Impact of Microbiota Depletion by Antibiotics on SARS-CoV-2 Infection of K18-hACE2 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibiotic Treatment
2.3. Virus
2.4. SARS-CoV-2 Infection and Clinical Analysis of Mice
2.5. Bacterial DNA Isolation from Mice Feces
2.6. rRNA Sequencing and Analysis
2.7. RNA Extraction and Quantification
2.8. Viral Load Quantification
2.9. Histopathological Score
2.10. Bronchoalveolar Lavage Fluid (BALF)
2.11. Flow Cytometry
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Quantitative Real-Time PCR Analysis
2.14. Statistical Analysis
3. Results
3.1. Microbiota Depletion Does Not Change Mortality of SARS-CoV-2 Infection, but Alters Clinical Symptoms
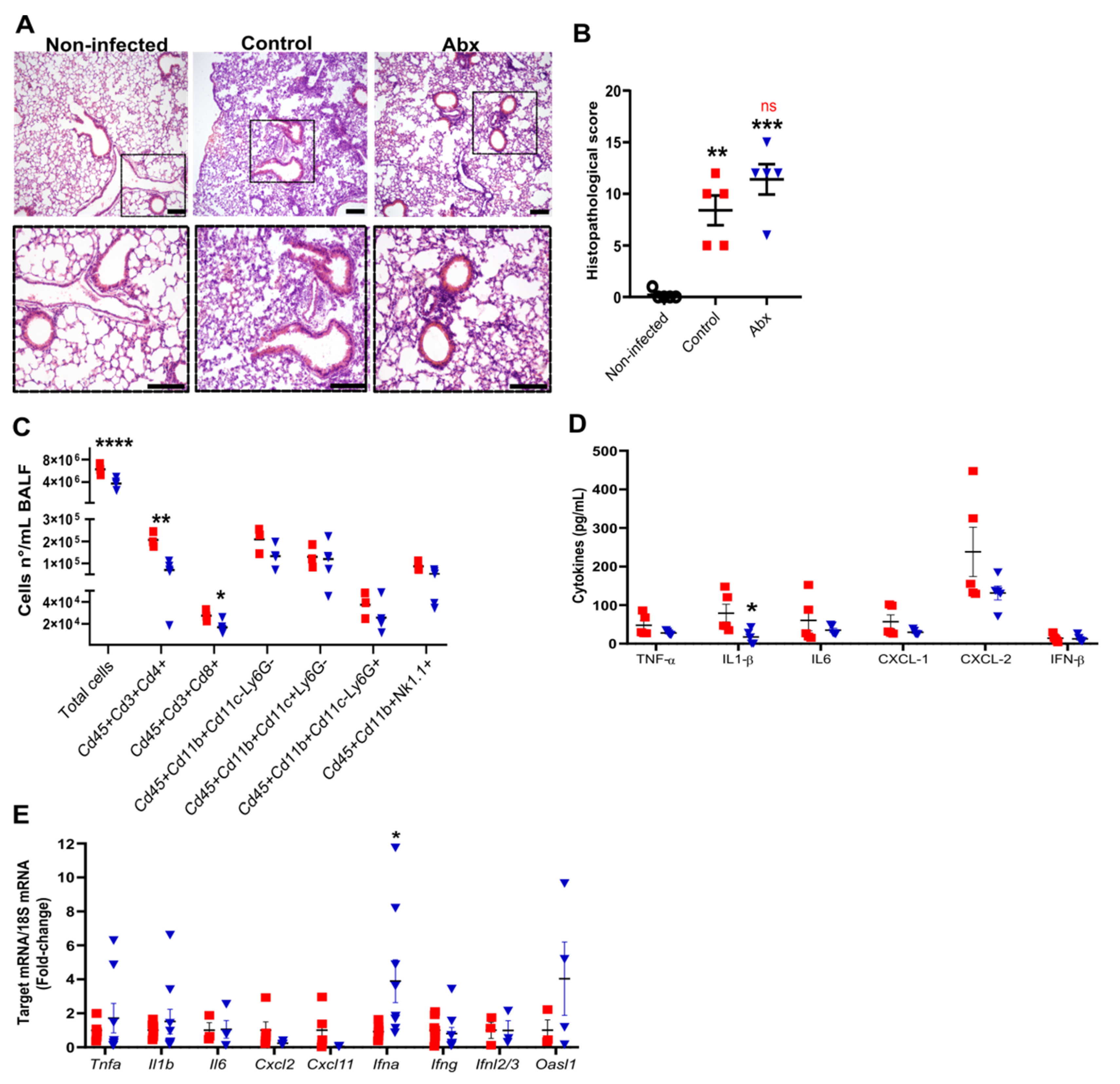
3.2. Antibiotic Treatment Does Not Affect Lung Histopathology but Promotes Changes in Production of Immune Molecules Following SARS-CoV-2 Infection
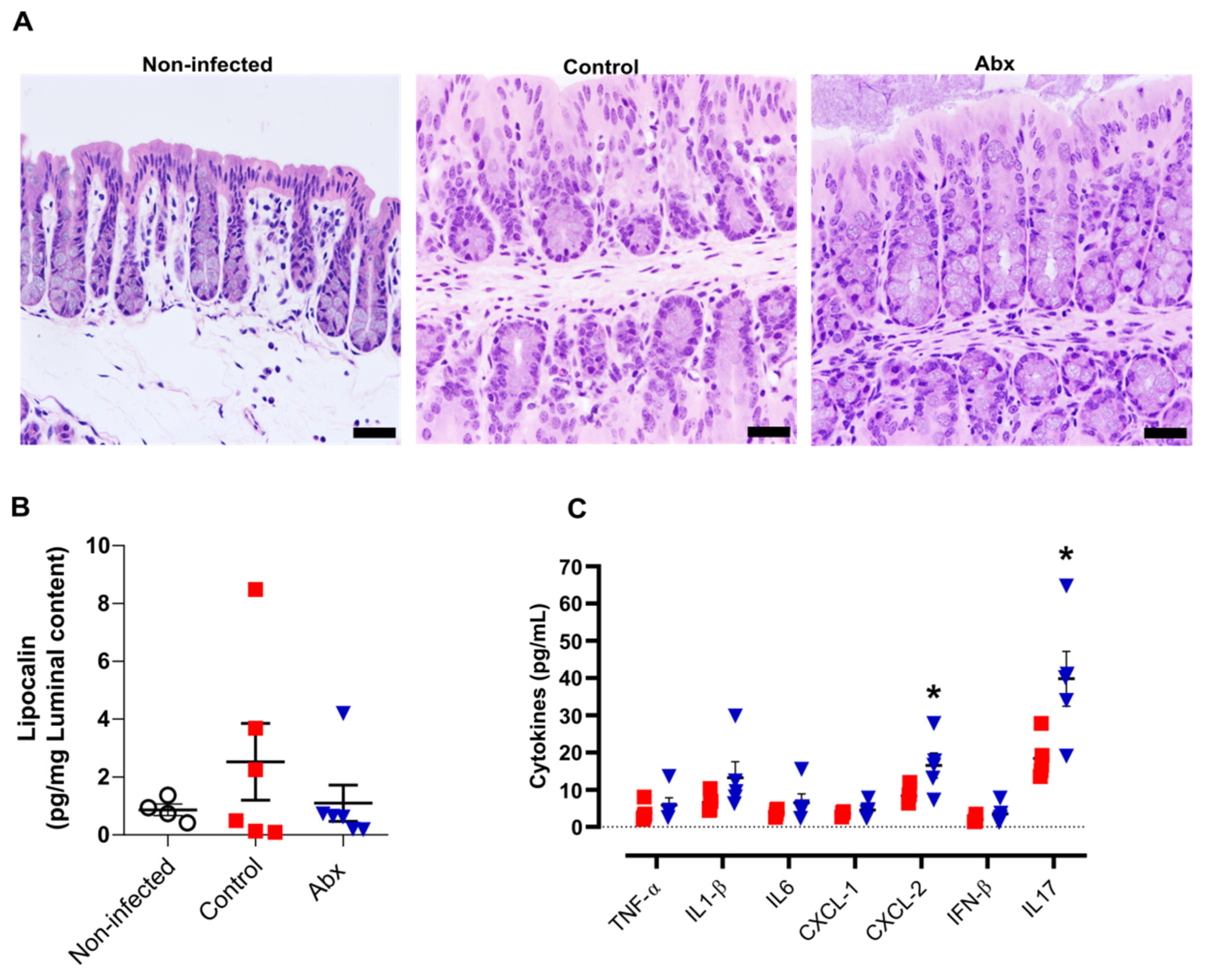
3.3. Antibiotic Treatment Increases Inflammatory Cytokines in Colon but This Effect Is Not Associated with Significant Alterations in Colon Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J.; Li, F.; Shi, Z.L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- COVID Live-Coronavirus Statistics–Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 26 May 2022).
