Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation
Abstract
1. Introduction
| Main hypotheses of the review: |
|
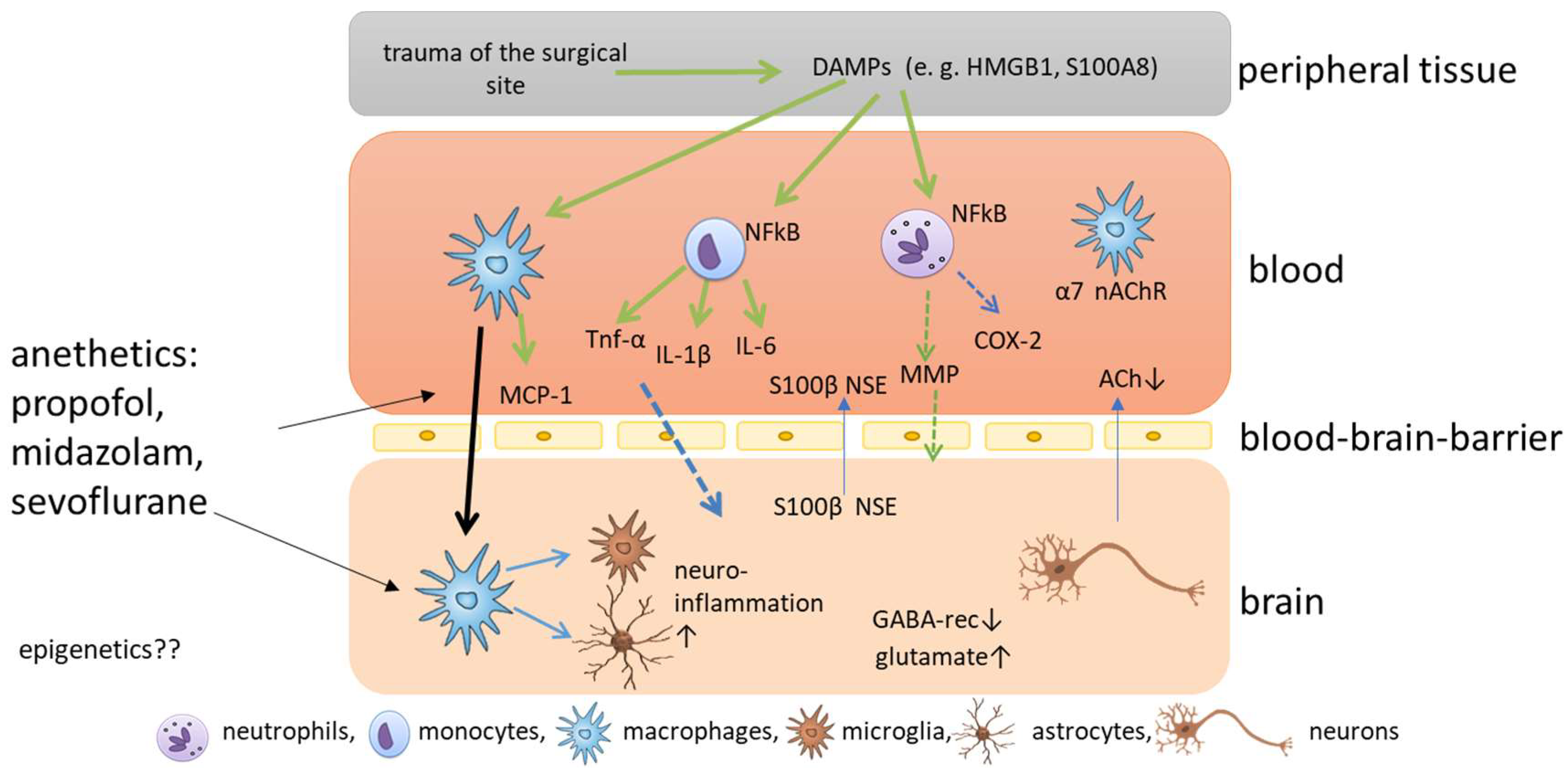
2. Molecular Mechanisms of Postoperative Cognitive Impairment
3. Anesthetics Related to Cognitive Impairment
4. Epigenetic Mechanisms
Epigenetics Mechanisms Induced by Anesthetics
5. Postoperative Cognitive Impairment and Epigenetic Mechanisms
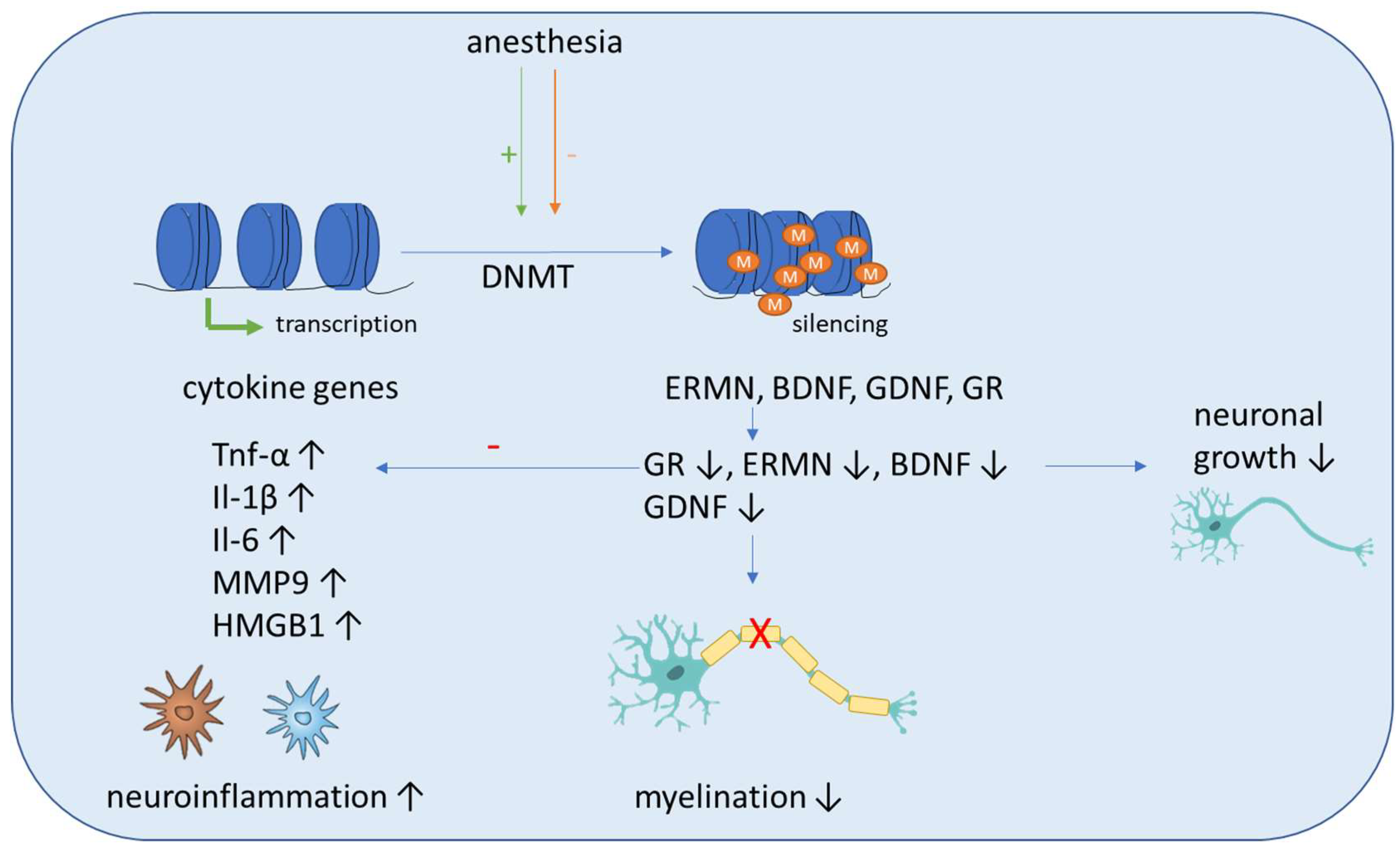
5.1. DNA Methylation
5.1.1. Sevoflurane Effects in Animal Studies
5.1.2. Isoflurane Anesthesia Effects in Animal Studies
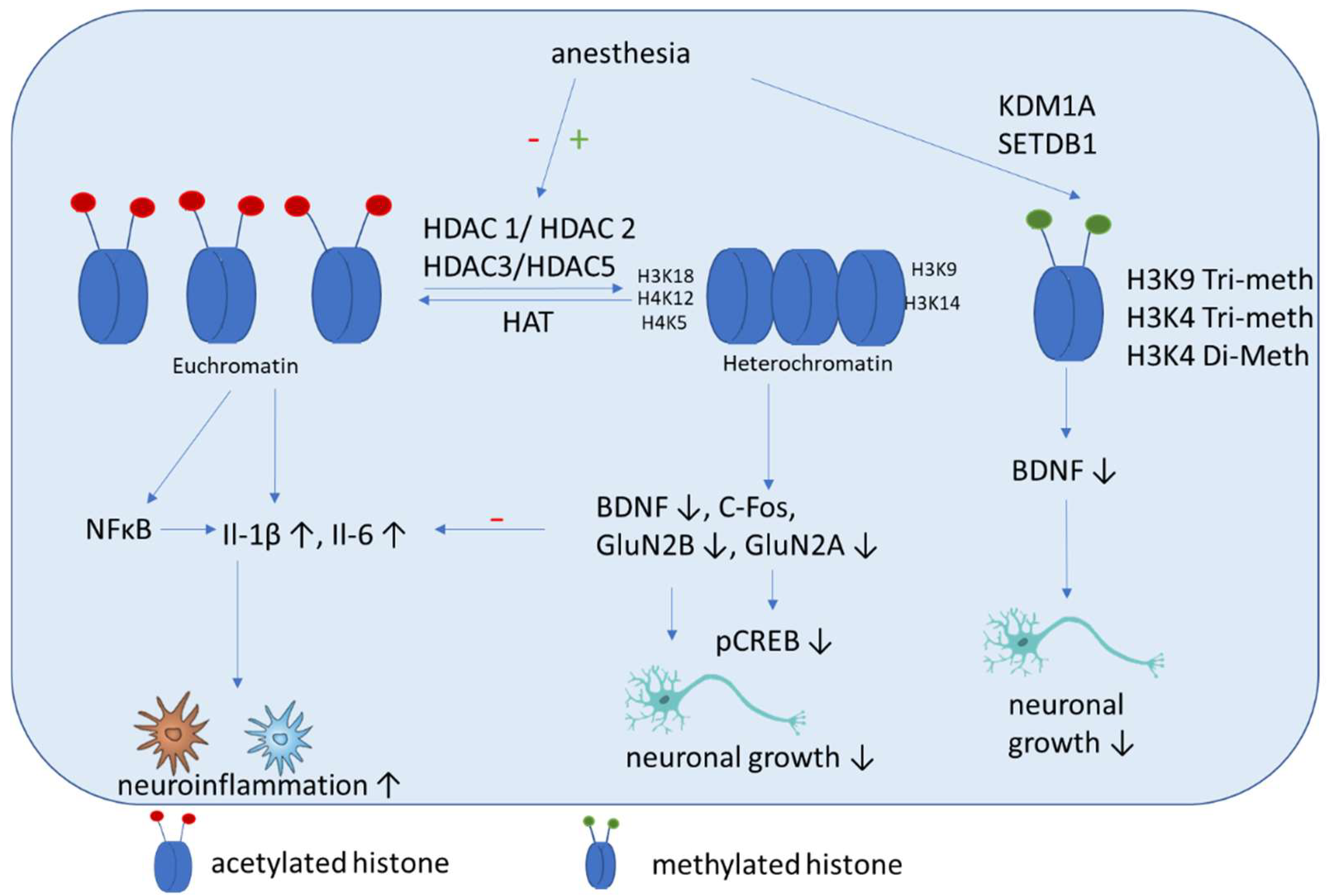
5.2. Histone Acetylation
5.2.1. Propofol-Induced Effects
5.2.2. Midazolam-Induced Effects
5.2.3. Isoflurane-Induced Effects
5.2.4. Sevoflurane-Induced Effects
5.3. Histone Methylation
6. Therapeutic Usage of Epigenetics in Postoperative Cognitive Impairment
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedford, P.D. Adverse cerebral effects of anaesthesia on old people. Lancet 1955, 269, 259–263. [Google Scholar] [CrossRef]
- Daiello, L.A.; Racine, A.M.; Yun Gou, R.; Marcantonio, E.R.; Xie, Z.; Kunze, L.J.; Vlassakov, K.V.; Inouye, S.K.; Jones, R.N.; Alsop, D.; et al. Postoperative Delirium and Postoperative Cognitive Dysfunction: Overlap and Divergence. Anesthesiology 2019, 131, 477–491. [Google Scholar] [CrossRef]
- Hartholt, K.A.; van der Cammen, T.J.; Klimek, M. Postoperative cognitive dysfunction in geriatric patients. Z Gerontol. Geriatr. 2012, 45, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.V.; Irkal, J.N.; Srinivasamurthy, A. Postoperative delirium in elderly citizens and current practice. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 291–299. [Google Scholar] [CrossRef] [PubMed]
- González, M.; de Pablo, J.; Valdés, M. [Delirium: The clinical confusion]. Rev. Med. Chil. 2003, 131, 1051–1060. [Google Scholar] [PubMed]
- Hogan, K.J. Hereditary vulnerabilities to post-operative cognitive dysfunction and dementia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 47, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Petersen, R.C. Mild cognitive impairment and mild dementia: A clinical perspective. Mayo Clin. Proc. 2014, 89, 1452–1459. [Google Scholar] [CrossRef]
- Devinney, M.J.; Mathew, J.P.; Berger, M. Postoperative Delirium and Postoperative Cognitive Dysfunction: Two Sides of the Same Coin? Anesthesiology 2018, 129, 389–391. [Google Scholar] [CrossRef] [PubMed]
- MacLullich, A.M.; Beaglehole, A.; Hall, R.J.; Meagher, D.J. Delirium and long-term cognitive impairment. Int. Rev. Psychiatry 2009, 21, 30–42. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Riker, R.R.; Fraser, G.L. Delirium-Beyond the CAM-ICU. Crit Care Med. 2020, 48, 134–136. [Google Scholar] [CrossRef]
- Saller, T.; Hofmann-Kiefer, K.F.; Saller, I.; Zwissler, B.; von Dossow, V. Implementation of strategies to prevent and treat postoperative delirium in the post-anesthesia caring unit: A German survey of current practice. J. Clin. Monit. Comput. 2021, 35, 599–605. [Google Scholar] [CrossRef]
- Maldonado, J.R. Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am. J. Geriatr. Psychiatry 2013, 21, 1190–1222. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, J.H.; Timberger, M.; Reich, D.L.; Uysal, S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology 2007, 106, 622–628. [Google Scholar] [CrossRef]
- Schaefer, S.T.; Koenigsperger, S.; Olotu, C.; Saller, T. Biomarkers and postoperative cognitive function: Could it be that easy? Curr. Opin. Anaesthesiol. 2019, 32, 92–100. [Google Scholar] [CrossRef]
- Monk, T.G.; Weldon, B.C.; Garvan, C.W.; Dede, D.E.; van der Aa, M.T.; Heilman, K.M.; Gravenstein, J.S. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008, 108, 18–30. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Y.; Zhang, P.; Chen, G.; Zhou, Y.; Yu, X. The potential mechanism of postoperative cognitive dysfunction in older people. Exp. Gerontol. 2020, 130, 110791. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Terrando, N.; Smith, S.K.; Browndyke, J.N.; Newman, M.F.; Mathew, J.P. Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology 2018, 129, 829–851. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhao, Y.; Shi, R.; Wang, Y.; Xu, J.; Wu, A.; Johns, R.A.; Yue, Y. Epigenetics as a new therapeutic target for postoperative cognitive dysfunction. Med. Hypotheses 2013, 80, 249–251. [Google Scholar] [CrossRef]
