; et al. Transfer of cGAMP into Bystander Cells via LRRC8 Volume-Regulated Anion Channels Augments STING-Mediated Interferon Responses and Anti-viral Immunity. Immunity 2020, 52, 767–781.e766. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.F.; Ritchie, C.; Böhnert, V.; Li, L. Human SLC46A2 Is the Dominant cGAMP Importer in Extracellular cGAMP-Sensing Macrophages and Monocytes. ACS Cent. Sci. 2021, 7, 1073–1088. [Google Scholar] [CrossRef]
- Gentili, M.; Kowal, J.; Tkach, M.; Satoh, T.; Lahaye, X.; Conrad, C.; Boyron, M.; Lombard, B.; Durand, S.; Kroemer, G.; et al. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science 2015, 349, 1232–1236. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity 2020, 52, 357–373.e359. [Google Scholar] [CrossRef]
- Wu, J.; Dobbs, N.; Yang, K.; Yan, N. Interferon-Independent Activities of Mammalian STING Mediate Antiviral Response and Tumor Immune Evasion. Immunity 2020, 53, 115–126.e115. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, L.H.; Wilson, S.C.; Morrison, H.M.; Karalis, V.; Chung, J.J.; Chen, K.J.; Bateup, H.S.; Szpara, M.L.; Lee, A.Y.; Cox, J.S.; et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020, 11, 3382. [Google Scholar] [CrossRef]
- Watson, R.O.; Manzanillo, P.S.; Cox, J.S.; Extracellular, M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 2012, 150, 803–815. [Google Scholar] [CrossRef]
- Liu, D.; Wu, H.; Wang, C.; Li, Y.; Tian, H.; Siraj, S.; Sehgal, S.A.; Wang, X.; Wang, J.; Shang, Y.; et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019, 26, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Gulen, M.F.; Koch, U.; Haag, S.M.; Schuler, F.; Apetoh, L.; Villunger, A.; Radtke, F.; Ablasser, A. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 2017, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171, 1110–1124.e1118. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, A.R.; Wagner, L.E., 2nd; Zhu, J.; Tao, A.Y.; Yang, J.; Khodadadi-Jamayran, A.; Wang, Y.H.; Liu, M.; Rose, R.E.; Jones, D.R.; et al. The volume-regulated anion channel LRRC8C suppresses T cell function by regulating cyclic dinucleotide transport and STING-p53 signaling. Nat. Immunol. 2022, 23, 287–302. [Google Scholar] [CrossRef]
- Pokatayev, V.; Yang, K.; Tu, X.; Dobbs, N.; Wu, J.; Kalb, R.G.; Yan, N. Homeostatic regulation of STING protein at the resting state by stabilizer TOLLIP. Nat. Immunol. 2020, 21, 158–167. [Google Scholar] [CrossRef]
- Parvatiyar, K.; Zhang, Z.; Teles, R.M.; Ouyang, S.; Jiang, Y.; Iyer, S.S.; Zaver, S.A.; Schenk, M.; Zeng, S.; Zhong, W.; et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 2012, 13, 1155–1161. [Google Scholar] [CrossRef]
- McFarland, A.P.; Luo, S.; Ahmed-Qadri, F.; Zuck, M.; Thayer, E.F.; Goo, Y.A.; Hybiske, K.; Tong, L.; Woodward, J.J. Sensing of Bacterial Cyclic Dinucleotides by the Oxidoreductase RECON Promotes NF-κB Activation and Shapes a Proinflammatory Antibacterial State. Immunity 2017, 46, 433–445. [Google Scholar] [CrossRef]
- Abdul-Sater, A.A.; Tattoli, I.; Jin, L.; Grajkowski, A.; Levi, A.; Koller, B.H.; Allen, I.C.; Beaucage, S.L.; Fitzgerald, K.A.; Ting, J.P.; et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO Rep. 2013, 14, 900–906. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Wu, J.; Zhang, X.; Sun, L.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 2013, 51, 226–235. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Rohl, I.; Hopfner, K.P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Lioux, T.; Mauny, M.A.; Lamoureux, A.; Bascoul, N.; Hays, M.; Vernejoul, F.; Baudru, A.S.; Boularan, C.; Lopes-Vicente, J.; Qushair, G.; et al. Design, Synthesis, and Biological Evaluation of Novel Cyclic Adenosine-Inosine Monophosphate (cAIMP) Analogs That Activate Stimulator of Interferon Genes (STING). J. Med. Chem. 2016, 59, 10253–10267. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhao, L.; Han, B.B.; Hu, H.G.; Zhang, B.D.; Li, W.H.; Chen, Y.X.; Li, Y.M. A novel STING agonist for cancer immunotherapy and a SARS-CoV-2 vaccine adjuvant. Chem. Commun. (Camb. Engl.) 2021, 57, 504–507. [Google Scholar] [CrossRef]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.Y.; Tran, J.L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018, 564, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Prantner, D.; Perkins, D.J.; Lai, W.; Williams, M.S.; Sharma, S.; Fitzgerald, K.A.; Vogel, S.N. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J. Biol. Chem. 2012, 287, 39776–39788. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Li, L.; Maliga, Z.; Yin, Q.; Wu, H.; Mitchison, T.J. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem. Biol. 2013, 8, 1396–1401. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Pei, J.; Luo, Q.; Zeng, X.; Li, Q.; Yang, Z.; Quan, J. Identification of α-Mangostin as an Agonist of Human STING. ChemMedChem 2018, 13, 2057–2064. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Xu, W.; Deng, W.; Wang, Y.; Wang, M.; Wang, Q.; Hsieh, M.; Dong, J.; Wang, X.; et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022, 32, 269–287. [Google Scholar] [CrossRef]
- Cavlar, T.; Deimling, T.; Ablasser, A.; Hopfner, K.P.; Hornung, V. Species-specific detection of the antiviral small-molecule compound CMA by STING. EMBO J. 2013, 32, 1440–1450. [Google Scholar] [CrossRef]
- Liu, B.; Tang, L.; Zhang, X.; Ma, J.; Sehgal, M.; Cheng, J.; Zhang, X.; Zhou, Y.; Du, Y.; Kulp, J.; et al. A cell-based high throughput screening assay for the discovery of cGAS-STING pathway agonists. Antivir. Res. 2017, 147, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; van der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, E.V.; Zhang, X.; Remillard, D.; Lazar, D.C.; Suciu, R.M.; Wang, Y.; Bianco, G.; Yamashita, Y.; Crowley, V.M.; Schafroth, M.A.; et al. An Activity-Guided Map of Electrophile-Cysteine Interactions in Primary Human T Cells. Cell 2020, 182, 1009–1026.e1029. [Google Scholar] [CrossRef]