- Yuen, K.S.; Ye, Z.W.; Fung, S.Y.; Chan, C.P.; Jin, D.Y. SARS-CoV-2 and COVID-19: The Most Important Research Questions. Cell Biosci. 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.E.; Adab, P.; Cheng, K.K. COVID-19: Risk Factors for Severe Disease and Death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Respiratory Medicine. Realising the Potential of SARS-CoV-2 Vaccines-a Long Shot? Lancet Respir. Med. 2021, 9, 117. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334.e27. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal Symptoms of 95 Cases with SARS-CoV-2 Infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, G.Y.; Myoung, J.; Kim, S.J.; Ahn, D.G. Robust and Persistent SARS-CoV-2 Infection in the Human Intestinal Brush Border Expressing Cells. Emerg. Microbes Infect. 2020, 9, 2169–2179. [Google Scholar] [CrossRef]
- Lamers, M.M.; Beumer, J.; Van Der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 Productively Infects Human Gut Enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Allers, K.; Heldt, C.; Meinhardt, J.; Schmidt, F.; Rodriguez-Sillke, Y.; Kunkel, D.; Schumann, M.; Böttcher, C.; Stahl-Hennig, C.; et al. Human Small Intestinal Infection by SARS-CoV-2 Is Characterized by a Mucosal Infiltration with Activated CD8 + T Cells. Mucosal Immunol. 2021, 14, 1381–1392. [Google Scholar] [CrossRef]
- Yantiss, R.K.; Qin, L.; He, B.; Crawford, C.V.; Seshan, S.; Patel, S.; Wahid, N.; Jessurun, J. Intestinal Abnormalities in Patients With SARS-CoV-2 Infection: Histopathologic Changes Reflect Mechanisms of Disease. Am. J. Surg. Pathol. 2022, 46, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Jansen, T.; Jacobs, L.; Bonder, M.J.; Kurilshikov, A.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, Metabolites, and the Gut-Lung Axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Antunes, K.H.; Stein, R.T.; Franceschina, C.; da Silva, E.F.; de Freitas, D.N.; Silveira, J.; Mocellin, M.; Leitão, L.; Fachi, J.L.; Pral, L.P.; et al. Short-Chain Fatty Acid Acetate Triggers Antiviral Response Mediated by RIG-I in Cells from Infants with Respiratory Syncytial Virus Bronchiolitis. EBioMedicine 2022, 77, 103891. [Google Scholar] [CrossRef]
- Song, L.; Huang, Y.; Liu, G.; Li, X.; Xiao, Y.; Liu, C.; Zhang, Y.; Li, J.; Xu, J.; Lu, S.; et al. A Novel Immunobiotics Bacteroides Dorei Ameliorates Influenza Virus Infection in Mice. Front. Immunol. 2022, 12, 828887. [Google Scholar] [CrossRef]
- Machado, M.G.; Patente, T.A.; Rouillé, Y.; Heumel, S.; Melo, E.M.; Deruyter, L.; Pourcet, B.; Sencio, V.; Teixeira, M.M.; Trottein, F. Acetate Improves the Killing of Streptococcus Pneumoniae by Alveolar Macrophages via NLRP3 Inflammasome and Glycolysis-HIF-1α Axis. Front. Immunol. 2022, 13, 773261. [Google Scholar] [CrossRef]
- Sencio, V.; Machado, M.G.; Trottein, F. The Lung-Gut Axis during Viral Respiratory Infections: The Impact of Gut Dysbiosis on Secondary Disease Outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 during Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.Y.; Zhang, F.; Liu, Q.; Li, A.Y.L.; Chung, A.C.K.; Cheung, C.P.; Tso, E.Y.K.; Fung, K.S.C.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates with Severe COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 747816. [Google Scholar] [CrossRef] [PubMed]