- Joris, J.; Kehlet, H.; Slim, K. Postoperative cognitive dysfunction: Time for enhanced recovery after surgery programmes. Eur. J. Anaesthesiol. 2022, 39, 733–734. [Google Scholar] [CrossRef]
- Vutskits, L.; Xie, Z. Lasting impact of general anaesthesia on the brain: Mechanisms and relevance. Nat. Rev. Neurosci. 2016, 17, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.E.; Hemmings, H.C., Jr. Are anaesthetics toxic to the brain? Br. J. Anaesth 2011, 107, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Safavynia, S.A.; Goldstein, P.A. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front Psychiatry 2018, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Dunne, S.S.; Coffey, J.C.; Konje, S.; Gasior, S.; Clancy, C.C.; Gulati, G.; Meagher, D.; Dunne, C.P. Biomarkers in delirium: A systematic review. J. Psychosom. Res. 2021, 147, 110530. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Y.; Zhu, S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS ONE 2018, 13, e0195659. [Google Scholar] [CrossRef] [PubMed]
- Androsova, G.; Krause, R.; Winterer, G.; Schneider, R. Biomarkers of postoperative delirium and cognitive dysfunction. Front. Aging Neurosci. 2015, 7, 112. [Google Scholar] [CrossRef]
- Müller, A.; Olbert, M.; Heymann, A.; Zahn, P.K.; Plaschke, K.; von Dossow, V.; Bitzinger, D.; Barth, E.; Meister, M.; Kranke, P.; et al. Relevance of peripheral cholinesterase activity on postoperative delirium in adult surgical patients (CESARO): A prospective observational cohort study. Eur. J. Anaesthesiol. 2019, 36, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Hana, Z.; Jin, Z.; Suen, K.C.; Ma, D. Surgery, neuroinflammation and cognitive impairment. EBio. Med. 2018, 37, 547–556. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Wang, Y.; Le, Y.; Duan, K.M.; Yan, X.B.; Liao, Q.; Liao, Y.; Tong, J.B.; Terrando, N.; Ouyang, W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012, 18, 994–1002. [Google Scholar] [CrossRef]
- Yang, H.; Tracey, K.J. Targeting HMGB1 in inflammation. Biochim. Biophys Acta 2010, 1799, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Skvarc, D.R.; Berk, M.; Byrne, L.K.; Dean, O.M.; Dodd, S.; Lewis, M.; Marriott, A.; Moore, E.M.; Morris, G.; Page, R.S.; et al. Post-Operative Cognitive Dysfunction: An exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav. Rev. 2018, 84, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Terrando, N.; Eriksson, L.I.; Ryu, J.K.; Yang, T.; Monaco, C.; Feldmann, M.; Jonsson Fagerlund, M.; Charo, I.F.; Akassoglou, K.; Maze, M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Tickets to the brain: Role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J. Neuroimmunol. 2010, 224, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Zhou, H.; Zhang, F.; Wilson, B.C.; Kam, W.; Hong, J.S. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J. Neurosci. 2011, 31, 1081–1092. [Google Scholar] [CrossRef]
- Bayram, H.; Hidiroglu, M.; Cetin, L.; Kucuker, A.; Iriz, E.; Uguz, E.; Saglam, F.; Sener, E. Comparing S-100 beta protein levels and neurocognitive functions between patients undergoing on-pump and off-pump coronary artery bypass grafting. J. Surg. Res. 2013, 182, 198–202. [Google Scholar] [CrossRef]
- Pribiag, H.; Stellwagen, D. TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J. Neurosci. 2013, 33, 15879–15893. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Xiong, J.; Xue, F.S.; Liu, J.H.; Xu, Y.C.; Liao, X.; Zhang, Y.M.; Wang, W.L.; Li, S. Transcutaneous vagus nerve stimulation may attenuate postoperative cognitive dysfunction in elderly patients. Med. Hypotheses 2009, 73, 938–941. [Google Scholar] [CrossRef]
- Kalb, A.; von Haefen, C.; Sifringer, M.; Tegethoff, A.; Paeschke, N.; Kostova, M.; Feldheiser, A.; Spies, C.D. Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS ONE 2013, 8, e62679. [Google Scholar] [CrossRef]
- Schiemann, A.; Hadzidiakos, D.; Spies, C. Managing ICU delirium. Curr. Opin. Crit. Care 2011, 17, 131–140. [Google Scholar] [CrossRef]