- Hansen, A.L.; Buchan, G.J.; Rühl, M.; Mukai, K.; Salvatore, S.R.; Ogawa, E.; Andersen, S.D.; Iversen, M.B.; Thielke, A.L.; Gunderstofte, C.; et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc. Natl. Acad. Sci. USA 2018, 115, e7768–e7775. [Google Scholar] [CrossRef] [PubMed]
- Siu, T.; Altman, M.D.; Baltus, G.A.; Childers, M.; Ellis, J.M.; Gunaydin, H.; Hatch, H.; Ho, T.; Jewell, J.; Lacey, B.M.; et al. Discovery of a Novel cGAMP Competitive Ligand of the Inactive Form of STING. ACS Med. Chem. Lett. 2019, 10, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, Z.; Wang, Z.; Li, F.; Mei, J.; Huang, L.; Lou, X.; Zhao, S.; Song, L.; Chen, W.; et al. The Cyclopeptide Astin C Specifically Inhibits the Innate Immune CDN Sensor STING. Cell Rep. 2018, 25, 3405–3421.e3407. [Google Scholar] [CrossRef]
- Hong, Z.; Mei, J.; Li, C.; Bai, G.; Maimaiti, M.; Hu, H.; Yu, W.; Sun, L.; Zhang, L.; Cheng, D.; et al. STING inhibitors target the cyclic dinucleotide binding pocket. Proc. Natl. Acad. Sci. USA 2021, 118, e2105465118. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, L.; Ruan, Y.; Deng, B.; Yang, Z.; Ren, Y.; Li, L.; Liu, T.; Zhao, H.; Mai, R.; et al. Novel CRBN-Recruiting Proteolysis-Targeting Chimeras as Degraders of Stimulator of Interferon Genes with In Vivo Anti-Inflammatory Efficacy. J. Med. Chem. 2022, 65, 6593–6611. [Google Scholar] [CrossRef]
- Li, X.D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Stülke, J.; Krüger, L. Cyclic di-AMP Signaling in Bacteria. Annu. Rev. Microbiol. 2020, 74, 159–179. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yin, W.; Galperin, M.Y.; Chou, S.H. Cyclic di-AMP, a second messenger of primary importance: Tertiary structures and binding mechanisms. Nucleic Acids Res. 2020, 48, 2807–2829. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.W.; Bogard, R.W.; Young, T.S.; Mekalanos, J.J. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 2012, 149, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, A.T.; Eaglesham, J.B.; de Oliveira Mann, C.C.; Morehouse, B.R.; Lowey, B.; Nieminen, E.A.; Danilchanka, O.; King, D.S.; Lee, A.S.Y.; Mekalanos, J.J.; et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 2019, 567, 194–199. [Google Scholar] [CrossRef]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef]
- Wang, C.; Sinn, M.; Stifel, J.; Heiler, A.C.; Sommershof, A.; Hartig, J.S. Synthesis of All Possible Canonical (3′-5′-Linked) Cyclic Dinucleotides and Evaluation of Riboswitch Interactions and Immune-Stimulatory Effects. J. Am. Chem. Soc. 2017, 139, 16154–16160. [Google Scholar] [CrossRef]
- Yan, H.; Chen, W. The Promise and Challenges of Cyclic Dinucleotides as Molecular Adjuvants for Vaccine Development. Vaccines 2021, 9, 917. [Google Scholar] [CrossRef]
- Karaolis, D.K.; Means, T.K.; Yang, D.; Takahashi, M.; Yoshimura, T.; Muraille, E.; Philpott, D.; Schroeder, J.T.; Hyodo, M.; Hayakawa, Y.; et al. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 2007, 178, 2171–2181. [Google Scholar] [CrossRef]
- Ebensen, T.; Libanova, R.; Schulze, K.; Yevsa, T.; Morr, M.; Guzmán, C.A. Bis-(3′,5′)-cyclic dimeric adenosine monophosphate: Strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 2011, 29, 5210–5220. [Google Scholar] [CrossRef]
- Mansouri, S.; Katikaneni, D.S.; Gogoi, H.; Jin, L. Monocyte-Derived Dendritic Cells (moDCs) Differentiate into Bcl6(+) Mature moDCs to Promote Cyclic di-GMP Vaccine Adjuvant-Induced Memory T(H) Cells in the Lung. J. Immunol. 2021, 206, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.L.; Jee, J.; Kim, E.; Steiner, H.E.; Cormet-Boyaka, E.; Boyaka, P.N. Sublingual targeting of STING with 3′3′-cGAMP promotes systemic and mucosal immunity against anthrax toxins. Vaccine 2017, 35, 2511–2519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, M.M.; Crute, B.W.; Cambier, J.C.; Getahun, A. B Cell-Intrinsic STING Signaling Triggers Cell Activation, Synergizes with B Cell Receptor Signals, and Promotes Antibody Responses. J. Immunol. 2018, 201, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Benoit-Lizon, I.; Jacquin, E.; Rivera Vargas, T.; Richard, C.; Roussey, A.; Dal Zuffo, L.; Martin, T.; Melis, A.; Vinokurova, D.; Shahoei, S.H.; et al. CD4 T cell-intrinsic STING signaling controls the differentiation and effector functions of T(H)1 and T(H)9 cells. J. Immunother. Cancer 2022, 10, e003459. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kuolee, R.; Yan, H. The potential of 3′,5′-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine 2010, 28, 3080–3085. [Google Scholar] [CrossRef] [PubMed]
- Karanja, C.W.; Yeboah, K.S.; Sintim, H.O. Identification of a Mycobacterium tuberculosis Cyclic Dinucleotide Phosphodiesterase Inhibitor. ACS Infect. Dis. 2021, 7, 309–317. [Google Scholar] [CrossRef]
- Li, L.; Yin, Q.; Kuss, P.; Maliga, Z.; Millán, J.L.; Wu, H.; Mitchison, T.J. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 2014, 10, 1043–1048. [Google Scholar] [CrossRef]
- Kato, K.; Nishimasu, H.; Oikawa, D.; Hirano, S.; Hirano, H.; Kasuya, G.; Ishitani, R.; Tokunaga, F.; Nureki, O. Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat. Commun. 2018, 9, 4424. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhao, L.; Hu, H.G.; Li, W.H.; Li, Y.M. Agonists and inhibitors of the STING pathway: Potential agents for immunotherapy. Med. Res. Rev. 2019, 40, 1117–1141. [Google Scholar] [CrossRef]
- Fu, J.; Kanne, D.B.; Leong, M.; Glickman, L.H.; McWhirter, S.M.; Lemmens, E.; Mechette, K.; Leong, J.J.; Lauer, P.; Liu, W.; et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015, 7, 283ra252. [Google Scholar] [CrossRef]