- Venzon, M.; Bernard-Raichon, L.; Klein, J.; Axelrad, J.E.; Zhang, C.; Hussey, G.A.; Sullivan, A.P.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut Microbiome Dysbiosis during COVID-19 Is Associated with Increased Risk for Bacteremia and Microbial Translocation. bioRxiv, 2022; preprint. [Google Scholar] [CrossRef]
- Sokol, H.; Contreras, V.; Maisonnasse, P.; Desmons, A.; Delache, B.; Sencio, V.; Machelart, A.; Brisebarre, A.; Humbert, L.; Deryuter, L.; et al. SARS-CoV-2 Infection in Nonhuman Primates Alters the Composition and Functional Activity of the Gut Microbiota. Gut Microbes 2021, 13, 1893113. [Google Scholar] [CrossRef]
- Sencio, V.; Machelart, A.; Robil, C.; Benech, N.; Hoffmann, E.; Galbert, C.; Deryuter, L.; Heumel, S.; Hantute-Ghesquier, A.; Flourens, A.; et al. Alteration of the Gut Microbiota Following SARS-CoV-2 Infection Correlates with Disease Severity in Hamsters. Gut Microbes 2022, 14, 2018900. [Google Scholar] [CrossRef]
- Seibert, B.; Cáceres, C.J.; Cardenas-Garcia, S.; Carnaccini, S.; Geiger, G.; Rajao, D.S.; Ottesen, E.; Perez, D.R. Mild and Severe SARS-CoV-2 Infection Induces Respiratory and Intestinal Microbiome Changes in the K18-HACE2 Transgenic Mouse Model. Microbiol. Spectr. 2021, 9, e00536-21. [Google Scholar] [CrossRef]
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; dos Santos, A.Á.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-Derived Acetate Protects against Respiratory Syncytial Virus Infection through a GPR43-Type 1 Interferon Response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef]
- Rocco, P.R.M.; Silva, P.L.; Cruz, F.F.; Melo, M.A.C.; Tierno, P.F.G.M.M.; Moura, M.A.; de Oliveira, L.F.G.; Lima, C.C.; Dos Santos, E.A.; Junior, W.F.; et al. Early Use of Nitazoxanide in Mild COVID-19 Disease: Randomised, Placebo-Controlled Trial. Eur. Respir. J. 2021, 58, 2003725. [Google Scholar] [CrossRef]
- Moreau, G.B.; Burgess, S.L.; Sturek, J.M.; Donlan, A.N.; Petri, W.A.; Mann, B.J. Evaluation of K18- HACE2 Mice as a Model of SARS-CoV-2 Infection. Am. J. Trop. Med. Hyg. 2020, 103, 1215–1219. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-HACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Andrews, S.; Krueger, F.; Segonds-Pichon, A.; Biggins, L.; Krueger, C.; Wingett, S. Babraham Bioinformatics-FastQC. A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 14 July 2022).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and Microbial Differential Abundance Strategies Depend upon Data Characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3, e00021-18. [Google Scholar] [CrossRef]