- Rundshagen, I. Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 2014, 111, 119–125. [Google Scholar] [CrossRef]
- Cui, Y.; Li, G.; Cao, R.; Luan, L.; Kla, K.M. The effect of perioperative anesthetics for prevention of postoperative delirium on general anesthesia: A network meta-analysis. J. Clin. Anesth. 2020, 59, 89–98. [Google Scholar] [CrossRef]
- Carr, Z.J.; Cios, T.J.; Potter, K.F.; Swick, J.T. Does Dexmedetomidine Ameliorate Postoperative Cognitive Dysfunction? A Brief Review of the Recent Literature. Curr. Neurol. Neurosci. Rep. 2018, 18, 64. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.B. Which medications to avoid in people at risk of delirium: A systematic review. Age Ageing 2011, 40, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.J.; Pandharipande, P.P.; Hughes, C.G. Intensive Care Unit Delirium: A Review of Diagnosis, Prevention, and Treatment. Anesthesiology 2016, 125, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Kowark, A.; Rossaint, R.; Keszei, A.P.; Bischoff, P.; Czaplik, M.; Drexler, B.; Kienbaum, P.; Kretzschmar, M.; Rex, C.; Saller, T.; et al. Impact of PReOperative Midazolam on OuTcome of Elderly patients (I-PROMOTE): Study protocol for a multicentre randomised controlled trial. Trials 2019, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.; Meng, G.; Xu, G.; Cheng, X.; Chen, S.; Zhang, Y.; Zhang, M.; Liu, X.; Gu, E. Intraoperative Sedation With Dexmedetomidine is Superior to Propofol for Elderly Patients Undergoing Hip Arthroplasty: A Prospective Randomized Controlled Study. Clin. J. Pain 2018, 34, 811–817. [Google Scholar] [CrossRef]
- Dressler, I.; Fritzsche, T.; Cortina, K.; Pragst, F.; Spies, C.; Rundshagen, I. Psychomotor dysfunction after remifentanil/propofol anaesthesia. Eur. J. Anaesthesiol. 2007, 24, 347–354. [Google Scholar] [CrossRef]
- Kapoor, I.; Prabhakar, H.; Mahajan, C. Postoperative Cognitive Dysfunction. Indian J. Crit. Care Med. 2019, 23, S162–S164. [Google Scholar] [CrossRef]
- Ali, Z.; Prabhakar, H.; Bithal, P.K.; Dash, H.H. Bispectral index-guided administration of anesthesia for transsphenoidal resection of pituitary tumors: A comparison of 3 anesthetic techniques. J. Neurosurg. Anesthesiol. 2009, 21, 10–15. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Li, X.B.; Yang, Z.X.; Zhou, J.M.; Wu, Y.N.; Zhao, Z.H.; Liu, X.Z.; Hu, C.L. Impact of inhalational anesthetics on postoperative cognitive function: Study protocol of a systematic review and meta-analysis. Med. Baltim. 2018, 97, e9316. [Google Scholar] [CrossRef]
- Cortese, G.P.; Burger, C. Neuroinflammatory challenges compromise neuronal function in the aging brain: Postoperative cognitive delirium and Alzheimer’s disease. Behav. Brain Res. 2017, 322, 269–279. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, P. Epigenetic Alterations in Anesthesia-Induced Neurotoxicity in the Developing Brain. Front Physiol. 2018, 9, 1024. [Google Scholar] [CrossRef]
- Gao, X.; Mi, Y.; Guo, N.; Luan, J.; Xu, H.; Hu, Z.; Wang, N.; Zhang, D.; Gou, X.; Xu, L. The mechanism of propofol in cancer development: An updated review. Asia Pac. J. Clin. Oncol. 2020, 16, e3–e11. [Google Scholar] [CrossRef]
- Freire, C.M.M.; Braz, M.G.; Marcondes, J.P.C.; Arruda, N.M.; Braz, J.R.C.; Rainho, C.A.; Braz, L.G.; Salvadori, D.M.F. Expression and promoter methylation status of two DNA repair genes in leukocytes from patients undergoing propofol or isoflurane anaesthesia. Mutagenesis 2018, 33, 147–152. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Yan, J.; Yang, Y.; Sun, Y.; Huang, Y.; Hu, R.; Zhang, Y.; Jiang, H. Sevoflurane attenuate hypoxia-induced VEGF level in tongue squamous cell carcinoma cell by upregulating the DNA methylation states of the promoter region. Biomed Pharm. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Li, Q.; Mathena, R.P.; Xu, J.; Eregha, O.N.; Wen, J.; Mintz, C.D. Early Postnatal Exposure to Isoflurane Disrupts Oligodendrocyte Development and Myelin Formation in the Mouse Hippocampus. Anesthesiology 2019, 131, 1077–1091. [Google Scholar] [CrossRef]
- Liu, Y.; Song, F.; Yang, Y.; Yang, S.; Jiang, M.; Zhang, W.; Ma, Z.; Gu, X. Mitochondrial DNA methylation drift and postoperative delirium in mice. Eur. J. Anaesthesiol. 2022, 39, 133–144. [Google Scholar] [CrossRef]