- Downey, C.M.; Aghaei, M.; Schwendener, R.A.; Jirik, F.R. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2′3′-cGAMP, induces M2 macrophage repolarization. PLoS ONE 2014, 9, e99988. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N., Jr.; Douillard, J.Y.; Nakagawa, K.; von Pawel, J.; McKeage, M.J.; Albert, I.; Losonczy, G.; Reck, M.; Heo, D.S.; Fan, X.; et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xu, M.M.; Fan, C.; Feng, C.L.; Lu, Q.K.; Lu, H.M.; Xiang, C.G.; Bai, F.; Wang, H.Y.; Wu, Y.W.; et al. STING inhibitor ameliorates LPS-induced ALI by preventing vascular endothelial cells-mediated immune cells chemotaxis and adhesion. Acta Pharmacol. Sin. 2021, 43, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.J.; Cerikan, B.; Cortese, M.; Frankish, J.; Lee, J.Y.; Plociennikowska, A.; Heigwer, F.; Prasad, V.; Joecks, S.; Burkart, S.S.; et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun. Biol. 2022, 5, 45. [Google Scholar] [CrossRef]
- Steiner, A.; Hrovat-Schaale, K.; Prigione, I.; Yu, C.H.; Laohamonthonkul, P.; Harapas, C.R.; Low, R.R.J.; De Nardo, D.; Dagley, L.F.; Mlodzianoski, M.J.; et al. Deficiency in coatomer complex I causes aberrant activation of STING signalling. Nat. Commun. 2022, 13, 2321. [Google Scholar] [CrossRef]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Sanchez, G.A.M.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef]
- Jeremiah, N.; Neven, B.; Gentili, M.; Callebaut, I.; Maschalidi, S.; Stolzenberg, M.C.; Goudin, N.; Fremond, M.L.; Nitschke, P.; Molina, T.J.; et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Investig. 2014, 124, 5516–5520. [Google Scholar] [CrossRef]
- McKee, A.S.; Burchill, M.A.; Munks, M.W.; Jin, L.; Kappler, J.W.; Friedman, R.S.; Jacobelli, J.; Marrack, P. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc. Natl. Acad. Sci. USA 2013, 110, E1122–E1131. [Google Scholar] [CrossRef]
- Nunokawa, H.; Murakami, Y.; Ishii, T.; Narita, T.; Ishii, H.; Takizawa, H.; Yamashita, N. Crucial role of stimulator of interferon genes-dependent signaling in house dust mite extract-induced IgE production. Sci. Rep. 2021, 11, 13157. [Google Scholar] [CrossRef]
- Ozasa, K.; Temizoz, B.; Kusakabe, T.; Kobari, S.; Momota, M.; Coban, C.; Ito, S.; Kobiyama, K.; Kuroda, E.; Ishii, K.J. Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor. Front. Immunol. 2019, 10, 2212. [Google Scholar] [CrossRef]
- She, L.; Barrera, G.D.; Yan, L.; Alanazi, H.H.; Brooks, E.G.; Dube, P.H.; Sun, Y.; Zan, H.; Chupp, D.P.; Zhang, N.; et al. STING activation in alveolar macrophages and group 2 innate lymphoid cells suppresses IL-33-driven type 2 immunopathology. JCI Insight 2021, 6, e143509. [Google Scholar] [CrossRef] [PubMed]
- Cavagnero, K.J.; Badrani, J.H.; Naji, L.H.; Amadeo, M.B.; Leng, A.S.; Lacasa, L.D.; Strohm, A.N.; Renusch, S.R.; Gasparian, S.S.; Doherty, T.A. Cyclic-di-GMP Induces STING-Dependent ILC2 to ILC1 Shift During Innate Type 2 Lung Inflammation. Front. Immunol. 2021, 12, 618807. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, D.Q.; Xiao, Q.; Liu, Y.B.; Song, J.; Liang, Y.; Ruan, J.W.; Wang, Z.Z.; Li, J.X.; Pan, L.; et al. Defective STING expression potentiates IL-13 signaling in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.; Gombault, A.; Lacerda-Queiroz, N.; Panek, C.; Savigny, F.; Sbeity, M.; Bourinet, M.; Le Bert, M.; Riteau, N.; Ryffel, B.; et al. Self-DNA release and STING-dependent sensing drives inflammation to cigarette smoke in mice. Sci. Rep. 2019, 9, 14848. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Sharma, R.; O’Sullivan, K.M.; Callaghan, J.; Dousha, L.; Thomas, B.; Ruwanpura, S.; Lim, S.; Farmer, M.W.; Jennings, B.R.; et al. Deoxyribonuclease 1 reduces pathogen.nic effects of cigarette smoke exposure in the lung. Sci. Rep. 2017, 7, 12128. [Google Scholar] [CrossRef]
- Qin, H.; Huang, G.; Gao, F.; Huang, B.; Wang, D.; Hu, X.; Wang, Y.; Peng, L.; Luo, D.; Mo, B.; et al. Diminished stimulator of interferon genes production with cigarette smoke-exposure contributes to weakened anti-adenovirus vectors response and destruction of lung in chronic obstructive pulmonary disease model. Exp. Cell Res. 2019, 384, 111545. [Google Scholar] [CrossRef]
- Ma, C.; Ma, X.; Jiang, B.; Pan, H.; Liao, X.; Zhang, L.; Li, W.; Luo, Y.; Shen, Z.; Cheng, X.; et al. A novel inactivated whole-cell Pseudomonas aeruginosa vaccine that acts through the cGAS-STING pathway. Signal. Transduct Target 2021, 6, 353. [Google Scholar] [CrossRef]
- Savigny, F.; Schricke, C.; Lacerda-Queiroz, N.; Meda, M.; Nascimento, M.; Huot-Marchand, S.; Da Gama Monteiro, F.; Ryffel, B.; Gombault, A.; Le Bert, M.; et al. Protective Role of the Nucleic Acid Sensor STING in Pulmonary Fibrosis. Front. Immunol. 2020, 11, 588799. [Google Scholar] [CrossRef]
- Benmerzoug, S.; Rose, S.; Bounab, B.; Gosset, D.; Duneau, L.; Chenuet, P.; Mollet, L.; Le Bert, M.; Lambers, C.; Geleff, S.; et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018, 9, 5226. [Google Scholar] [CrossRef]
- Messaoud-Nacer, Y.; Culerier, E.; Rose, S.; Maillet, I.; Rouxel, N.; Briault, S.; Ryffel, B.; Quesniaux, V.F.J.; Togbe, D. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis. 2022, 13, 269. [Google Scholar] [CrossRef]
- Han, B.; Wang, X.; Wu, P.; Jiang, H.; Yang, Q.; Li, S.; Li, J.; Zhang, Z. Pulmonary inflammatory and fibrogenic response induced by graphitized multi-walled carbon nanotube involved in cGAS-STING signaling pathway. J. Hazard. Mater. 2021, 417, 125984. [Google Scholar] [CrossRef] [PubMed]
- Humphries, F.; Shmuel-Galia, L.; Jiang, Z.; Wilson, R.; Landis, P.; Ng, S.L.; Parsi, K.M.; Maehr, R.; Cruz, J.; Morales-Ramos, A.; et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Thaiss, A.M.; Diamond, M.S.; Cherry, S. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci. Immunol. 2021, 6, eabi9007. [Google Scholar] [CrossRef]
- Liu, W.; Reyes, H.M.; Yang, J.F.; Li, Y.; Stewart, K.M.; Basil, M.C.; Lin, S.M.; Katzen, J.; Morrisey, E.E.; Weiss, S.R.; et al. Activation of STING Signaling Pathway Effectively Blocks Human Coronavirus Infection. J. Virol. 2021, 95. [Google Scholar] [CrossRef] [PubMed]