- Halko, N.; Martinsson, P.G.; Shkolnisky, Y.; Tygert, M. An Algorithm for the Principal Component Analysis of Large Data Sets. SIAM J. Sci. Comput. 2010, 33, 2580–2594. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 Years of Serving the Community with Ribosomal RNA Gene Reference Databases and Tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Euro Surveill. 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Yinda, C.K.; Port, J.R.; Bushmaker, T.; Owusu, I.O.; Purushotham, J.N.; Avanzato, V.A.; Fischer, R.J.; Schulz, J.E.; Holbrook, M.G.; Hebner, M.J.; et al. K18-HACE2 Mice Develop Respiratory Disease Resembling Severe COVID-19. PLoS Pathog. 2021, 17, e1009195. [Google Scholar] [CrossRef]
- Dong, W.; Mead, H.; Tian, L.; Park, J.-G.; Garcia, J.I.; Jaramillo, S.; Barr, T.; Kollath, D.S.; Coyne, V.K.; Stone, N.E.; et al. The K18-Human ACE2 Transgenic Mouse Model Recapitulates Non-Severe and Severe COVID-19 in Response to an Infectious Dose of the SARS-CoV-2 Virus. J. Virol. 2022, 96, e00964-21. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; Macfadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Bendala Estrada, A.D.; Calderón Parra, J.; Fernández Carracedo, E.; Muiño Míguez, A.; Ramos Martínez, A.; Muñez Rubio, E.; Rubio-Rivas, M.; Agudo, P.; Arnalich Fernández, F.; Estrada Perez, V.; et al. Inadequate Use of Antibiotics in the Covid-19 Era: Effectiveness of Antibiotic Therapy. BMC Infect. Dis. 2021, 21, 1144. [Google Scholar] [CrossRef]
- Dessein, R.; Bauduin, M.; Grandjean, T.; Le Guern, R.; Figeac, M.; Beury, D.; Faure, K.; Faveeuw, C.; Guery, B.; Gosset, P.; et al. Antibiotic-Related Gut Dysbiosis Induces Lung Immunodepression and Worsens Lung Infection in Mice. Crit. Care 2020, 24, 611. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota Regulates Immune Defense against Respiratory Tract Influenza A Virus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Thackray, L.B.; Handley, S.A.; Gorman, M.J.; Poddar, S.; Bagadia, P.; Briseño, C.G.; Theisen, D.J.; Tan, Q.; Hykes, B.L.; Lin, H.; et al. Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell Rep. 2018, 22, 3440–3453.e6. [Google Scholar] [CrossRef]
- Fumagalli, V.; Ravà, M.; Marotta, D.; Di Lucia, P.; Laura, C.; Sala, E.; Grillo, M.; Bono, E.; Giustini, L.; Perucchini, C.; et al. Administration of Aerosolized SARS-CoV-2 to K18-HACE2 Mice Uncouples Respiratory Infection from Fatal Neuroinvasion. Sci. Immunol. 2022, 7, eabl9929. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, L.; Richner, J.M.; Class, J.; Rehman, J.; Malik, A.B. Interleukin-1ra Mitigates Sars-Cov-2-Induced Inflammatory Lung Vascular Leakage and Mortality in Humanized K18-Hace-2 Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2773–2785. [Google Scholar] [CrossRef]
- Bessière, P.; Wasniewski, M.; Picard-Meyer, E.; Servat, A.; Figueroa, T.; Foret-Lucas, C.; Coggon, A.; Lesellier, S.; Boué, F.; Cebron, N.; et al. Intranasal Type I Interferon Treatment Is Beneficial Only When Administered before Clinical Signs Onset in the SARS-CoV-2 Hamster Model. PLoS Pathog. 2021, 17, e1009427. [Google Scholar] [CrossRef]