- Schenning, K.J.; Holden, S.; Davis, B.A.; Mulford, A.; Nevonen, K.A.; Quinn, J.F.; Raber, J.; Carbone, L.; Alkayed, N.J. Gene-Specific DNA Methylation Linked to Postoperative Cognitive Dysfunction in Apolipoprotein E3 and E4 Mice. J. Alzheimers Dis. 2021, 83, 1251–1268. [Google Scholar] [CrossRef]
- Liang, B.; Fang, J. Postnatal Isoflurane Exposure Induces Cognitive Impairment and Abnormal Histone Acetylation of Glutamatergic Systems in the Hippocampus of Adolescent Rats. J. Mol. Neurosci. 2016, 60, 11–20. [Google Scholar] [CrossRef]
- Sen, T.; Sen, N. Isoflurane-induced inactivation of CREB through histone deacetylase 4 is responsible for cognitive impairment in developing brain. Neurobiol. Dis. 2016, 96, 12–21. [Google Scholar] [CrossRef]
- Yang, C.X.; Bao, F.; Zhong, J.; Zhang, L.; Deng, L.B.; Sha, Q.; Jiang, H. The inhibitory effects of class I histone deacetylases on hippocampal neuroinflammatory regulation in aging mice with postoperative cognitive dysfunction. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10194–10202. [Google Scholar] [CrossRef]
- Chen, L.; Xie, W.; Xie, W.; Zhuang, W.; Jiang, C.; Liu, N. Apigenin attenuates isoflurane-induced cognitive dysfunction via epigenetic regulation and neuroinflammation in aged rats. Arch. Gerontol. Geriatr. 2017, 73, 29–36. [Google Scholar] [CrossRef]
- Luo, F.; Min, J.; Wu, J.; Zuo, Z. Histone Deacetylases May Mediate Surgery-Induced Impairment of Learning, Memory, and Dendritic Development. Mol. Neurobiol. 2020, 57, 3702–3711. [Google Scholar] [CrossRef]
- Min, J.; Lai, Z.; Wang, H.; Zuo, Z. Preoperative environment enrichment preserved neuroligin 1 expression possibly via epigenetic regulation to reduce postoperative cognitive dysfunction in mice. CNS Neurosci. Ther. 2022, 28, 619–629. [Google Scholar] [CrossRef]
- Martynyuk, A.E.; Ju, L.S.; Morey, T.E. The potential role of stress and sex steroids in heritable effects of sevoflurane†. Biol. Reprod 2021, 105, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, J.; Zhang, P.; Wang, J.; Cui, J.; Wang, H. Enriched environment improves sevoflurane-induced cognitive impairment during late-pregnancy via hippocampal histone acetylation. Braz. J. Med. Biol. Res. 2020, 53, e9861. [Google Scholar] [CrossRef]
- Li, G.; Du, J.; Wang, L.; Shi, X. Developmental neurotoxicity in the context of multiple sevoflurane exposures: Potential role of histone deacetylase 6. Neurotoxicol. Teratol. 2019, 74, 106813. [Google Scholar] [CrossRef]
- Braz, M.G.; Braz, L.G.; Mazoti, M.A.; Pinotti, M.F.; Pardini, M.I.; Braz, J.R.; Salvadori, D.M. Lower levels of oxidative DNA damage and apoptosis in lymphocytes from patients undergoing surgery with propofol anesthesia. Environ. Mol. Mutagen. 2012, 53, 70–77. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, Y.; Zeng, Y.; Zhang, X.P.; Chen, J.Y. Dexmedetomidine reduces propofol-induced hippocampal neuron injury by modulating the miR-377-5p/Arc pathway. BMC Pharmacol. Toxicol. 2022, 23, 18. [Google Scholar] [CrossRef]
- Holownia, A.; Mroz, R.M.; Wielgat, P.; Skiepko, A.; Sitko, E.; Jakubow, P.; Kolodziejczyk, A.; Braszko, J.J. Propofol protects rat astroglial cells against tert-butyl hydroperoxide-induced cytotoxicity; the effect on histone and cAMP-response-element-binding protein (CREB) signalling. J. Physiol. Pharmacol. 2009, 60, 63–69. [Google Scholar] [PubMed]
- Chuang, S.M.; Lu, J.H.; Lin, K.L.; Long, C.Y.; Lee, Y.C.; Hsiao, H.P.; Tsai, C.C.; Wu, W.J.; Yang, H.J.; Juan, Y.S. Epigenetic regulation of COX-2 expression by DNA hypomethylation via NF-κB activation in ketamine-induced ulcerative cystitis. Int. J. Mol. Med. 2019, 44, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.S.; Yang, J.J.; Lei, L.; Xia, J.Y.; Luo, D.; Ji, M.H.; Martynyuk, A.E.; Yang, J.J. The Combination of Long-term Ketamine and Extinction Training Contributes to Fear Erasure by Bdnf Methylation. Front Cell Neurosci. 2017, 11, 100. [Google Scholar] [CrossRef]
- Li, X.; Saiyin, H.; Zhou, J.H.; Yu, Q.; Liang, W.M. HDAC6 is critical for ketamine-induced impairment of dendritic and spine growth in GABAergic projection neurons. Acta Pharmacol. Sin. 2021, 42, 861–870. [Google Scholar] [CrossRef]