- Domizio, J.D.; Gulen, M.F.; Saidoune, F.; Thacker, V.V.; Yatim, A.; Sharma, K.; Nass, T.; Guenova, E.; Schaller, M.; Conrad, C.; et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature 2022, 603, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, L.; Xu, F.; Zhao, F.; Huang, Y.; Fan, Z.; Mei, S.; Hu, Y.; Zhai, L.; Guo, J.; et al. SARS-CoV-2 spike protein–induced cell fusion activates the cGAS-STING pathway and the interferon response. Sci. Signal. 2022, 15, eabg8744. [Google Scholar] [CrossRef]
- Holm, C.K.; Rahbek, S.H.; Gad, H.H.; Bak, R.O.; Jakobsen, M.R.; Jiang, Z.; Hansen, A.L.; Jensen, S.K.; Sun, C.; Thomsen, M.K.; et al. Influenza A virus targets a cGAS-independent STING pathway that controls enveloped RNA viruses. Nat. Commun. 2016, 7, 10680. [Google Scholar] [CrossRef]
- Moriyama, M.; Koshiba, T.; Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 2019, 10, 4624. [Google Scholar] [CrossRef]
- Lv, N.; Zhao, Y.; Liu, X.; Ye, L.; Liang, Z.; Kang, Y.; Dong, Y.; Wang, W.; Kolliputi, N.; Shi, L. Dysfunctional telomeres through mitostress-induced cGAS/STING activation to aggravate immune senescence and viral pneumonia. Aging Cell 2022, 21, e13594. [Google Scholar] [CrossRef]
- Watson, R.O.; Bell, S.L.; MacDuff, D.A.; Kimmey, J.M.; Diner, E.J.; Olivas, J.; Vance, R.E.; Stallings, C.L.; Virgin, H.W.; Cox, J.S. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe 2015, 17, 811–819. [Google Scholar] [CrossRef]
- Dey, B.; Dey, R.J.; Cheung, L.S.; Pokkali, S.; Guo, H.; Lee, J.H.; Bishai, W.R. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat. Med. 2015, 21, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Cambier, C.J.; O’Leary, S.M.; O’Sullivan, M.P.; Keane, J.; Ramakrishnan, L. Phenolic Glycolipid Facilitates Mycobacterial Escape from Microbicidal Tissue-Resident Macrophages. Immunity 2017, 47, 552–565.e554. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.C.; Cai, H.; Li, T.; Franco, L.H.; Li, X.D.; Nair, V.R.; Scharn, C.R.; Stamm, C.E.; Levine, B.; Chen, Z.J.; et al. Cyclic GMP-AMP Synthase Is an Innate Immune DNA Sensor for Mycobacterium tuberculosis. Cell Host Microbe 2015, 17, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.S.; Hamann, L.; Jin, L.; Sander, L.E.; Puzianowska-Kuznicka, M.; Cambier, J.; Witzenrath, M.; Schumann, R.R.; Suttorp, N.; Opitz, B. The cGAS/STING Pathway Detects Streptococcus pneumoniae but Appears Dispensable for Antipneumococcal Defense in Mice and Humans. Infect. Immun. 2018, 86, e00849-17. [Google Scholar] [CrossRef]
- Patel, S.; Tucker, H.R.; Gogoi, H.; Mansouri, S.; Jin, L. cGAS–STING and MyD88 Pathways Synergize in Ly6Chi Monocyte to Promote Streptococcus pneumoniae-Induced Late-Stage Lung IFNγ Production. Front. Immunol. 2021, 12, 699702. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, X.; Ma, C.; Xu, W.; Gan, L.; Cui, J.; Yin, Y.; Wang, H. Nontypeable Haemophilus influenzae DNA stimulates type I interferon expression via STING signaling pathway. Biochim. Et Biophys. Acta Mol. Cell Res. 2018, 1865, 665–673. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Q.; Huang, L.; Jin, J.; Zuo, X.; Zhang, Q.; Ye, L.; Zhu, S.; Zhan, P.; Ren, J.; et al. Low-dose carboplatin reprograms tumor immune microenvironment through STING signaling pathway and synergizes with PD-1 inhibitors in lung cancer. Cancer Lett. 2021, 500, 163–171. [Google Scholar] [CrossRef]
- Lemos, H.; Mohamed, E.; Huang, L.; Ou, R.; Pacholczyk, G.; Arbab, A.S.; Munn, D.; Mellor, A.L. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016, 76, 2076–2081. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wu, X.; Ma, D.; Wu, J.; Wang, L.; Jiang, Y.; Fei, Y.; Zhu, C.; Tan, R.; et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 2018, 563, 131–136. [Google Scholar] [CrossRef]
- Raaby Gammelgaard, K.; Sandfeld-Paulsen, B.; Godsk, S.H.; Demuth, C.; Meldgaard, P.; Sorensen, B.S.; Jakobsen, M.R. cGAS-STING pathway expression as a prognostic tool in NSCLC. Transl. Lung Cancer Res. 2021, 10, 340–354. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Sen, T.; Gay, C.M.; Ramkumar, K.; Diao, L.; Cardnell, R.J.; Rodriguez, B.L.; Stewart, C.A.; Papadimitrakopoulou, V.A.; Gibson, L.; et al. STING Pathway Expression Identifies NSCLC With an Immune-Responsive Phenotype. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, R.M.; Muirhead, G.; Krastev, D.B.; Adam, J.; Morel, D.; Garrido, M.; Lamb, A.; Hénon, C.; Dorvault, N.; Rouanne, M.; et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J. Clin. Investig. 2019, 129, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Ivanova, E.; Guo, S.; Yoshida, R.; Campisi, M.; Sundararaman, S.K.; Tange, S.; Mitsuishi, Y.; Thai, T.C.; Masuda, S.; et al. Suppression of STING Associated with LKB1 Loss in KRAS-Driven Lung Cancer. Cancer Discov. 2019, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Frémond, M.L.; Hadchouel, A.; Berteloot, L.; Melki, I.; Bresson, V.; Barnabei, L.; Jeremiah, N.; Belot, A.; Bondet, V.; Brocq, O.; et al. Overview of STING-Associated Vasculopathy with Onset in Infancy (SAVI) Among 21 Patients. J. Allergy Clin. Immunol. Pract. 2021, 9, 803–818.e811. [Google Scholar] [CrossRef]
- Clarke, S.L.; Pellowe, E.J.; de Jesus, A.A.; Goldbach-Mansky, R.; Hilliard, T.N.; Ramanan, A.V. Interstitial Lung Disease Caused by STING-associated Vasculopathy with Onset in Infancy. Am. J. Respir. Crit. Care Med. 2016, 194, 639–642. [Google Scholar] [CrossRef]
- Picard, C.; Thouvenin, G.; Kannengiesser, C.; Dubus, J.C.; Jeremiah, N.; Rieux-Laucat, F.; Crestani, B.; Belot, A.; Thivolet-Béjui, F.; Secq, V.; et al. Severe Pulmonary Fibrosis as the First Manifestation of Interferonopathy (TMEM173 Mutation). Chest 2016, 150, e65–e71. [Google Scholar] [CrossRef]
- Lin, B.; Torreggiani, S.; Kahle, D.; Rumsey, D.G.; Wright, B.L.; Montes-Cano, M.A.; Silveira, L.F.; Alehashemi, S.; Mitchell, J.; Aue, A.G.; et al. Case Report: Novel SAVI-Causing Variants in STING1 Expand the Clinical Disease Spectrum and Suggest a Refined Model of STING Activation. Front. Immunol. 2021, 12, 636225. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.J.; Dobbs, N.; Sakai, T.; Liou, J.; Miner, J.J.; Yan, N. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 2019, 216, 867–883. [Google Scholar] [CrossRef]