- Oliva, A.; Miele, M.C.; Di Timoteo, F.; De Angelis, M.; Mauro, V.; Aronica, R.; Al Ismail, D.; Ceccarelli, G.; Pinacchio, C.; d’Ettorre, G.; et al. Persistent Systemic Microbial Translocation and Intestinal Damage During Coronavirus Disease-19. Front. Immunol. 2021, 12, 708149. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Punjabi Katiyar, C.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and Fungal Coinfections in COVID-19 Patients Hospitalized during the New York City Pandemic Surge. Infect. Control. Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef]
- Watanabe, K.; Gilchrist, C.A.; Uddin, M.J.; Burgess, S.L.; Abhyankar, M.M.; Moonah, S.N.; Noor, Z.; Donowitz, J.R.; Schneider, B.N.; Arju, T.; et al. Microbiome-Mediated Neutrophil Recruitment via CXCR2 and Protection from Amebic Colitis. PLoS Pathog. 2017, 13, e1006513. [Google Scholar] [CrossRef]
- Fachi, J.L.; de Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; de Sales e Souza, É.L.; et al. Butyrate Protects Mice from Clostridium Difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761.e7. [Google Scholar] [CrossRef]
- Reeves, A.E.; Theriot, C.M.; Bergin, I.L.; Huffnagle, G.B.; Schloss, P.D.; Young, V.B. The Interplay between Microbiome Dynamics and Pathogen Dynamics in a Murine Model of Clostridium Difficile Infection. Gut Microbes 2011, 2, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, L.B.; Rodrigues, P.B.; Genaro, L.M.; dos Santos Pereira Gomes, A.B.; Toledo-Teixeira, D.A.; Parise, P.L.; Bispo-Dos-Santos, K.; Simeoni, C.L.; Guimarães, P.V.; Buscaratti, L.I.; et al. Microbiota-Derived Short-Chain Fatty Acids Do Not Interfere with SARS-CoV-2 Infection of Human Colonic Samples. Gut Microbes 2021, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Tan, J.; Niewold, P.; Spiteri, A.G.; Pinget, G.V.; Stanley, D.; King, N.J.C.; Macia, L. Impact of Dietary Fiber on West Nile Virus Infection. Front. Immunol. 2022, 13, 784486. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Morgan, M.E.; Chen, S.; Vos, A.P.; Garssen, J.; van Bergenhenegouwen, J.; Boon, L.; Georgiou, N.A.; Kraneveld, A.D.; Folkerts, G. Bifidobacterium Breve and Lactobacillus Rhamnosus Treatment Is as Effective as Budesonide at Reducing Inflammation in a Murine Model for Chronic Asthma. Respir. Res. 2014, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; Takahashi, H.; Kitazawa, H.; Villena, J. Alveolar Macrophages Are Key Players in the Modulation of the Respiratory Antiviral Immunity Induced by Orally Administered Lacticaseibacillus Rhamnosus CRL1505. Front. Immunol. 2020, 11, 568636. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Yang, Y.; Gong, W.; Sun, X.; Yang, L.; Zhang, Q.; Jin, M. Akkermansia Muciniphila Improves Host Defense Against Influenza Virus Infection. Front. Microbiol. 2021, 11, 586476. [Google Scholar] [CrossRef]
- Jang, Y.O.; Lee, S.H.; Choi, J.J.; Kim, D.H.; Choi, J.M.; Kang, M.J.; Oh, Y.M.; Park, Y.J.; Shin, Y.; Lee, S.W. Fecal Microbial Transplantation and a High Fiber Diet Attenuates Emphysema Development by Suppressing Inflammation and Apoptosis. Exp. Mol. Med. 2020, 52, 1128–1139. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c-Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef]