- Liu, Y.F.; Hu, R.; Zhang, L.F.; Fan, Y.; Xiao, J.F.; Liao, X.Z. Effects of dexmedetomidine on cognitive dysfunction and neuroinflammation via the HDAC2/HIF-1α/PFKFB3 axis in a murine model of postoperative cognitive dysfunction. J. Biochem. Mol. Toxicol. 2022, 36, e23044. [Google Scholar] [CrossRef]
- Caputi, F.F.; Carboni, L.; Rullo, L.; Alessandrini, I.; Balzani, E.; Melotti, R.M.; Romualdi, P.; Candeletti, S.; Fanelli, A. An Exploratory Pilot Study of Changes in Global DNA Methylation in Patients Undergoing Major Breast Surgery Under Opioid-Based General Anesthesia. Front Pharmacol. 2021, 12, 733577. [Google Scholar] [CrossRef]
- Gong, S.; Ying, L.; Fan, Y.; Sun, Z. Fentanyl Inhibits Lung Cancer Viability and Invasion via Upregulation of miR-331-3p and Repression of HDAC5. Onco. Targets Ther. 2020, 13, 13131–13141. [Google Scholar] [CrossRef]
- Echavarría, R.; Garcia, D.; Figueroa, F.; Franco-Acevedo, A.; Palomino, J.; Portilla-Debuen, E.; Goldaraz-Monraz, M.P.; Moreno-Carranza, B.; Melo, Z. Anesthetic preconditioning increases sirtuin 2 gene expression in a renal ischemia reperfusion injury model. Minerva. Urol. Nefrol. 2020, 72, 243–249. [Google Scholar] [CrossRef]
- Li, T.; Huang, Z.; Wang, X.; Zou, J.; Tan, S. Role of the GABAA receptors in the long-term cognitive impairments caused by neonatal sevoflurane exposure. Rev. Neurosci. 2019, 30, 869–879. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Naguib, M. Epigenetic Manipulation of Brain-derived Neurotrophic Factor Improves Memory Deficiency Induced by Neonatal Anesthesia in Rats. Anesthesiology 2016, 124, 624–640. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Yamanashi, T.; Nagao, T.; Wahba, N.E.; Marra, P.S.; Crutchley, K.J.; Meyer, A.A.; Andreasen, A.J.; Hellman, M.M.; Jellison, S.S.; Hughes, C.G.; et al. DNA methylation in the inflammatory genes after neurosurgery and diagnostic ability of post-operative delirium. Transl. Psychiatry 2021, 11, 627. [Google Scholar] [CrossRef]
- Li, H.; Wu, T.T.; Tang, L.; Liu, Q.; Mao, X.Z.; Xu, J.M.; Dai, R.P. Association of global DNA hypomethylation with post-operative cognitive dysfunction in elderly patients undergoing hip surgery. Acta Anaesthesiol. Scand. 2020, 64, 354–360. [Google Scholar] [CrossRef]
- Sadahiro, R.; Knight, B.; James, F.; Hannon, E.; Charity, J.; Daniels, I.R.; Burrage, J.; Knox, O.; Crawford, B.; Smart, N.J.; et al. Major surgery induces acute changes in measured DNA methylation associated with immune response pathways. Sci. Rep. 2020, 10, 5743. [Google Scholar] [CrossRef]
- Shinozaki, G.; Braun, P.R.; Hing, B.W.Q.; Ratanatharathorn, A.; Klisares, M.J.; Duncan, G.N.; Jellison, S.S.; Heinzman, J.T.; Nagahama, Y.; Close, L.; et al. Epigenetics of Delirium and Aging: Potential Role of DNA Methylation Change on Cytokine Genes in Glia and Blood Along With Aging. Front Aging Neurosci. 2018, 10, 311. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, Z.; Liu, Q.; Liu, Y.; Xi, S.; Cheng, Y.; Li, J.; Yan, J.; Shen, Y.; Xiao, C.; et al. Disrupted folate metabolism with anesthesia leads to myelination deficits mediated by epigenetic regulation of ERMN. EBio Med. 2019, 43, 473–486. [Google Scholar] [CrossRef]
- Neal, M.; Richardson, J.R. Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim. Biophys Acta Mol. Basis Dis. 2018, 1864, 432–443. [Google Scholar] [CrossRef]
- Barter, J.D.; Foster, T.C. Aging in the Brain: New Roles of Epigenetics in Cognitive Decline. Neuroscientist 2018, 24, 516–525. [Google Scholar] [CrossRef]
- Wang, J.Y.; Feng, Y.; Fu, Y.H.; Liu, G.L. Effect of Sevoflurane Anesthesia on Brain Is Mediated by lncRNA HOTAIR. J. Mol. Neurosci. 2018, 64, 346–351. [Google Scholar] [CrossRef]
- Zhong, J.; Xu, W. Characterization of DNA hydroxymethylation in the hypothalamus of elderly mice with post-operative cognitive dysfunction. Exp. Ther. Med. 2019, 18, 4002–4010. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Yao, R.; Hao, T.; Cao, J.; Huang, H.; Wang, L.; Wu, Y. Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period. J. Neuroinflammation 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Chastain-Potts, S.E.; Tesic, V.; Tat, Q.L.; Cabrera, O.H.; Quillinan, N.; Jevtovic-Todorovic, V. Sevoflurane Exposure Results in Sex-Specific Transgenerational Upregulation of Target IEGs in the Subiculum. Mol. Neurobiol. 2020, 57, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Klenke, S.; Specking, C.; Stegen, M.; Engler, A.; Peters, J. Methylation in HT22 cells and primary hippocampal neurons with and without isoflurane exposurewhether isoflurane causes. BMC Anesthesiol. 2020, 20, 66. [Google Scholar] [CrossRef]