- Warner, J.D.; Irizarry-Caro, R.A.; Bennion, B.G.; Ai, T.L.; Smith, A.M.; Miner, C.A.; Sakai, T.; Gonugunta, V.K.; Wu, J.; Platt, D.J.; et al. STING-associated vasculopathy develops independently of IRF3 in mice. J. Exp. Med. 2017, 214, 3279–3292. [Google Scholar] [CrossRef]
- Bouis, D.; Kirstetter, P.; Arbogast, F.; Lamon, D.; Delgado, V.; Jung, S.; Ebel, C.; Jacobs, H.; Knapp, A.M.; Jeremiah, N.; et al. Severe combined immunodeficiency in stimulator of interferon genes (STING) V154M/wild-type mice. J. Allergy Clin. Immunol. 2019, 143, 712–725.e715. [Google Scholar] [CrossRef]
- Luksch, H.; Stinson, W.A.; Platt, D.J.; Qian, W.; Kalugotla, G.; Miner, C.A.; Bennion, B.G.; Gerbaulet, A.; Rosen-Wolff, A.; Miner, J.J. STING-associated lung disease in mice relies on T cells but not type I interferon. J. Allergy Clin. Immunol. 2019, 144, 254–266.e258. [Google Scholar] [CrossRef] [PubMed]
- Stinson, W.A.; Miner, C.A.; Zhao, F.R.; Lundgren, A.J.; Poddar, S.; Miner, J.J. The IFN-γ receptor promotes immune dysregulation and disease in STING gain-of-function mice. JCI Insight 2022, 7, e155250. [Google Scholar] [CrossRef] [PubMed]
- Balci, S.; Ekinci, R.M.K.; de Jesus, A.A.; Goldbach-Mansky, R.; Yilmaz, M. Baricitinib experience on STING-associated vasculopathy with onset in infancy: A representative case from Turkey. Clin. Immunol. 2020, 212, 108273. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Mulla, J.; Saheb Sharif-Askari, N.; Guzmán-Vega, F.J.; Arold, S.T.; Abd-Alwahed, M.; Alharbi, N.; Kashour, T.; Halwani, R. A Novel Biallelic STING1 Gene Variant Causing SAVI in Two Siblings. Front Immunol. 2020, 11, 599564. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.A.M.; Reinhardt, A.; Ramsey, S.; Wittkowski, H.; Hashkes, P.J.; Berkun, Y.; Schalm, S.; Murias, S.; Dare, J.A.; Brown, D.; et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J. Clin. Investig. 2018, 128, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- David, C.; Frémond, M.L. Lung Inflammation in STING-Associated Vasculopathy with Onset in Infancy (SAVI). Cells 2022, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Volpi, S.; Insalaco, A.; Caorsi, R.; Santori, E.; Messia, V.; Sacco, O.; Terheggen-Lagro, S.; Cardinale, F.; Scarselli, A.; Pastorino, C.; et al. Efficacy and Adverse Events During Janus Kinase Inhibitor Treatment of SAVI Syndrome. J. Clin. Immunol. 2019, 39, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Watkin, L.B.; Jessen, B.; Wiszniewski, W.; Vece, T.J.; Jan, M.; Sha, Y.; Thamsen, M.; Santos-Cortez, R.L.; Lee, K.; Gambin, T.; et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 2015, 47, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Arakel, E.C.; Schwappach, B. Formation of COPI-coated vesicles at a glance. J. Cell Sci. 2018, 131, jcs209890. [Google Scholar] [CrossRef]
- Volpi, S.; Tsui, J.; Mariani, M.; Pastorino, C.; Caorsi, R.; Sacco, O.; Ravelli, A.; Shum, A.K.; Gattorno, M.; Picco, P. Type I interferon pathway activation in COPA syndrome. Clin. Immunol. 2018, 187, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Lepelley, A.; Martin-Niclós, M.J.; Le Bihan, M.; Marsh, J.A.; Uggenti, C.; Rice, G.I.; Bondet, V.; Duffy, D.; Hertzog, J.; Rehwinkel, J.; et al. Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chong, Z.; Law, C.S.; Mukai, K.; Ho, F.O.; Martinu, T.; Backes, B.J.; Eckalbar, W.L.; Taguchi, T.; Shum, A.K. A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Ogawa, E.; Uematsu, R.; Kuchitsu, Y.; Kiku, F.; Uemura, T.; Waguri, S.; Suzuki, T.; Dohmae, N.; Arai, H.; et al. Homeostatic regulation of STING by retrograde membrane traffic to the ER. Nat. Commun. 2021, 12, 61. [Google Scholar] [CrossRef]
- Deng, Z.; Law, C.S.; Ho, F.O.; Wang, K.M.; Jones, K.D.; Shin, J.S.; Shum, A.K. A Defect in Thymic Tolerance Causes T Cell-Mediated Autoimmunity in a Murine Model of COPA Syndrome. J. Immunol. 2020, 204, 2360–2373. [Google Scholar] [CrossRef]
- Frémond, M.L.; Legendre, M.; Fayon, M.; Clement, A.; Filhol-Blin, E.; Richard, N.; Berdah, L.; Roullaud, S.; Rice, G.I.; Bondet, V.; et al. Use of ruxolitinib in COPA syndrome manifesting as life-threatening alveolar haemorrhage. Thorax 2020, 75, 92–95. [Google Scholar] [CrossRef]
- Krutzke, S.; Rietschel, C.; Horneff, G. Baricitinib in therapy of COPA syndrome in a 15-year-old girl. Eur. J. Rheumatol. 2019, 7, 1–4. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Seo, S.U.; Jeong, J.H.; Baek, B.S.; Choi, J.M.; Choi, Y.S.; Ko, H.J.; Kweon, M.N. Bleomycin-Induced Lung Injury Increases Resistance to Influenza Virus Infection in a Type I Interferon-Dependent Manner. Front. Immunol. 2021, 12, 697162. [Google Scholar] [CrossRef]
- Newling, M.; Hoepel, W.; Vogelpoel, L.T.C.; Heineke, M.H.; van Burgsteden, J.A.; Taanman-Kueter, E.W.M.; Eggink, D.; Kuijpers, T.W.; Beaumont, T.; van Egmond, M.; et al. Fc gamma receptor IIa suppresses type I and III interferon production by human myeloid immune cells. Eur. J. Immunol. 2018, 48, 1796–1809. [Google Scholar] [CrossRef] [PubMed]
- Wiens, K.E.; Ernst, J.D. The Mechanism for Type I Interferon Induction by Mycobacterium tuberculosis is Bacterial Strain-Dependent. PLoS Pathog. 2016, 12, e1005809. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.F.; Faden, H.; Bakaletz, L.O.; Kyd, J.M.; Forsgren, A.; Campos, J.; Virji, M.; Pelton, S.I. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatric Infect. Dis. J. 2009, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, J.; Müller, H.C.; Naujoks, J.; Tabeling, C.; Shin, S.; Witzenrath, M.; Hellwig, K.; Kirschning, C.J.; Taylor, G.A.; Barchet, W.; et al. Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice. Cell. Microbiol. 2011, 13, 1668–1682. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.S.; Hamann, L.; Shah, J.A.; Verbon, A.; Mockenhaupt, F.P.; Puzianowska-Kuznicka, M.; Naujoks, J.; Sander, L.E.; Witzenrath, M.; Cambier, J.C.; et al. The common HAQ STING variant impairs cGAS-dependent antibacterial responses and is associated with susceptibility to Legionnaires’ disease in humans. PLoS Pathog. 2018, 14, e1006829. [Google Scholar] [CrossRef]