- Jang, Y.O.; Kim, O.H.; Kim, S.J.; Lee, S.H.; Yun, S.; Lim, S.E.; Yoo, H.J.; Shin, Y.; Lee, S.W. High-Fiber Diets Attenuate Emphysema Development via Modulation of Gut Microbiota and Metabolism. Sci. Rep. 2021, 11, 7008. [Google Scholar] [CrossRef]

| Clinical Parameters | Degree | Score Points |
|---|---|---|
| Body weight loss | Normal | 0 |
| <5% | 1 | |
| 6–10% | 2 | |
| 11–15% | 3 | |
| 16–20% | 4 | |
| >20% | 5 | |
| Appearance | No piloerection | 0 |
| Piloerection | 1 | |
| Spontaneous behavior | Alert | 0 |
| Slow-moving | 1 | |
| Lethargic | 2 | |
| Immobile | 3 | |
| Eyes | Normal | 0 |
| Squinted | 1 | |
| Closed | 2 | |
| Provoked behavior | Quickly moves away | 0 |
| Slow to move away | 1 | |
| Does not respond | 2 | |
| Breathing | Normal | 0 |
| Elevated | 1 |
| Gene 1 | Sequences |
|---|---|
| Tnfa | F: 5′-TCT TCT CAT TCC TGC TTG TGG C-3′ R: 5′-CAC TTG GTG GTT TGC TAC GAC G-3′ |
| Il1b | F: 5′-GGC AGC TAC CTG TGT CTT TCC C-3′ R: 5′-ATA TGG GTC CGA CAG CAC GAG-3′ |
| Cxcl2 | F: 5′-GGGACAAATAGCTGCAGTCGG-3′ R: 5′-CTACTCTCCTCGGTGCTTAC-3′ |
| Cxcl11 | F: 5′-CCGAGTAACGGCTGCGACAAAG-3′ R: 5′-CCTGCATTATGAGGCGAGCTTG-3′ |
| Ifna | F: 5-CCTGAGAGAGAAGAAACACAGCC-3 R:5-TCTGCTCTGACCACYTCCCAG-3 |
| Ifng | F: 5-ACTGGCAAAAGGATGGTGAC-3 R: 5-TGAGCTCATTGAATGCTTGG-3 |
| Ifnl2/3 | F: 5-AGC TGC AGG CCT TCA AAA AG-3 R: 5-TGG GAG TGA ATG TGG CTC AG-3 |
| Oasl1 | F: 5-GGATGCCTGGGAGAGAATCG-3 R: 5-TCGCCTGCTCTTCGAAACTG-3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.B.; Gomes, G.F.; Angelim, M.K.S.C.; Souza, G.F.; Muraro, S.P.; Toledo-Teixeira, D.A.; Rattis, B.A.C.; Passos, A.S.; Pral, L.P.; de Rezende Rodovalho, V.; et al. Impact of Microbiota Depletion by Antibiotics on SARS-CoV-2 Infection of K18-hACE2 Mice. Cells 2022, 11, 2572. https://doi.org/10.3390/cells11162572
Rodrigues PB, Gomes GF, Angelim MKSC, Souza GF, Muraro SP, Toledo-Teixeira DA, Rattis BAC, Passos AS, Pral LP, de Rezende Rodovalho V, et al. Impact of Microbiota Depletion by Antibiotics on SARS-CoV-2 Infection of K18-hACE2 Mice. Cells. 2022; 11(16):2572. https://doi.org/10.3390/cells11162572
Chicago/Turabian StyleRodrigues, Patrícia Brito, Giovanni Freitas Gomes, Monara K. S. C. Angelim, Gabriela F. Souza, Stefanie Primon Muraro, Daniel A. Toledo-Teixeira, Bruna Amanda Cruz Rattis, Amanda Stephane Passos, Laís Passarielo Pral, Vinícius de Rezende Rodovalho, and et al. 2022. "Impact of Microbiota Depletion by Antibiotics on SARS-CoV-2 Infection of K18-hACE2 Mice" Cells 11, no. 16: 2572. https://doi.org/10.3390/cells11162572
APA StyleRodrigues, P. B., Gomes, G. F., Angelim, M. K. S. C., Souza, G. F., Muraro, S. P., Toledo-Teixeira, D. A., Rattis, B. A. C., Passos, A. S., Pral, L. P., de Rezende Rodovalho, V., dos Santos P. Gomes, A. B., Matheus, V. A., Antunes, A. S. L. M., Crunfli, F., Antunes, K. H., de Souza, A. P. D., Consonni, S. R., Leiria, L. O., Alves-Filho, J. C., ... R. Vinolo, M. A. (2022). Impact of Microbiota Depletion by Antibiotics on SARS-CoV-2 Infection of K18-hACE2 Mice. Cells, 11(16), 2572. https://doi.org/10.3390/cells11162572