- Grégoire, S.; Millecamps, M.; Naso, L.; Do Carmo, S.; Cuello, A.C.; Szyf, M.; Stone, L.S. Therapeutic benefits of the methyl donor S-adenosylmethionine on nerve injury-induced mechanical hypersensitivity and cognitive impairment in mice. Pain 2017, 158, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Gräff, J.; Tsai, L.H. Histone acetylation: Molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 2013, 14, 97–111. [Google Scholar] [CrossRef]
- Ji, M.; Dong, L.; Jia, M.; Liu, W.; Zhang, M.; Ju, L.; Yang, J.; Xie, Z.; Yang, J. Epigenetic enhancement of brain-derived neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol. Neurobiol. 2014, 50, 937–944. [Google Scholar] [CrossRef]
- Dalla Massara, L.; Osuru, H.P.; Oklopcic, A.; Milanovic, D.; Joksimovic, S.M.; Caputo, V.; DiGruccio, M.R.; Ori, C.; Wang, G.; Todorovic, S.M.; et al. General Anesthesia Causes Epigenetic Histone Modulation of c-Fos and Brain-derived Neurotrophic Factor, Target Genes Important for Neuronal Development in the Immature Rat Hippocampus. Anesthesiology 2016, 124, 1311–1327. [Google Scholar] [CrossRef]
- Garden, G.A. Epigenetics and the modulation of neuroinflammation. Neurotherapeutics 2013, 10, 782–788. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Feng, Y.; Zhao, W.; Zhao, W.; Luo, F.; Feng, N. Propofol exposure during early gestation impairs learning and memory in rat offspring by inhibiting the acetylation of histone. J. Cell Mol. Med. 2018, 22, 2600–2611. [Google Scholar] [CrossRef]
- Guo, X.; Deng, J.; Zheng, B.; Liu, H.; Zhang, Y.; Ying, Y.; Jia, J.; Ruan, X. HDAC1 and HDAC2 regulate anti-inflammatory effects of anesthetic isoflurane in human monocytes. Immunol. Cell Biol. 2020, 98, 318–331. [Google Scholar] [CrossRef]
- Zhong, T.; Guo, Q.; Zou, W.; Zhu, X.; Song, Z.; Sun, B.; He, X.; Yang, Y. Neonatal isoflurane exposure induces neurocognitive impairment and abnormal hippocampal histone acetylation in mice. PLoS ONE 2015, 10, e0125815. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, M.; Wang, J.; Cao, H.; Niu, W.; Du, L. Paeonol attenuates isoflurane anesthesia-induced hippocampal neurotoxicity via modulation of JNK/ERK/P38MAPK pathway and regulates histone acetylation in neonatal rat. J. Matern. Fetal. Neonatal. Med. 2020, 33, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, J.R.; Gao, Y.; Yang, X.; Shen, X.R.; Wang, H.Y.; Sun, N.; Gao, C. HDAC3 of dorsal hippocampus induces postoperative cognitive dysfunction in aged mice. Behav. Brain Res. 2022, 433, 114002. [Google Scholar] [CrossRef]
- Zhong, T.; Ren, F.; Huang, C.S.; Zou, W.Y.; Yang, Y.; Pan, Y.D.; Sun, B.; Wang, E.; Guo, Q.L. Swimming exercise ameliorates neurocognitive impairment induced by neonatal exposure to isoflurane and enhances hippocampal histone acetylation in mice. Neuroscience 2016, 316, 378–388. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, J.M.T.; Ran, Y.; Zhang, Y.; Chang, R.C.C.; Wong, G.T.C. Prehabilitative resistance exercise reduces neuroinflammation and improves mitochondrial health in aged mice with perioperative neurocognitive disorders. J. Neuroinflammation 2022, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Wu, J.; Fang, R.; Liu, Y.; Wang, X.; Wang, X.; Zhang, J.; Zhou, J.; Jiang, Z.; Yi, H.; et al. Sevoflurane inhibits histone acetylation and contributes to cognitive dysfunction by enhancing the expression of ANP32A in aging mice. Behav. Brain Res. 2022, 431, 113949. [Google Scholar] [CrossRef] [PubMed]
- Hsing, C.H.; Hung, S.K.; Chen, Y.C.; Wei, T.S.; Sun, D.P.; Wang, J.J.; Yeh, C.H. Histone Deacetylase Inhibitor Trichostatin A Ameliorated Endotoxin-Induced Neuroinflammation and Cognitive Dysfunction. Mediat. Inflamm. 2015, 2015, 163140. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, B.; You, J.; Zhang, B.; Chai, G. Suberoylanilide hydroxamic acid reversed cognitive and synaptic plasticity impairments induced by sevoflurane exposure in adult mice. Neuroreport 2019, 30, 274–279. [Google Scholar] [CrossRef]