- Nandakumar, R.; Tschismarov, R.; Meissner, F.; Prabakaran, T.; Krissanaprasit, A.; Farahani, E.; Zhang, B.C.; Assil, S.; Martin, A.; Bertrams, W.; et al. Intracellular bacteria engage a STING-TBK1-MVB12b pathway to enable paracrine cGAS-STING signalling. Nat. Microbiol. 2019, 4, 701–713. [Google Scholar] [CrossRef]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Munoz-Wolf, N.; Oleszycka, E.; Moran, H.B.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Riteau, N.; Sher, A. Chitosan: An Adjuvant with an Unanticipated STING. Immunity 2016, 44, 522–524. [Google Scholar] [CrossRef]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Decramer, M.; Janssens, W.; Miravitlles, M. Chronic obstructive pulmonary disease. Lancet 2012, 379, 1341–1351. [Google Scholar] [CrossRef]
- Barnes, P.J. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat. Rev. Drug Discov. 2013, 12, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis Primers 2015, 1, 15076. [Google Scholar] [CrossRef]
- Shak, S.; Capon, D.J.; Hellmiss, R.; Marsters, S.A.; Baker, C.L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 1990, 87, 9188–9192. [Google Scholar] [CrossRef]
- Lazarus, R.A.; Wagener†, J.S. Recombinant Human Deoxyribonuclease I. In Pharmaceutical Biotechnology: Fundamentals and Applications; Crommelin, D.J.A., Sindelar, R.D., Meibohm, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 471–488. [Google Scholar]
- D’Anna, S.E.; Maniscalco, M.; Carriero, V.; Gnemmi, I.; Caramori, G.; Nucera, F.; Righi, L.; Brun, P.; Balbi, B.; Adcock, I.M.; et al. Evaluation of Innate Immune Mediators Related to Respiratory Viruses in the Lung of Stable COPD Patients. J. Clin. Med. 2020, 9, 1807. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Seemungal, T.A. COPD exacerbations: Defining their cause and prevention. Lancet 2007, 370, 786–796. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Strieter, R.M.; Mehrad, B. New mechanisms of pulmonary fibrosis. Chest 2009, 136, 1364–1370. [Google Scholar] [CrossRef]
- Qiu, H.; Weng, D.; Chen, T.; Shen, L.; Chen, S.S.; Wei, Y.R.; Wu, Q.; Zhao, M.M.; Li, Q.H.; Hu, Y.; et al. Stimulator of Interferon Genes Deficiency in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Front. Immunol. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Ryu, C.; Sun, H.; Gulati, M.; Herazo-Maya, J.D.; Chen, Y.; Osafo-Addo, A.; Brandsdorfer, C.; Winkler, J.; Blaul, C.; Faunce, J.; et al. Extracellular Mitochondrial DNA Is Generated by Fibroblasts and Predicts Death in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 1571–1581. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA: A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Obenauf, A.C.; Massagué, J. Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer 2015, 1, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; An, X.; Zhang, X.; Qiao, Y.; Zheng, T.; Li, X. STING: A master regulator in the cancer-immunity cycle. Mol. Cancer 2019, 18, 152. [Google Scholar] [CrossRef]
- Westcott, P.M.; To, M.D. The genetics and biology of KRAS in lung cancer. Chin. J. Cancer 2013, 32, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef]
- Mai, J.; Li, Z.; Xia, X.; Zhang, J.; Li, J.; Liu, H.; Shen, J.; Ramirez, M.; Li, F.; Li, Z.; et al. Synergistic Activation of Antitumor Immunity by a Particulate Therapeutic Vaccine. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2021, 8, 2100166. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, W.; Zhu, J.; Zhu, J.; Shen, J.; Liu, Z.; Yang, Y.; Chen, Q. Nanoparticle-Mediated Delivery of Inhaled Immunotherapeutics for Treating Lung Metastasis. Adv. Mater. (Deerfield Beach Fla.) 2021, 33, e2007557. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.P.; Xiong, F.F.; Gao, F.X.; Yi, Y.L.; Zhang, M.; Chen, Z.; Tan, W.S. Enhancing Immune Response and Heterosubtypic Protection Ability of Inactivated H7N9 Vaccine by Using STING Agonist as a Mucosal Adjuvant. Front. Immunol. 2019, 10, 2274. [Google Scholar] [CrossRef]
- Takaki, H.; Takashima, K.; Oshiumi, H.; Ainai, A.; Suzuki, T.; Hasegawa, H.; Matsumoto, M.; Seya, T. cGAMP Promotes Germinal Center Formation and Production of IgA in Nasal-Associated Lymphoid Tissue. Med. Sci. (Basel Switz.) 2017, 5, 35. [Google Scholar] [CrossRef]
- Lirussi, D.; Ebensen, T.; Schulze, K.; Trittel, S.; Duran, V.; Liebich, I.; Kalinke, U.; Guzmán, C.A. Type I IFN and not TNF, is Essential for Cyclic Di-nucleotide-elicited CTL by a Cytosolic Cross-presentation Pathway. EBioMedicine 2017, 22, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Junkins, R.D.; Gallovic, M.D.; Johnson, B.M.; Collier, M.A.; Watkins-Schulz, R.; Cheng, N.; David, C.N.; McGee, C.E.; Sempowski, G.D.; Shterev, I.; et al. A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. J. Control. Release Off. J. Control. Release Soc. 2018, 270, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Wu, M.X. Natural STING Agonist as an "Ideal" Adjuvant for Cutaneous Vaccination. J. Investig. Derm. 2016, 136, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef] [PubMed]
- Van Dis, E.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Jong, R.M.; Van Dis, E.; Berry, S.B.; Nguyenla, X.; Baltodano, A.; Pastenkos, G.; Xu, C.; Fox, D.; Yosef, N.; McWhirter, S.M.; et al. Mucosal Vaccination with Cyclic Dinucleotide Adjuvants Induces Effective T Cell Homing and IL-17-Dependent Protection against Mycobacterium tuberculosis Infection. J. Immunol. 2021, 208, 407–419. [Google Scholar] [CrossRef]
- An, X.; Martinez-Paniagua, M.; Rezvan, A.; Sefat, S.R.; Fathi, M.; Singh, S.; Biswas, S.; Pourpak, M.; Yee, C.; Liu, X.; et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience 2021, 24, 103037. [Google Scholar] [CrossRef]