- Santos-Rosa, H.; Schneider, R.; Bannister, A.J.; Sherriff, J.; Bernstein, B.E.; Emre, N.C.; Schreiber, S.L.; Mellor, J.; Kouzarides, T. Active genes are tri-methylated at K4 of histone H3. Nature 2002, 419, 407–411. [Google Scholar] [CrossRef]
- Wu, T.; Sun, X.Y.; Yang, X.; Liu, L.; Tong, K.; Gao, Y.; Hao, J.R.; Cao, J.; Gao, C. Histone H3K9 Trimethylation Downregulates the Expression of Brain-Derived Neurotrophic Factor in the Dorsal Hippocampus and Impairs Memory Formation During Anaesthesia and Surgery. Front Mol. Neurosci. 2019, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, C.; Koos, B.; Unterberg, M.; Rahmel, T.; Bergmann, L.; Bazzi, Z.; Bazzi, M.; Bukhari, H.; Adamzik, M.; Rump, K. A novel understanding of postoperative complications: In vitro study of the impact of propofol on epigenetic modifications in cholinergic genes. PLoS ONE 2019, 14, e0217269. [Google Scholar] [CrossRef]
- Rump, K.; Holtkamp, C.; Bergmann, L.; Nowak, H.; Unterberg, M.; Orlowski, J.; Thon, P.; Bazzi, Z.; Bazzi, M.; Adamzik, M.; et al. Midazolam impacts acetyl-And butyrylcholinesterase genes: An epigenetic explanation for postoperative delirium? PLoS ONE 2022, 17, e0271119. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, P.; Chuwdhury, G.S.; Sun, Z.; Chen, Y.; Dong, H.; Ahmed, F.F.; Nana, L.; Rahman, M.H.; Qian, Y. Network Biology Approaches to Uncover Therapeutic Targets Associated with Molecular Signaling Pathways from circRNA in Postoperative Cognitive Dysfunction Pathogenesis. J. Mol. Neurosci 2022, 72, 1875–1901. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yao, R.Q.; Li, Y.X.; Zhao, P.Y.; Ren, C.; Du, X.H.; Yao, Y.M. The Role and Regulatory Mechanism of Transcription Factor EB in Health and Diseases. Front Cell Dev. Biol. 2021, 9, 667750. [Google Scholar] [CrossRef]
- Adwan, L.; Zawia, N.H. Epigenetics: A novel therapeutic approach for the treatment of Alzheimer’s disease. Pharmacol. Ther. 2013, 139, 41–50. [Google Scholar] [CrossRef]
- Coppedè, F. The potential of epigenetic therapies in neurodegenerative diseases. Front Genet. 2014, 5, 220. [Google Scholar] [CrossRef]
- Wu, Y.; Dou, J.; Wan, X.; Leng, Y.; Liu, X.; Chen, L.; Shen, Q.; Zhao, B.; Meng, Q.; Hou, J. Histone Deacetylase Inhibitor MS-275 Alleviates Postoperative Cognitive Dysfunction in Rats by Inhibiting Hippocampal Neuroinflammation. Neuroscience 2019, 417, 70–80. [Google Scholar] [CrossRef]
- Jia, M.; Liu, W.X.; Sun, H.L.; Chang, Y.Q.; Yang, J.J.; Ji, M.H.; Yang, J.J.; Feng, C.Z. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, attenuates postoperative cognitive dysfunction in aging mice. Front Mol. Neurosci. 2015, 8, 52. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zheng, T.; Yang, X.; Liu, L.; Gao, S.S.; Xu, H.B.; Song, Y.T.; Tong, K.; Yang, L.; Gao, Y.; et al. HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J. Neuroinflammation 2019, 16, 249. [Google Scholar] [CrossRef]
- Joksimovic, S.M.; Osuru, H.P.; Oklopcic, A.; Beenhakker, M.P.; Jevtovic-Todorovic, V.; Todorovic, S.M. Histone Deacetylase Inhibitor Entinostat (MS-275) Restores Anesthesia-induced Alteration of Inhibitory Synaptic Transmission in the Developing Rat Hippocampus. Mol. Neurobiol. 2018, 55, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Lovrecic, L.; de la Garza, A.L.; Migliore, L.; Peterlin, B.; Milagro, F.I.; Martinez, A.J.; Haslberger, A.G. Therapeutic perspectives of epigenetically active nutrients. Br. J. Pharmacol. 2015, 172, 2756–2768. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.; Zhou, X.; Deng, Y.; Liu, T.; Weng, G.; Yu, D.; Pan, S. DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor. Mol. Med. Rep. 2015, 12, 1435–1442. [Google Scholar] [CrossRef]
- Mohd Murshid, N.; Aminullah Lubis, F.; Makpol, S. Epigenetic Changes and Its Intervention in Age-Related Neurodegenerative Diseases. Cell Mol. Neurobiol. 2022, 42, 577–595. [Google Scholar] [CrossRef]