- Blaauboer, S.M.; Mansouri, S.; Tucker, H.R.; Wang, H.L.; Gabrielle, V.D.; Jin, L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. elife 2015, 4, e06670. [Google Scholar] [CrossRef]
- Koshy, S.T.; Cheung, A.S.; Gu, L.; Graveline, A.R.; Mooney, D.J. Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst. 2017, 1, 1600013. [Google Scholar] [CrossRef]
- Liu, Y.; Crowe, W.N.; Wang, L.; Lu, Y.; Petty, W.J.; Habib, A.A.; Zhao, D. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat. Commun. 2019, 10, 5108. [Google Scholar] [CrossRef]
- Karaolis, D.K.; Cheng, K.; Lipsky, M.; Elnabawi, A.; Catalano, J.; Hyodo, M.; Hayakawa, Y.; Raufman, J.P. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem. Biophys. Res. Commun. 2005, 329, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; KuoLee, R.; Tram, K.; Qiu, H.; Zhang, J.; Patel, G.B.; Chen, W. 3′,5′-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem. Biophys. Res. Commun. 2009, 387, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Blaauboer, S.M.; Gabrielle, V.D.; Jin, L. MPYS/STING-Mediated TNF-α, Not Type I IFN, Is Essential for the Mucosal Adjuvant Activity of (3′–5′)-Cyclic-Di-Guanosine-Monophosphate In Vivo. J. Immunol. 2014, 192, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Amouzegar, A.; Chelvanambi, M.; Filderman, J.N.; Storkus, W.J.; Luke, J.J. STING Agonists as Cancer Therapeutics. Cancers 2021, 13, 2695. [Google Scholar] [CrossRef]
| Agonists | Mouse | Ref. | Human | Ref. | |
|---|---|---|---|---|---|
| Endogenous CDN | 2′3′ cGAMP | YES | [50,51] | YES | [50,51] |
| Bacterial-CDNs | c-di-GMP | YES | [12] | YES | [12] |
| c-di-AMP | YES | [12] | YES | [12] | |
| 3′3′ cGAMP | YES | [50] | YES | [50] | |
| CDN analogs | cAIMP | YES | [52] | YES | [52] |
| CDGSF | YES | [53] | |||
| ML RR-S2 CDA (ADU-S100) | YES | [54] | YES | [54] | |
| Non-nucleotide based | diABZI | YES | [55] | YES | [55] |
| DMXAA | YES | [56] | NO | [57] | |
| α-mangostin | (YES) | [58] | YES | [58] | |
| CF501 | YES | [59] | |||
| CMA | YES | [60] | NO | [60] | |
| DSDP | NO | [61] | YES | [61] | |
| Antagonists | Mouse | Ref. | Human | Ref. | |
| Palmitoylation sites | C-176 | YES | [62] | NO | [62] |
| C-178 | YES | [62] | NO | [62] | |
| H-151 | YES | [62] | YES | [62] | |
| BPK-25 | YES | [63] | |||
| NO2-FAs | YES | [64] | YES | [64] | |
| Competitive inhibitors | Compound 18 | YES | [65] | ||
| Astin C | YES | [66] | YES | [66] | |
| SN-011 | YES | [67] | YES | [67] | |
| STING degrader | SP23 | YES | [68] | YES | [68] |
| Category | Disease/Model | Species | Trigger/Pathway | Main Effects/Findings | Ref. | ||
|---|---|---|---|---|---|---|---|
| STING Agonist | STING Antagonist | ||||||
| Autoimmunity | COPA | H | Coatomer protein complex dysfunction | STING-dependent inflammation, with varying degree of interstitial lung disease | [95] | ||
| COPA | H (Φ), M (Φ) | Coatomer protein complex dysfunction | H-151 | H-151 reduces IFN-β and ISG inductions | [95] | ||
| SAVI | H | Gain of function mutation in STING | Interferonopathy associated with skin lesions, perivascular inflammation and interstitial lung disease | [96,97] | |||
| Inflammatory diseases | Asthma (OVA/ALUM) | M | Self DNA release | STING deficiency leads to IFNAR independent reduction of antigen specific CD4+ T cell priming and IgE | [98] | ||
| Asthma (HDM) | M | cGAMP | cGAMP increases HDM-specific IgE levels by promoting T follicular helper cells (Tfh) responses | [99] | |||
| Asthma (HDM) | M | cGAMP | cGAMP increases IL-33-dependent asthma and Th2 responses | [100] | |||
| Asthma (IL-33 or Aspergillus flavus) | M | cGAMP | cGAMP decreases Th2-associated lung immunopathology and airway hyperreactivity by inhibiting ILC2 cell activation | [101] | |||
| Asthma (Alternaria alternata or IL-33) | M | ci-di-GMP | ci-di-GMP suppresses ILC2s and type 2 lung inflammation, while promoting ILC1s in a STING/type I IFN-dependent manner | [102] | |||
| Asthma (ILC2) | H (Φ), M (Φ) | cGAMP | cGAMP suppresses proliferation and cytokine production of ILC2 | [101] | |||
| CRSwNP | H | Reduced STING/type I IFN expressions within eosinophilic nasal polyps leading to IL-13 signaling and eosinophilic inflammation | [103] | ||||
| COPD | M | Self DNA release | cGAS/STING-dependent neutrophilic influx and inflammatory response | [104] | |||
| COPD | M | Self DNA release | DNAse I treatment alleviates cigarette smoke-induced lung inflammation | [105] | |||
| COPD | M | Decreased STING lung expression limiting subsequent immune response to infection | [106] | ||||
| COPD exacerbation | M | PAO1 vaccine | cGAS/STING-dependent protection to P. aeruginosa infection | [107] | |||
| IPF | M | Self-DNA release | STING decreases lung fibrosis in a type I IFN-independent manner | [108] | |||
| Silicosis | M | Self-DNA release | STING-dependent type I IFN responses promoting lung inflammation | [109] | |||
| ARDS | M | diABZI | diABZI induces PANoptosis and promotes ARDS | [110] | |||
| GMWCNTs | M | C-176 | C-176 decreases pulmonary inflammation and fibrosis | [111] | |||
| Infectious diseases | SARS-CoV-2 | M | diABZI | Strong protection from SARS-CoV-2-triggered lethality | [112,113] | ||
| SARS-CoV-2 | H (Φ) | cGAMP; diABZI | Inhibition of SARS-CoV-2 replication | [113,114] | |||
| SARS-CoV-2 | M | H-151 | H-151 reduces severe lung inflammation and improves survival | [115] | |||
| SARS-CoV-2 | H (Φ) | Mitochondrial DNA release | H-151 | H-151 reduces type I IFN/ISG production and cell death | [115] | ||
| SARS-CoV-2 | H (Φ) | Cell fusion-induced micronuclei | Activation of the cGAS/STING/type I IFN pathway | [116] | |||
| SARS-CoV-2 | H (Φ) | H-151; VS-X4 | H-151 or VS-X4 limit cGAS/STING-driven NF-κB activation and inflammatory immune response | [94] | |||
| Influenza | M (Φ) | cGAS-independent STING-mediated type I IFN production | [117] | ||||