- Cerejeira, J.; Nogueira, V.; Luís, P.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. The cholinergic system and inflammation: Common pathways in delirium pathophysiology. J. Am. Geriatr. Soc. 2012, 60, 669–675. [Google Scholar] [CrossRef]
- Adam, E.H.; Haas, V.; Lindau, S.; Zacharowski, K.; Scheller, B. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open 2020, 10, e031212. [Google Scholar] [CrossRef]
- Yu, A.; Wu, S.; Zhang, Z.; Dening, T.; Zhao, S.; Pinner, G.; Xia, J.; Yang, D. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst. Rev. 2018, 6, Cd012494. [Google Scholar] [CrossRef] [PubMed]
- Zujalovic, B.; Barth, E. Delirium Accompanied by Cholinergic Deficiency and Organ Failure in a 73-Year-Old Critically Ill Patient: Physostigmine as a Therapeutic Option. Case Rep. Crit. Care 2015, 2015, 793015. [Google Scholar] [CrossRef][Green Version]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzym. Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Cerejeira, J.; Batista, P.; Nogueira, V.; Firmino, H.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age Ageing 2011, 40, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Odaka, H. The Role of Neurotrophin Signaling in Age-Related Cognitive Decline and Cognitive Diseases. Int. J. Mol. Sci. 2022, 23, 7726. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Inouye, S.K. The inter-relationship between delirium and dementia: The importance of delirium prevention. Nat. Rev. Neurol. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, M.; Jakhete, S.M.; Manekar, A.G.; Sasikumar, S. Specific epigenetic regulators serve as potential therapeutic targets in idiopathic pulmonary fibrosis. Heliyon 2022, 8, e09773. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.; Spadotto, V.; Pozzi, P.; Stevenazzi, A.; Vergani, B.; Marchini, M.; Sandrone, G.; Bonetti, E.; Mazzarella, L.; Minucci, S.; et al. HDAC Inhibition as Potential Therapeutic Strategy to Restore the Deregulated Immune Response in Severe COVID-19. Front Immunol. 2022, 13, 841716. [Google Scholar] [CrossRef] [PubMed]
- Brocks, D.; Schmidt, C.R.; Daskalakis, M.; Jang, H.S.; Shah, N.M.; Li, D.; Li, J.; Zhang, B.; Hou, Y.; Laudato, S.; et al. DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats. Nat. Genet. 2017, 49, 1052–1060. [Google Scholar] [CrossRef]
| Anesthetic | DNA Methylation | Histone Modification | Association with Cognitive POD/POCD |
|---|---|---|---|
| Desflurane | n. a. | n. a. | |
| Isoflurane | DNMT1 ↓ [58] Methylation ↓ Cxcl12-methyl. ↑ methylation levels at the D-loop ↓ [59] Ephrin pathway gene methylation ↑ [60] | Acetylation ↓ [61] HDAC2 ↑ HDAC4 ↑ [62] HDAC8 ↑ [63] GLT-1 act. ↓ H3K9 and H4K12 acetylation ↓ [64] BDNF ↓ [65] H3K9 trimethy ↑ HDAC ↑ and H3-ac ↓ H4-ac ↓ neuroligin 1 ↓ [66] | Cognitive impairment in rats and mice |
| Sevoflurane | Methylation in hippocampus ↑ Kcc2 gene methylation ≠ [67] ↑ VEGF-methyl. ↑ HMGB1-methyl. ↓ MMP9-methyl. ↓ GLUR2-methyl. ↑ DNMT ↑ BDNF-methyl. ↑ | HDAC3 ↑ [68] HDAC6 ↑ [69] HDAC8 ↑ acetyl-H3K9 ↓ and acetyl-H3K14 ↓ H4-acetyl. ↓ histone acetyltransferase (HAT) ↓ | Cognitive impairment in rats |
| Midazolame | n.a. | n.a. alone (comination with isoflurane) | |
| Propofol | XRCC1-methyl-. = [70] hOGG1-methyl. = DNTM3A ↓ DNMT3B ↓ [71] | HDAC2 ↑ [72] H3-actetyl. ↓ H4-acetyl. ↓ H3K27 trimethy. ↓ HDAC1 ↓ | n.a. |
| Ketamine | COX2-methyl. ↓ [73] Hypermethylation hyper-methylation of Bdnf gene[74] | HDAC ↑ HDAC5 ↑ HDAC6 ↓ [75] H3K27m3 ↓ H3K36m3 ↓ | |
| pentobarbital sodium | HDAC2 ↑ [76] | Cognitive dysfunction in mice | |
| fentanyl | DNMT1 ↓ DNMT3A ↓ DNMT3B ↓ [77] | HDAC5 ↓ [78] Sirtuin 2 [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rump, K.; Adamzik, M. Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation. Cells 2022, 11, 2954. https://doi.org/10.3390/cells11192954
Rump K, Adamzik M. Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation. Cells. 2022; 11(19):2954. https://doi.org/10.3390/cells11192954
Chicago/Turabian StyleRump, Katharina, and Michael Adamzik. 2022. "Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation" Cells 11, no. 19: 2954. https://doi.org/10.3390/cells11192954
APA StyleRump, K., & Adamzik, M. (2022). Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation. Cells, 11(19), 2954. https://doi.org/10.3390/cells11192954