| Influenza | H (Φ), M (Φ) | M2 protein-mediated mtDNA release | cGAS- and DDX41-mediated STING-dependent antiviral responses | [118] | |||
| Influenza | M | Aging/senescence-induced mitochondrial stress | cGAS/STING activation and increased susceptibility to IAV infection | [119] | |||
| Influenza | M | H-151 | H-151 decreases viral loads and histopathology | [119] | |||
| M. tuberculosis | M | M. tuberculosis DNA | STING-mediated autophagy decreases bacterial replication | [41] | |||
| M. tuberculosis | M | M. tuberculosis DNA | cGAS/STING activation leading to type I IFN production | [120] | |||
| M. tuberculosis | M | M. tuberculosis c-di-AMP | STING-dependent autophagy and type I IFN production limiting virulence and pathogenicity | [121] | |||
| M. tuberculosis | Z | M. tuberculosis phenolic glycolipids | STING-mediated CCL-2 production and growth-permissive monocyte recruitment in a type I IFN independent manner | [122] | |||
| M. tuberculosis | M | In contrast to cGAS deficiency, STING deficiency shows no effect on mouse survival | [123] | ||||
| S. pneumonia | M | STING is dispensable for initial control of bacterial burden | [124] | ||||
| S. pneumonia | M | cGAS/STING and MyD88 pathway-mediated late IFN-γ production | [125] | ||||
| NTHI | M | NTHI DNA | cGAS/STING-dependent type I IFN induction | [126] | |||
| L. pneumophila | M (Φ) | cGAS/STING-mediated bacterial clearance | [120] | ||||
| Lung cancer | LLC | M | DNA damage by carboplatin | Synergizes with PD-1 inhibitors to promote protective CD8+ T cells infiltration | [127] | ||
| LLC | M | STING-triggered IDO | Promotes tumor growth and limits CD8+ T cell-mediated tumor cell killing | [128] | |||
| LLC | M | Nuclear cGAS suppresses DNA repair | cGAS promotes tumor expansion | [129] | |||
| NSCLC | H | Increased cGAS-STING expression levels correlating with overall survival | [130] | ||||
| NSCLC | H | Increased cGAS expression correlates with tumor stage | [129] | ||||
| NSCLC | H | STING pathway activation correlates with efficient immunotherapy | [131] | ||||
| NSCLC | H, H (Φ) | PARP inhibition-triggered micronuclei | cGAS/STING activation in ERCC1-defective NSCLC | [132] | |||
| NSCLC | H, H (Φ) | STING suppression in KRAS-LKB1 mutant | Decreased STING-mediated tumor cell cytotoxicity | [133] | |||
| SCLC | M | DDR inhibition + anti-PD-L1 | cGAS/STING-dependent anti-tumor effect | [134] | |||
| Category | Disease/Model | STING Agonist | Antigen/Costimulant | Carrier | Route | Main Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Infectious diseases | Influenza | cGAMP | HA (H7N9) or inactivated H7N9 | PBS | IN | Protection against a lethal dose of influenza virus | [190] |
| Influenza | cGAMP | HA | PBS | IN | Increased germinal center formation and IgA production | [191] | |
| Influenza | c-di-AMP | OVA-expressing H1N1 | PBS | IN | Increased CTL immune memory and reduced weight loss upon viral challenge | [192] | |
| Influenza | 3′3′-cGAMP | HA (H1N1) | Acetalated dextran polymeric microparticles | IM | Protective immunity against a lethal influenza challenge | [193] | |
| Influenza | cGAMP | HA (H1N1) | ID (not IM) | Protective immunity against a lethal influenza challenge | [194] | ||
| Influenza | cGAMP | Inactivated H1N1, H5N1, H7N9 | Pulmonary surfactant biomimetic liposomes | IN | Protective immunity against a lethal influenza challenge | [195] | |
| M. tuberculosis | RR-CDG, ML-RR-cGAMP | 5Ag | AddaVax | SC | Type I IFN-independent Th1 immune response and protection | [196] | |
| M. tuberculosis | RR-CDG, ML-RR-cGAMP | 5Ag | PBS | IN | Th17 immune response and enhanced protection | [196] | |
| M. tuberculosis | ML-RR-cGAMP | 5Ag or H1 | PBS | IN | IL-17-dependent Protection | [197] | |
| SARS-CoV-2 | cGAMP | Spike protein | Negatively charged liposomes | IN | Increased B and T cell responses | [198] | |
| SARS-CoV-2 | CF501 | RBD-Fc protein | PBS | IM | Long term immunity against SARS-CoV-2 challenge | [59] | |
| SARS-CoV-2 | CDGSF | spike protein | SC | Increased IFN-γ and SARS-CoV-2 specific IgG | [53] | ||
| S. pneumoniae | c-di-GMP (>cGAMP) | PspA | IN | Enhanced antigen uptake and protection | [199] | ||
| Lung Cancer | Lung metastases (melanoma, breast, colon) | DMXAA; ML RR-S2 CDA | NaHCO3; HBSS | IT | Systemic antitumor immunity | [54] | |
| Lung adenocarcinoma, lung metastasis (breast) | DMXAA | DMSO | IP | M1 macrophage polarization-associated antitumor immunity | [91] | ||
| Lung metastases (melanoma) | cGAMP | Anti-CTLA-4 and anti-PD-1 antibodies (IP) | PEG-containing cationic liposomes | IV | Synergistic antitumor immunity | [200] | |
| Lung metastases (melanoma) | c-di-GMP | Anti-PD-1 antibody (IP) | Lipid nanoparticle | IV | NK cell-dependent synergistic antitumor effect | [187] | |
| Lung metastases (melanoma, breast) | cGAMP | Radiotherapy | Phosphatidylserine coated liposome | IN | Synergistic antitumor immunity | [201] | |
| Lung metastases (melanoma) | cGAMP | CpG and tumor antigen peptides | Nanoporous microparticles | Increased DC maturation and enhanced survival | [188] | ||
| Lung metastases (melanoma) | Chitosan and anti-PD-1 antibody (aerosol) | IN | cGAS–STING–Type I IFN pathway enhancing DC activation and metastasis regression | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Moura Rodrigues, D.; Lacerda-Queiroz, N.; Couillin, I.; Riteau, N. STING Targeting in Lung Diseases. Cells 2022, 11, 3483. https://doi.org/10.3390/cells11213483
de Moura Rodrigues D, Lacerda-Queiroz N, Couillin I, Riteau N. STING Targeting in Lung Diseases. Cells. 2022; 11(21):3483. https://doi.org/10.3390/cells11213483
Chicago/Turabian Stylede Moura Rodrigues, Dorian, Norinne Lacerda-Queiroz, Isabelle Couillin, and Nicolas Riteau. 2022. "STING Targeting in Lung Diseases" Cells 11, no. 21: 3483. https://doi.org/10.3390/cells11213483
APA Stylede Moura Rodrigues, D., Lacerda-Queiroz, N., Couillin, I., & Riteau, N. (2022). STING Targeting in Lung Diseases. Cells, 11(21), 3483. https://doi.org/10.3390/cells11213483






